Abstract
Objectives: To identify the toxicity profile of snakebites and to assess clinical severity.
Methods: An analysis of all patients admitted to Ngwelezane Hospital’s Emergency Department with a diagnosis of snakebite over five years was done. All patients were admitted, assessed and standard haematological and biochemical tests were done. Patients were observed for a minimum of 12 hours’ observation.
Results: In total, 879 cases were analysed. Envenomation was identified in over two-thirds of admissions. Cytotoxic snakebites accounted for 98% of envenomations. Only four cases of haemotoxic bleeding and five cases of neurotoxicity were admitted. Abnormal laboratory indices correlated with severity: INR > 1.5 (odds ratio 2.25, CI 1.12–4.53; p = 0.023), platelets < 100x109/L (OR 2.35, CI 1.01– 5.49; p = 0.048), haemoglobin concentration < 8.0 g/dL (OR 5.68, CI 2.15–15.00; p < 0.001) and leucocyte count > 10x109 (OR 3.15, CI 1.89– 5.26, p < 0.001). Children and delays to admission correlated to and were predictors of severity.
Conclusion: Two-thirds of patients who present to hospital with snakebite will have symptoms of envenomation, with the overwhelming majority having been bitten by cytotoxic species. Some factors correlate to severity and may be useful for anticipating the patient’s clinical course.
Introduction
Snakebite constitutes a serious and neglected public health problem in much of the developing world.Citation1 Of 3 496 species of snake identified worldwide as of April 2015,Citation2 approximately 600 are venomous.Citation3 In South Africa there are some 38 venomous species, of which approximately half pose a significant threat to humans and a potentially fatal bite.Citation4
There is extensive variability in snakebite from region to region, dependent on the range of species locally present, and environmental and population factors. Given the variability, however, it is essential that experience is reported with reference to a specific area, the characteristics of the snake species inhabiting the region, and also the specific characteristics of the population of that area.Citation5
In terms of global burden of disease, Southeast Asia carries the highest burden, followed by sub-Saharan Africa.Citation6 The difficulty inherent in establishing reliable and representative data for sub-Saharan Africa has been highlighted in a recent meta-analysis.Citation7 The highest incidence of snakebite in South Africa is in the rural north-eastern coastal belt of KwaZulu-Natal. Small local studies have suggested an annual incidence of snakebite in north-eastern parts of the province of 28–96.5 per 100 000.Citation5,8–10 Our own recent studies, extrapolated from antivenom utilisation, suggest an incidence within this range (manuscript currently under review).
The snake species most commonly responsible for serious snakebites in KwaZulu-Natal are the puff adder (Bitis arietans), a viperid, and the Mozambican spitting cobra (Naja mossambica), an elapid. Both produce potent cytotoxic venom. Other snakes responsible for severe bites include four elapids with neurotoxic venom: the forest cobra (Naja melanoleuca), black mamba (Dendroaspis polylepis), green mamba (Dendroaspis angusticeps) and snouted cobra (Naja annulifera); species with cytotoxic venom, the gaboon adder (Bitis gabonica) and the rinkhals (Hemachatus hemachatus) and a colubrid with haemotoxic venom, the boomslang (Dispholidus typus).Citation4 Deaths due to snakebite appears to be infrequent and the reported mortality from these small studies in South Africa is low (0.08–2.67 per 100 000).Citation7
Studies over the past 50 years in KwaZulu-Natal have reported similar results with respect to demographics, seasonal distribution, bite characteristics, antivenom use and mortality,Citation5,8–11 suggesting that little progress has been made in terms of prevention strategies or treatment practice over the past few decades, and confirming snakebite’s status as a neglected disease.Citation12
We have previously reported our experience of 243 snakebite admissions over a year period in 2007–2008.Citation11 We now follow this up with a larger study over a five-year period. The purpose of our study was to identify the presenting features of snakebites, to determine the proportion of envenomation, to assess clinical severity, and to identify possible factors that correlate to severity.
Subjects and methods
Setting
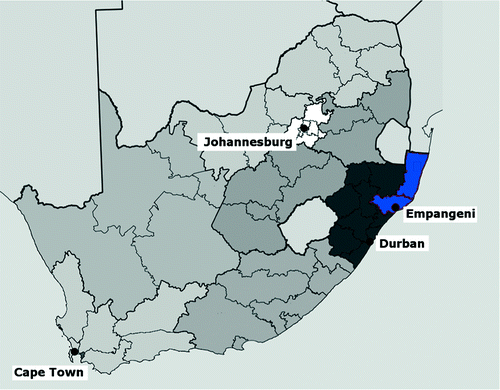
Ngwelezane Hospital is the major referral hospital in the subtropical north-east of KwaZulu-Natal (Figure ) and serves close to 3 million people. It serves the local population of the uThungulu district and provides regional and tertiary care to 20 district hospitals in the uMkhanyekhude and Zululand districts. The specialist-led Emergency Department (ED) admits all snakebites for a period of observation and workup from the local area and takes referrals for more severe snakebites from district hospitals.
Figure 1: High-incidence snakebite setting in southern Africa. The province of KwaZulu-Natal is shaded in dark grey and abuts on Swaziland and Mozambique in the north. The areas in blue are the Uthungulu (south) and Ukhanyakude (north) administrative districts. These two districts constitute a significant part of the catchment area for Ngwelezane Hospital, situated in the town of Empangeni, are coastal and subtropical in climate, and have a high incidence of snakebite.
Study sample
Over a five-year period, all patients admitted to Ngwelezane Hospital’s Emergency Department with a diagnosis of snakebite were studied. Baseline demographic data were captured on a protected database prospectively and files were retrieved retrospectively from hospital records for more detail such as blood test results and treatment received. Patients whose case files could not be located were excluded from the study. In our local setting patients who are less than 12 years of age are treated as paediatrics cases and are thus grouped as children in this study. Patients were categorised according to clinical presentation. Three categories were identified as Painful Progressive Swelling, ± necrosis (PPS), Bleeding (B) or Progressive Weakness (PW). Patients were further categorised according to severity at presentation: mild, moderate or severe.
Standardised patient care
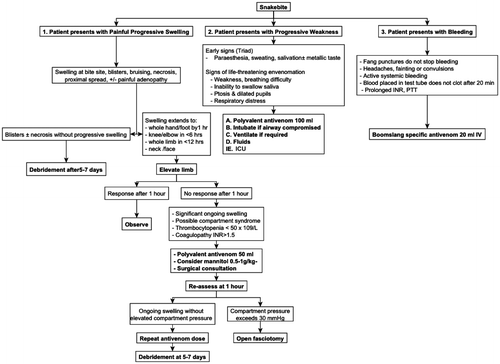
All patients were treated using a standard institution protocol adapted from the guidelines published by BlaylockCitation13 (Figure ). Bites from snakes were confirmed by identifying the presence of characteristic fang marks. All patients were admitted, assessed and standard haematological and biochemical tests were done. Patients received tetanus prophylaxis and, where indicated, analgesia, fluid therapy, antibiotics, and blood products. All patients were observed for a minimum of 12 hours.
Figure 2: Treatment algorithm for management of snakebite in northern KwaZulu-Natal.
Statistical analysis
All data were collected and entered into EpiData. Analysis was performed using Stata version 13 (StataCorp LP, College Station, TX, USA) and MedCalc version 15.4 (MedCalc Software bvba, Ostend, Belgium). Significant differences in categorical data were assessed using standard Pearson chi-square or Fisher’s exact test. Ordinal data were compared using Student’s t-test or, for non-parametric parameters, the Mann–Whitney U-test. Correlations were analysed using Spearman rank order correlation. Multivariable logistic regression was used to assess factors associated with severity to adjust for the influence of other risk factors and potential confounders. All confidence intervals (CI) are quoted at the 95% probability level. Abbreviations for relevant statistical data include inter quartile range (IQR) and odds ratios (OR). A p-value of < 0.05 was deemed significant.Ethics approval was granted by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal ref: BE034/14.
Results
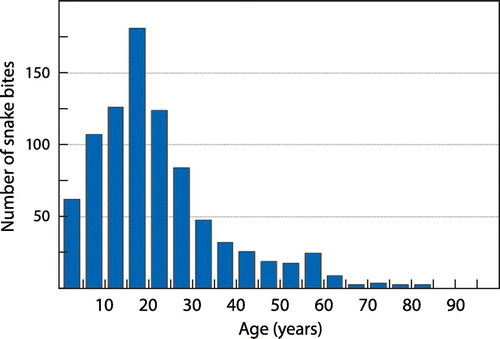
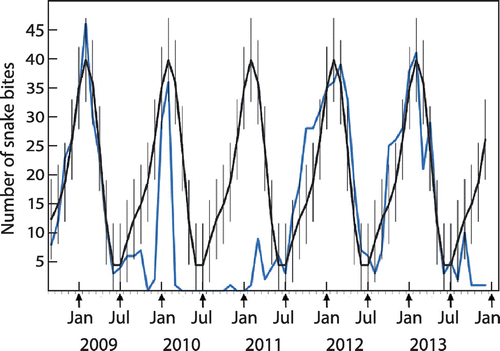
Data from 879 case files were analysed. Using cyclical regression modelling, we showed a strong repetitive seasonal cycle with a noticeable peak from December to March (Figure ). The age distribution is shown in Figure . The median age at admission was 18 years (IQR 12–28). Although the distribution was essentially similar for both males (447/879) and females (432/879), the median age was slightly higher in females (20 years, CI 18–21) versus males (17 years, CI 17–19) (p = 0.0009). We documented 222/879 admissions for children aged less than 12 years, representing a quarter of all admissions.
Figure 3: Seasonal distribution of snakebite incidence from September 2008 to December 2013 using cyclical regression. The incidence peaks in January (midsummer) and has a trough in July (midwinter).
Clinical features
Envenomation was identified in about two-thirds of admissions (534/879); 74% (164/222) for patients younger than 12 years versus 57% (370/657) of patients older than 12 years (odds ratio 2.19, CI 1.57–3.07, p < 0.001). The number of dry bites (no envenomation syndrome evident) was high (Figure ). There was a statistically significant decreasing trend in the risk of envenomation from the first through to the third decade. Isolated signs of bleeding (haemotoxic envenomation) and muscle weakness (neurotoxic envenomation) accounted for only 4 and 5 of the 879 patients respectively, while 98% (525/534) of envenomed patients presented with the syndrome of painful progressive swelling (PPS) following a cytotoxic bite. Three patients presented with painful inflamed eyes following ocular exposure to the venom of a spitting cobra.
Figure 5: Proportion of cases envenomed per decade of life. Confidence intervals for the proportion are shown by the white bars. The proportion for the second decade is significantly lower than that for the first decade (p < 0.0001); the proportion for the third decade is significantly lower than that for the first decade (p < 0.0001) and the second decade (p = 0.002). No significant differences are shown for the fourth to eighth decades: numbers of subjects are smaller and confidence intervals correspondingly wider.
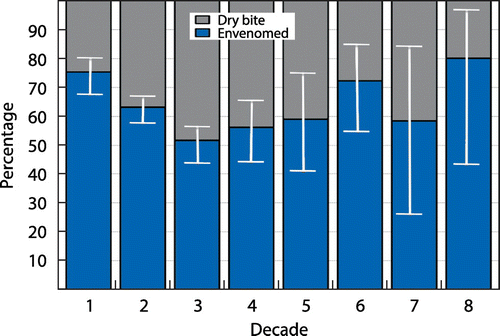
In order to study possible predictors of severity, we defined a subset of the patients with PPS as severe if they met the criteria for antivenom administration (see Figure ) or required surgery. Some 16% (137/879) of patients met the criteria for severe PPS, while the remainder of severe presentations were due to neurotoxicity (5/879) and haemotoxicity (4/879). The remainder of the envenomed patients had lesser degrees of severity (388/879 or 44%) and were classified as moderate. Patients with no local effects from the bite or a minimal inflammatory reaction and pain confined to the bite itself (345/879 or 39%) were classified as mild and termed as dry bite. In all, 45% of (73/164) children with envenomation were classified as severe, compared with 20% (73/370) in adults (odds ratio 3.26, CI 2.19–4.87, p < 0.001).
Laboratory values
Median INR was 1.08 (IQR 1.01–1.18). Of these, 57 (8.2%) were above 1.5. Haemoglobin was essentially normal, with a median value of 12.4 g/dL (IQR 11.1–13.5). Leucocytosis was common. The median WCC count was 9.0 x 109/L (n = 749, IQR 6.8–12.0); 217 had values greater than 11x109/l. The median urea of 3.4 mmol/L (n = 664, IQR 2.6–4.2). Only 16 subjects showed a urea greater than 7.1 mmol/L. The median creatinine concentration was 63 μmol/L (n = 652, IQR 47–78); 22 patients had a value exceeding 115 umol/L (3.4%). No patient in this study required dialysis.
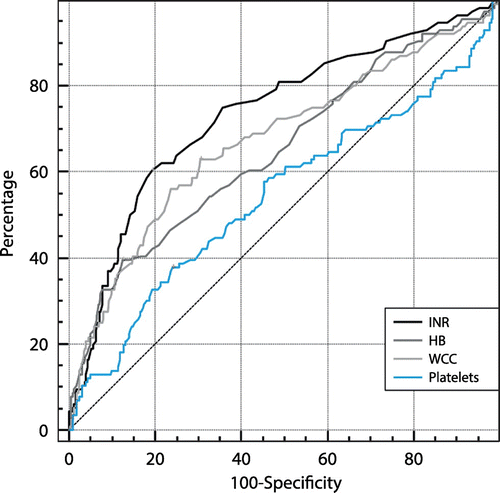
Abnormal laboratory indices correlated significantly with severity: INR > 1.5 (odds ratio 2.25, CI 1.12–4.53; p = 0.023), platelets < 100x109/L (OR 2.35, CI 1.01–5.49; p = 0.048), haemoglobin concentration < 8.0 g/dL (OR 5.68, CI 2.15– 15.00; p < 0.001) and leucocyte count > 10x109 (OR 3.15, CI 1.89– 5.26, p < 0.001). However, their value as accurate predictors for severity is limited by poor sensitivity and specificity. ROC curves are shown in Figure . The most predictive laboratory index for severity was the INR; the ROC curve analysis returned an AUC of 0.75 (CI 0.70–0.79).
Figure 6: Receiver operating characteristics (ROC) curves: laboratory indices as predictors of categorisation as severe. All indices are poorly predictive; the best performing is INR (AUC: 0.75, CI 0.70–0.79). The Youden index J corresponds to an INR value of 1.18, with a sensitivity of 60.8% and a specificity of 81.2%.
Delayed presentation from bite
Information on the time from bite to admission was available in 743/879 cases. The median time was 5 hours (IQR 3–10 hours). A delay in presentation was significantly associated with increasing severity. The median elapsed time of the patients with moderate acuity was 5 hours (IQR 3.8–10 hours), and for patients with severe acuity 10 hours (IQR 7–16.8 hours, p < 0.0001).
Referred patients
Most patients were residents of the immediate area (782/879) and only 11% (89/879) had been transferred from outlying district hospitals. Patients transferred from district hospitals to the Emergency Department had a significantly longer delay before presentation than local admissions; a median time of 14 hours (IQR 8–20 hours) versus 5 hours (IQR 8–20 hours, p < 0.001). Patients who were referred with envenomation from outlying hospitals for snakebite were 15 times more likely to be severe than admissions from the local area, 74% (78/106) versus 16% (68/427) respectively (OR 14.7, CI 8.89–24.30, p < 0.001) and 11 times more likely to require antivenom (OR 10.84, CI 6.56–17.89, p < 0.001). Logistic regression suggested that in both cases the elapsed time was redundant when the referral variable was included.
Management and outcome
Few patients presented with serious systemic toxicity. Fluid and repeated inotrope resuscitation was required in four patients, all of whom had had severe systemic allergic reactions to antivenom. One patient required ventilation. There were no deaths. A total of 96 patients (11.5%) received antivenom. Of these 4.2% (4/96) patients received monovalent antivenom following envenomation by Dispholidus typus, a haemotoxic species; the remaining 92 patients received polyvalent antivenom: 5 for neurotoxic envenomation, 87 for severe PPS following cytotoxic envenomation. Children were more likely to require antivenom (45/164 envenomations, 27%) than adults (51/370 envenomations, 14%) (OR 2.37, CI 1.5–3.72; p < 0.001). The median dose of antivenom administered per patient was 40 ml (IQR 40–100). Anaphylaxis was documented in 23% (23/96) of patients who received antivenom.
Surgery was performed in 16% (82/525) of patients with PPS; in some patients more than one procedure was performed during their admission. Of these, debridement of the wound with removal of necrotic tissue (76/128 procedures, 59%) and skin grafting (42/128 procedures, 31%) were the most common surgical interventions. Both fasciotomy and amputation were rare in this group: 2% and 6% respectively.
Discussion
Clinical presentation
Snakebites constitute almost 10% of all admissions to the Ngwelezane ED over the high incidence months of December to March. The snakebite incidence in our study for the local district, uThungulu, is 32 per 100 000 population, which is comparable to previous estimates.Citation5,8–10 This is considerably higher than the incidence of 2.34–3.3 per 100 000 extrapolated for southern sub-Saharan Africa as a whole and exceeds even the value of 12.94–22.61 per 100 000 predicted for eastern sub-Saharan Africa, the area immediately to the north of our province, by a global study.Citation6
The age distribution is interesting (see Figure ). The sex incidence is equal, and the patients young. This pattern does not appear to have changed significantly from earlier series in our areaCitation5,8–11 and in the Kangwane area of far north-east South Africa, which shares a similar climate.Citation14 The peak risk relative to population distribution is noted in the 10–14 age group, and risk tails off rapidly on either side of this, though females remain at increased risk longer than males, until the age of 24. This pattern contrasts sharply with that seen in both Southeast Asia and South America,Citation15–19 where it is predominantly young men at risk, bitten in the course of agricultural activities. In our area agricultural activities are not reserved for men, and women are responsible for the tending of agricultural fields and the drawing of water from rivers.
The phenomenon of dry bites from venomous snakes is well described and a third of all admissions in this study showed no signs of an envenomation syndrome. In south Asia rates of envenomation have been estimated at 10–40%,Citation15 and 23.2% in sub-Saharan Africa.Citation7 We are, however, surprised to note that the rate of dry bites we observed (39%) was three times that previously reported from our area (13%) in 2004.Citation8
Nearly all our patients exhibited PPS as a consequence of cytotoxic envenomation. We admitted very few patients with evidence of bites from neurotoxic or haemotoxic species. This pattern has not changed from the series in 2004Citation8 where a ratio of 7 PW:282 PPS was recorded. Indeed, in our environment and contrary to received wisdom, neurotoxic envenomation is rare.Citation5,8,11 The added rarity of haemotoxic envenomation contrasts with the Asian and North American experience where bites from various viperid and crotaline species commonly cause significant haemotoxicity, often in combination with cytotoxicity.
A striking finding is that serious systemic toxicity is extremely rare in our environment. Very few patients required fluid resuscitation or haemodynamic support, and this was always for allergic reactions to antivenom rather than for the effects of the venom itself. Few of our cases showed elevations in urea and creatinine, renal failure was not a significant factor and no patient required dialysis. Thus acute kidney injury secondary to hypovolaemia or rhabdomyolysis does not appear to be a significant complication of envenomation by southern African snakes in contrast with experience elsewhere, such as India and the Americas, where the incidence of snakebite-related acute renal failure may reach 30%.Citation20,21
Laboratory findings
A bleeding tendency and prothrombin time have shown to be predictive of an adverse outcome in India.Citation22 Our experience suggests that standard laboratory investigations may be helpful in guiding treatment in our population. Several simple abnormal laboratory indices (INR, WCC, platelets and haemoglobin) alluded to a positive correlation with severity; however, their predictive prognostic use could not be supported in this series. Further research on this is required.
Children
Children were nearly twice as likely to be envenomed as adults, and there was a significant decreasing trend in the likelihood of envenomation from the first to the third decade of life. Our clinical experience, though, does suggest that children are particularly vulnerable to snakebite.Citation11 This may be explained by the greater volume of venom to body size ratio in children compared with adults.Citation13 We found that children are significantly more likely to have severe presentations and to require antivenom, and almost half of all antivenom we prescribed was for children. The dose of antivenom required to neutralise venom-derived toxins is proportional to the potency and volume of the injected venom, not the bodyweight of the patient. Consequently children require the same volumes of antivenom as adults,Citation4 with a higher risk of adverse effects. Irrespective of risk, the management of envenomed children is particularly challenging.
Treatment
The treatment protocol we follow would appear to be satisfactory in that we observed zero mortality and only 4.3% of patients required amputation, fasciotomy or skin grafting. The proportion of antivenom prescribed was 11.5% (96/879 patients) with close to a quarter of patients experiencing anaphylaxis. This prescribing rate has remained remarkably constant across several studies in KwaZulu-Natal over the past 23 years, ranging from 9% to12% in 5 studies.Citation5,9–11
The majority of cases requiring antivenom administration in our series exhibited severe PPS, accounting for 87 of 96 cases. The protocol we follow, put forward by Blaylock,Citation13 is essentially based on empirical observation only. A significant proportion of our cases with PPS, 55 of 137, did not require surgery following antivenom administration. This finding supports the thesis that antivenom, when given early, can prevent the progression of swelling and necrosis significantly to the level where surgery may be unnecessary and simple wound care would suffice.
The polyvalent antivenom in use in southern Africa, currently produced by South African Vaccine Producers (Pty) Ltd (SAVP), is a polyvalent partially purified equine-derived antiserum, first introduced in 1928 and active against the venoms of two species,Citation23 which has been modified at intervals by the addition of further valences, such that it is currently marketed as being active against the venoms of 10 dangerous species. It has, however, not been changed in over 30 years. By modern standards, the degree of purity and specificity is not acceptable, as evidenced by the very high rate of anaphylaxis. The high cost of the added purification processes for antivenom precludes its introduction in many countries. Despite this, all were managed successfully with pre-dose and where required post-dose adrenaline. Only 4.2% manifested a persistent anaphylactic shocked state. This group of patients displayed a circulatory collapse with significant hypotension, loss of peripheral pulses and a depressed level of consciousness. Repeat doses of adrenaline and a fluid bolus immediately after onset of the shock was effective in all but one case, which required an adrenaline infusion and short-term ventilation.
Delays
Delayed presentation and referral from an outlying hospital were strongly correlated with severity. Though this may be due in part to referral bias, with more severely affected cases being preferentially referred, logistic regression analysis suggested a high degree of co-variation for referral and elapsed time since bite. Delays in transfer and in initiating treatment may worsen outcome. Antivenom should be administered as soon as possible to halt the effects of early tissue damage, and a delay in antivenom administration reduces its effectiveness: a recent study in India has confirmed a correlation between a shorter time to presentation and a less severe outcome.Citation24
Surgery
PPS is by far the commonest clinical presentation of snakebite in our setting. Patients must be monitored for progression of swelling, compartment syndrome and tissue necrosis. Less than a quarter of patients with PPS require surgery in our setting, resulting in prolonged hospital stays and rehabilitation. The symptoms of cytotoxic subcutaneous tissue damage can mimic an impending intramuscular compartment syndrome and may result in an unnecessary fasciotomy (only 2% in our series).
Envenomation within the muscle groups is very rare: in a series of 42 patients studied by us with ultrasound, we showed that only one had evidence of serious muscle swelling whereas in all other cases the swelling was confined to the subcutaneous tissues (manuscript currently under review), a finding in keeping with another recent study.Citation25 Current guidelines do not recommend performing a prophylactic fasciotomy merely on the basis of the clinical observation of an alarmingly swollen limb, without direct clinical or invasive evidence of an established compartment syndrome.Citation15,26−33 It has been shown that North American patients admitted to hospital following crotaline snakebite had a fivefold increased risk of fasciotomy if the primary care was provided by a surgeon rather than a non-surgical clinician.Citation31 However, limb-saving fasciotomy is indicated when there are sustained high compartment pressures exceeding 30 mmHg and an inadequate response to antivenom.
Limitations
The study is limited in that has been conducted at a single centre. Greater significance and a more reflective extrapolation of results could be achieved in a multi-centre study at sites throughout the province of KZN and even South Africa. The retrospective design for detailed analysis reduces the power and accuracy of the study. A prospective design would be preferable.
Conclusion
We believe that our experience is relevant to much of southern Africa, since many of the species involved are present throughout the region. Certain parameters such as delay to admission and being children are predictive of severity. Outcomes in snakebite have repeatedly been shown to be positively influenced by increased knowledge of snakebite amongst local practitioners.Citation34,35
Declaration
The contributing authors declare no competing interests.
Acknowledgements
Support funding by MEPI (Medical Education Partner Initiative); US Global Aids coordinator; US Department of Health and Human Services; National Institutes of Health. Grant number: R24W008863. The contents are solely the responsibility of the authors.
References
- Gutiérrez JM. Improving antivenom availability and accessibility: science, technology, and beyond. Toxicon. 2012;60(4):676–87.10.1016/j.toxicon.2012.02.008
- Uetz, P., Hošek, J. The reptile database. 2015 [cited 23 Jun, 2015]. Available from http://apps.who.int/bloodproducts/snakeantivenoms/database/.
- World Health Organization. (2015). Venomous snakes distribution and species risk categories [cited Jun 4 2015]. Available from: http://apps.who.int/bloodproducts/snakeantivenoms/database/.
- Müller GJ, Modler H, Wium CA, et al. Snake bite in southern Africa: diagnosis and management. Continuing Med Edu. 2012;30(10):362–82.
- Coetzer PW, Tilbury CR. The epidemiology of snakebite in northern Natal. S Afr Med J. 1982;62(7):206–12.
- Kasturiratne A, Wickremasinghe AR, de Silva N, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5(11):e218.10.1371/journal.pmed.0050218
- Chippaux J-P. Estimate of the burden of snakebites in sub-Saharan Africa: a meta-analytic approach. Toxicon. 2011;57(4):586–99.10.1016/j.toxicon.2010.12.022
- Blaylock R. Epidemiology of snakebite in Eshowe, KwaZulu-Natal, South Africa. Toxicon. 2004;43(2):159–166.10.1016/j.toxicon.2003.11.019
- Sloan DJ, Dedicoat MJ, Lalloo DG. Healthcare-seeking behaviour and use of traditional healers after snakebite in Hlabisa sub-district, KwaZulu Natal. KwaZulu Natal. Trop Med Int Health. 2007;12(11):1386–90.10.1111/tmi.2007.12.issue-11
- Wilkinson D. Retrospective analysis of snakebite at a rural hospital in Zululand. S Afr Med J. 1994;84(12):844–7.
- Wood D, Webb C, DeMeyer J. Severe snakebites in northern KwaZulu-Natal: treatment modalities and outcomes. S Afr Med J. 2009;99(11):814–8.
- Gutiérrez JM, Williams D, Fan HW, et al. Snakebite envenoming from a global perspective: towards an integrated approach. Toxicon. 2010;56(7):1223–35.10.1016/j.toxicon.2009.11.020
- Blaylock R. The identification and syndromic management of snakebite in South Africa. S Afr Fam Pract. 2005;47(9):48–53.10.1080/20786204.2005.10873288
- McNally SL, Reitz CJ. Victims of snakebite. A 5-year study at Shongwe Hospital, Kangwane, 1978-1982. S Afr Med J. 1987;72(12):855–60.
- Alirol E, Sharma SK, Bawaskar HS, et al. Snake bite in South Asia: a review. PLoS Negl Trop Dis. 2010;4(1):e603.10.1371/journal.pntd.0000603
- Chattopadhyay S, Sukul B. A profile of fatal snake bite cases in the Bankura district of West Bengal. J Forensic Leg Med. 2011;18(1):18–20.
- Dolab JA, de Roodt AR, de Titto EH, et al. Epidemiology of snakebite and use of antivenom in Argentina. Trans R Soc Trop Med Hyg. 2014;108(5):269–76.10.1093/trstmh/tru038
- González-Andrade F, Chippaux J-P. Snake bite envenomation in Ecuador. Trans R Soc Trop Med Hyg. 2010;104(9):588–91.10.1016/j.trstmh.2010.05.006
- Oliveira Nda R, Sousa AC, Belmino JF, et al. The epidemiology of envenomation via snakebite in the State of Piauí, Northeastern Brazil. Rev Soc Bras Med Trop. 2015;48(1):99–104.10.1590/0037-8682-0173-2014
- Chugh KS. Snake-bite-induced acute renal failure in India. Kidney Int. 1989 Mar;35(3):891–907.10.1038/ki.1989.70
- Pinho FM, Zanetta DMT, Burdmann EA. Acute renal failure after Crotalus durissus snakebite: a prospective survey on 100 patients. Kidney Int. 2005;67:659–667.10.1111/kid.2005.67.issue-2
- Chaudhari TS, Patil TB, Paithankar MM, et al. Predictors of mortality in patients of poisonous snake bite: experience from a tertiary care hospital in Central India. Int J Crit Illn Inj Sci. 2014;4(2):101–7.
- Christiansen PA. Snakebite and the use of antivenom in southern Africa. S Afr Med J. 1981;59(6):934–8.
- Yadavannavar MC, Patil AN. A study of morbidity and subsidence of symptoms: subject to time gap of snakebite and treatment. J Indian Med Assoc. 2013;111(1): 32, 34, 43.
- Vohra R, Rangan C, Bengiamin R. Sonographic signs of snakebite. Clin Toxicol. 2014;52(9):948–51.10.3109/15563650.2014.958613
- Anz AW, Schweppe M, Halvorson J, et al. Management of venomous snakebite injury to the extremities. J Am Acad Orthop Surg. 2010;18(12):749–59.
- Campbell BT, Corsi JM, Boneti C, et al. Pediatric snakebites: lessons learned from 114 cases. J Pediatr Surg. 2008;43(7):1338–41.10.1016/j.jpedsurg.2007.11.011
- Darracq MA, Cantrell FL, Klauk B, et al. A chance to cut is not always a chance to cure-fasciotomy in the treatment of rattlesnake envenomation: a retrospective poison center study. Toxicon. 2015;101:23–6.10.1016/j.toxicon.2015.04.014
- Garfin SR, Castilonia RR, Mubarak SJ, et al. Rattlesnake bites and surgical decompression: results using a laboratory model. Toxicon. 1984;22(2):177–82.10.1016/0041-0101(84)90018-7
- Gurucharri V, Henzel JH, Mitchell FL. Use of the Doppler flowmeter to monitor the peripheral bloodflow during the edema stage of snakebite. Plast Reconstr Surg. 1974;53(5):551–4.
- Lavonas EJ, Ruha AM, Banner W, et al. Unified treatment algorithm for the management of crotaline snakebite in the United States: results of an evidence-informed consensus workshop. BMC Emerg Med. 2011;11:1–15.10.1186/1471-227X-11-2
- Toschlog EA, Bauer CR, Hall EL, et al. Surgical considerations in the management of pit viper snake envenomation. J Am Coll Surg. 2013;217(4):726–35.10.1016/j.jamcollsurg.2013.05.004
- Mars M, Hadley GP, Aitchison JM. Direct intracompartmental pressure measurement in the management of snakebites in children. S Afr Med J. 1991;80(5):227–8.
- Chippaux, J. P., Diouf, A., Stock, R. P., et al (2011). Report of the 4th International Conference on envenomations by snakebites and scorpion stings in Africa, Dakar. Toxicon. 2011 25–29 Apr;58(5):426–9.
- Visser LE, Kyei-Faried S, Belcher DW. Protocol and monitoring to improve snake bite outcomes in rural Ghana. Trans R Soc Trop Med Hyg. 2004;98(5):278–83.10.1016/S0035-9203(03)00065-8