Abstract
Background: The South African Government has recently implemented the human papillomavirus (HPV) vaccination programme through the school health system. For the vaccination programme to be effective, it is important to investigate the acceptability of the vaccines among university academics. The objective of this study was to determine the awareness and acceptability of HPV vaccination among university academics, and to investigate whether health information increases the acceptability of the vaccines.
Materials and methods: This was a cross-sectional study conducted among academics from the University of KwaZulu-Natal — excluding medical school academics. Data were collected using a self-administered anonymous questionnaire, via an online survey.
Results: It was found that most academics were aware of cervical cancer and HPV infections. The health information regarding HPV infections and vaccines had significantly increased the acceptance of HPV vaccine for their daughters (79% to 88%, p < 0.05). There was a knowledge gap regarding the safety and effectiveness of the vaccines.
Conclusion: University academics need to be educated on this preventable disease so that they can provide accurate information to their students, who are in the high-risk population for cervical cancer.
Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infection (STI) among women worldwide. It is the main cause of cervical cancer — which is preventable. Globally, more than 500 000 new cases and 275 000 deaths occur due to cervical cancer every year.Citation1 More than half of sexually active people become infected by HPV during their lifetime.Citation2 It has been estimated that HPV infection among women aged 26 years to 30 years is 14%.Citation3 There are many types of HPV — but HPV types 16 and 18 are more prevalent, and account for more than 70% of cervical cancer cases.Citation4
The advent of an HPV vaccine plays a significant role in reducing cervical cancer incidence and mortality. Two prophylactic HPV vaccines have been commercially available since 2007 and 2009, respectively. Currently, more than 100 countries have given a licence to use them. Many countries are routinely using these vaccines. Clinical trial studies have proved a very high efficacy for both vaccines.Citation5–8 Globally, the vaccination rate is still inadequate.Citation9,10
In South Africa, cervical cancer is the second most common cancer among women. The South African health facilities are providing free cervical cancer screening for women who are 30 years or older. According to the policy, women should be screened once in every 10 years, if the screening result is normal. This policy was developed to screen at least 70% of the targeted population. But studies have reported low uptake of cervical cancer screening. Last year, the South African Government implemented an HPV vaccination programme through the school health system. A pilot study conducted in 39 schools in KwaZulu-Natal province reported a high completion rate of three doses of HPV vaccination.Citation11 To make the vaccination a success, all stakeholders in the country need to contribute in this regard, and university academics have a significant role to play. For example, they can provide accurate knowledge to their students and recommend vaccinating against this preventable disease. Very little is known about HPV vaccination acceptability among university academics in South Africa. Therefore, this study seeks to investigate university academics’ awareness and the acceptability of the HPV vaccine.
Methodology
This was a cross-sectional survey conducted among all the academics working at the University of KwaZulu-Natal, except those from the medical school. It is assumed that academics from medical schools would have sufficient knowledge regarding cervical cancer and HPV vaccination, and they were therefore excluded from this study. After approval from the university ethics committee, the questionnaire was posted on the university noticeboard and the questionnaire link was provided for academics to complete the questionnaire online via the QuestionPro program (QuestionPro Inc., Seattle, WA, USA). The link was provided between November 2013 and February 2014. Participation in this study was voluntary. The anonymity and confidentiality of the participants were maintained at all times. The researcher explained the aims and objectives of the study on the title page. By completing the questionnaire, participants consented to take part in this study. The questionnaire used for this study was previously used among educated parents in India.Citation12 It consisted of socio-demographic information, awareness regarding cervical cancer and HPV, a fact sheet providing basic information on cervical cancer, information on how HPV is transmitted and on the efficacy and safety of the HPV vaccine, and, finally, on the acceptability and perception of HPV vaccination. It took participants, on average, 15 minutes to complete the questionnaire.
Data were exported to the SPSS program from QuestionPro for analysis. The results were summarised using descriptive statistics, expressed as a mean (SD) for continuous variables and percentages for categorical variables. Student’s t-test was used to compare the proportion of acceptability rate for HPV vaccination between before and after the health education intervention. A p-value less than 0.05 was considered to be statistically significant.
Results
A total of 494 academics viewed the notice and 133 of them started to complete it. However, only 45 academics completed the questionnaire adequately. Therefore, the response rate was only 9%. Participants’ socio-demographic information is summarised in Table . Almost half of the academics were aged 31 to 40 years, with the average age being 38 years (SD = 9). It was found that more than two-thirds were male (71%), married (71%), and half of them had a master’s degree (51%), and were working in a lecturer position (55%) (Table ).
Table 1: Socio-demographic information on the participants
Most respondents (79%) indicated that they had vaccinated their children for their childhood vaccinations. Of those who vaccinated their children, 88% mentioned a vaccine preventing certain diseases as the main reason to vaccinate their children. With regard to concern about the vaccines, the seriousness of the disease (58%) followed by the effectiveness of the vaccines (23%) were mentioned by the academics. Of the six participants who did not vaccinate their children, 83% did so because they were anxious about the side effects of the vaccine and 17% thought the vaccines were of no use (Table ).
Table 2: Important reasons and concerns regarding vaccination of children
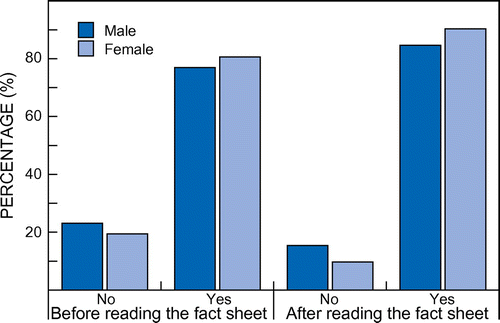
Almost all the academics (96%) had heard of cervical cancer and all had heard about HPV. Some 80% of the participants initially were willing to vaccinate their daughters against cervical cancer. After reading the factual information on cervical cancer and HPV, the acceptability rate (to vaccinate their daughter against cervical cancer) increased significantly (89%) (p < 0.05) (Figure ). When asked about the best time to vaccinate, 72.5% mentioned that HPV vaccination should be given before their daughters are mature enough to understand about sex, followed by waiting until the daughter is grown up and can decide for herself (22.5%), and suggesting vaccination just before a daughter gets married (5%). Those who still did not want to vaccinate their daughters gave the reason that the vaccines are new and that they were not sure if it would be safe for their child. All these academics (five) indicated that a more detailed report on the safety and effectiveness of the vaccine might change their minds with regard to vaccinating their daughters.
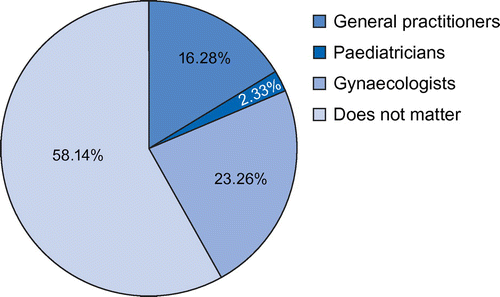
Table summarises participants’ perception regarding HPV vaccination. With regard to participants’ perception of the HPV vaccination, 73% agreed that it is necessary to explain to their daughters before vaccination that it protects them against sexually transmitted infection. Most academics disagreed that vaccination may convey a no-objection ‘message’ from the parents to start sexual relationships (84%), or that they would avoid discussing matters related to sex with a daughter if she wanted to know about the vaccine/papilloma virus (95%). When asked who should give the vaccines, more than a third indicated that it did not matter to them (58%), followed by vaccination by gynaecologists (23%) (Figure ).
Table 3: Participants’ perception of HPV vaccination (%)
Discussion
This study investigated the awareness and acceptability of HPV vaccination among academics at the University of KwaZulu-Natal, South Africa. Awareness and acceptability of cervical cancer and the HPV vaccine was high in this study population. Cervical cancer awareness and HPV vaccination acceptability varies globally. A South African study conducted among educated people such as master of business administration (MBA) students reported that most participants were aware of cervical cancer, but only about a quarter had heard about HPV.Citation13 A Chinese study conducted among employed women and undergraduate students found that 28% of employed women and 12% of university students had heard of HPV, while only 21% of employed women and 7.2% of students knew that HPV is related to cervical cancer.Citation14 The Indian study found that only a third of the educated parents of adolescents were aware of cervical cancer.Citation12
Vaccination is one of the most cost-effective public health interventions as it prevents serious diseases. In South Africa, vaccination coverage has increased considerably due to fully vaccinating one-year-olds. This includes oral polio vaccine (OPV) at birth and at 6 weeks, Bacille Calmette Guérin (BCG) at birth, rotavirus vaccine (RV) given at 6 and 14 weeks, both hepatitis B (Hep B) and a pentavalent (diphtheria, tetanus, acellular pertussis, inactivated polio virus, and Haemophilus influenzae type (b) vaccine (DTaP-IPV/Hib)) administered at weeks 6, 10 and 14; pneumococcal conjugate vaccine (PCV13) at weeks 6 and 14; and both PCV13 and the first dose of measles vaccine given at 9 months.Citation15,16 In this study, 79% of the academics vaccinated their children at the required ages. This finding is quite low, as the previous study conducted among educated people in South Africa reported 90% of the participants vaccinating their children using the regular vaccines.Citation13 It is expected that the current group of people are educated and aware of the benefits of the vaccines. The low rate of vaccination could be a result of the influence of the anti-vaccination lobby in South Africa. In South Africa, the media and anti-vaccination websites and blogs are evidence of such lobbies. One type in this lobby comprises affluent, relatively educated, mainly white individuals, including homeopaths, dentists, paediatricians, nurses, and their clients, who use mass media, including radio, TV, newspapers, popular magazines, websites and internet blogs to communicate misinformation about vaccines. The second type in the lobby are poor, relatively uneducated, mainly black, religious/cultural/traditional groups who do not use mass media.Citation17 Anti-vaccination groups normally provide incorrect information concerning the side effects of the vaccines.Citation18
Generally, HPV vaccine acceptability is high. Most (80%) of the academics were willing to vaccinate their daughters against cervical cancer. This finding is similar to that of a previous study conducted among educated people in South Africa.Citation13 A recent Nigerian study reported that 70% of mothers accepted HPV vaccination of their daughters.Citation19 A systematic review study conducted in sub-Saharan Africa regarding knowledge and awareness of HPV vaccine and acceptability to vaccinate highlighted that all 12 studies examined had high levels of acceptability of the HPV vaccine.Citation20
It has been proved that health education increases the acceptability of HPV vaccination. In the present study, the vaccine acceptability increased significantly, from 80% to 89%. A Chinese study, which conducted an informative group lecture, found a significant increase in HPV vaccine acceptability among employed women (77% to 90%) and university students (73% to 82%).Citation14 Studies conducted in many parts of the world reported the positive impact of educational intervention with regard to HPV vaccine acceptance. For example, an American study conducted using HPV education-intervention sessions among parents, healthcare staff and school staff reported that the education intervention increased HPV knowledge among all the participants.Citation21 Another study evaluated a narrative intervention aimed at increasing HPV vaccination among college women, and found that the combined peer–expert narrative intervention doubled vaccination acceptance compared with controls.Citation22 Studies in Malawi and Tanzania also found that HPV vaccination acceptance increased if basic information was provided.Citation23,24
The HPV vaccines should ideally be administered before becoming sexually active, which is the main exposure to HPV.Citation25 For this reason, the Advisory Committee on Immunization Practices (ACIP) recommended routine HPV vaccination for females in 11- to 12-year-olds and catch-up vaccination until 26 years.Citation25 In the present study, 72.5% of respondents mentioned that HPV vaccination should be given before their daughters are mature enough to understand about sex. This finding is similar to the previous South African study conducted among educated people.Citation13 Studies from the USA have reported that the proportion of HPV vaccination increases with age: between 11–12 years and 13–17 years.Citation26,27
The HPV vaccine is relatively new. Therefore, it will face some challenges. Effectiveness and side effects are the main concerns raised by the general public.Citation28 In this study, it was found that after providing accurate information about HPV vaccines, five participants still did not want to vaccinate their daughters because of possible side effects and ineffectiveness of the vaccines. Other studies in South Africa, Ghana and Tanzania reported the same.Citation24,29,30 Therefore, it is important to educate people regarding the side effects and effectiveness of the vaccines through the media and through health care workers as they are the most trusted people when it comes to health issues.
This study is not without limitations. The sample is very small. This is due to time constraints and non-response. The sample comes from one university and therefore the results cannot be generalised to a similar population group or the general population. Anonymity and confidentiality might have reduced the information bias as these were self-reported data. Despite all these limitations, the study had its strengths. For example, this was the first study that has investigated university academics’ awareness and acceptability of HPV vaccines in South Africa. The findings will be beneficial for future planning with regard to vaccination coverage in the country.
Conclusion
The academics of the University of KwaZulu-Natal were aware of cervical cancer. The educational information had significantly increased HPV vaccine acceptability for their daughters. There were, however, still some challenges among the academics with regard to vaccine safety and effectiveness. Therefore, it is important to educate university academics concerning the importance of HPV vaccines so that they can provide accurate information to the university community at large. Future studies should consider a larger sample size, and include more universities.
Conflict of interest
There is nothing to declare.
Acknowledgements
The author wishes to thank all the participants who voluntarily participated in the study.
References
- Forman D, de Martel C, Lacey CJ, et al. Global Burden of Human Papillomavirus and Related Diseases. Vaccine. 2012;30:F12–23.10.1016/j.vaccine.2012.07.055
- Centre for Disease Control and Prevention. 2010 [cited 2014 Sep 24]. Available from: http://www.cdc.gov/hpv/.
- Chan PKS, Ho WCS, Wong MC, et al. Epidemiologic risk profile of infection with different groups of human papillomaviruses. J Med Virol. 2009;81:635–44.
- Bruni L, Barrionuevo-Rosas L, Albero G, et al. ICO information centre on HPV and cancer (HPV information centre). Human Papillomavirus and Related Diseases in South Africa, 2014. Summary Report 2014-12-18. 2014 [cited 2015 Jan 15]. Available from: http://www.who.int/hpvcentre.
- Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–32.10.1002/(ISSN)1097-0215
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364(5):401–11.10.1056/NEJMoa0909537
- Lu B, Kumar A, Castellsagué X, et al. Efficacy and safety of prophylactic vaccines against cervical hpv infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;11:13.10.1186/1471-2334-11-13
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–85.10.1056/NEJMoa1010971
- US Department of Health and Human Services. Healthy people 2020. 2010 [cited 2014 Oct 17]. Available from: www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=23.
- Markowitz LE, Tsu V, Deeks SL, et al. Human papillomavirus vaccine introduction—the first five years. Vaccine. 2012;30(5):F139–48.10.1016/j.vaccine.2012.05.039
- Moodley I, Tathiah N, Mubaiwa V, et al. High uptake of Gardasil vaccine among 9–12-year-old schoolgirls participating in an HPV vaccination demonstration project in KwaZulu-Natal, South Africa. S Afr Med J. 2013;103:313–7.
- Basu P, Mittal S. Acceptability of human papillomavirus vaccine among the urban, affluent and educated parents of young girls residing in Kolkata, Eastern India. J Obstet Gynaecol Res. 2011;37(5):393–401.10.1111/jog.2011.37.issue-5
- Hoque ME, Van Hal G. Acceptability of human papillomavirus vaccine: a survey among Master of Business Administration students in KwaZulu-Natal, South Africa. Biomed Res Int. 2014:257807. doi: 10.1155/2014/257807.
- Chang IJ, Huang R, He W, et al. Effect of an educational intervention on HPV knowledge and vaccine attitudes among urban employed women and female undergraduate students in China: a cross-sectional study. BMC Pub Health. 2013;13:916.10.1186/1471-2458-13-916
- South African Department of Health. Expanded programme on immunisation — EPI (SA) revised childhood immunisation schedule from April 2009. [cited 2015 Jan 23]. Available from: http://www.kznhealth.gov.za/vaccinations.pdf.
- Health Systems Trust. The district health barometer 2008/09. Part A: indicator comparison by districts. output indicators: immunisation. 2010 [cited 2015 Feb 5]. Available from: http://www.hst.org.za/sites/default/files/dhb080931.pdf.
- Burnett RJ, Larson HJ, Moloi MH, et al. Addressing public questioning and concerns about vaccination in South Africa: A guide for healthcare workers. Vaccine. 2012;30:C72–8.10.1016/j.vaccine.2012.03.037
- Crowcroft NS, McKenzie KJ. Inoculating communities against vaccine scare stories. Lancet Infect Dis. 2013;13:564–5.10.1016/S1473-3099(13)70131-2
- Ezeanochie MC, Olagbuji BN. Human papilloma virus vaccine: determinants of acceptability by mothers for adolescents in Nigeria. Afr J Reprod Health. 2014;18(3):154–8.
- Perlman S, Wamai RG, Bain PA, et al. Knowledge and awareness of HPV vaccine and acceptability to vaccinate in Sub-Saharan Africa: a systematic review. PLoS ONE. 2014;9(3):e90912. doi:10.1371/journal.pone.0090912.
- Reiter PL, Stubbs B, Panozzo CA, et al. HPV and HPV vaccine education intervention: effects on parents, healthcare staff, and school staff. Cancer Epidemiol Biomarkers Prev. 2011;20(11):2354–61.10.1158/1055-9965.EPI-11-0562
- Hopfer S. Effects of a narrative HPV vaccination intervention aimed at reaching college women: a randomized controlled trial. Prev Sci. 2012;13:173–82.10.1007/s11121-011-0254-1
- Ports KA, Reddy DM, Rameshbabu A. Barriers and facilitators to HPV vaccination: perspectives from malawian women. Women Health. 2013;53(6):630–45.10.1080/03630242.2013.809046
- Remes P, Selestine V, Changalucha J, et al. A qualitative study of HPV vaccine acceptability among health workers, teachers, parents, female pupils, and religious leaders in northwest Tanzania. Vaccine. 2012;30(36):5363–7.10.1016/j.vaccine.2012.06.025
- Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2007;56(RR-2):1–24.
- Centre for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13-17 years — United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(34):685–93.
- Hofstetter AM, Stockwell MS, Al-Husayni N, et al. HPV vaccination: are we initiating too late? Vaccine. 2014;32(17):1939–45.10.1016/j.vaccine.2014.01.084
- Smith PJ, Humiston SG, Marcuse EK, et al. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the health belief model. Public Health Rep. 2011;126(2):135–46.
- Harries J, Moodley J, Barone MA, et al. Preparing for HPV vaccination in South Africa: key challenges and opinions. Vaccine. 2009;27(1):38–44.10.1016/j.vaccine.2008.10.033
- Coleman MA, Levison J, Sangi-Haghpeykar H. HPV vaccine acceptability in Ghana, West Africa. Vaccine. 2011;29(23):3945–50.10.1016/j.vaccine.2011.03.093