Abstract
Background: Meprobamate is a constituent of various combination analgesics in South Africa. Due to the lack of recent literature on the prescribing patterns of combination analgesics containing meprobamate and in the light of its possible higher re-scheduling, this study was conducted. The primary aim was to establish the extent of meprobamate-containing combination analgesic prescribing using a prescription claims database.
Methods: A retrospective, cross-sectional drug utilisation study was conducted on prescription data of a medical insurance scheme administrator in South Africa for 2011.
Results: A total of 31 854 patients received 97 491 analgesics during 2011. Within ATC category N02B, 62.10% of prescriptions were for analgesic combinations, of which 20 326 prescriptions were for meprobamate-containing analgesics. A total of 10 404 patients (53.00% males) were prescribed meprobamate-containing analgesics. Overall, 20.85% of all analgesics prescribed were therefore meprobamate-containing analgesics. Patients who received meprobamate-containing analgesics were slightly older (39.52 years) compared with patients who received analgesics in general (33.61 years). Twenty-two trade names of meprobamate-containing analgesics were prescribed. Seventeen of these products contained exactly the same strengths of active ingredients, namely 320 mg paracetamol, 8 mg codeine phosphate, 32 mg caffeine and 150 mg meprobamate. The originator product constituted 3.72% of prescribing frequency (average cost: R30.42) compared with 70.63% for the most popular generic (average cost: R11.65).
Conclusions: Prescribers should be conscious of the benefits and risks of the active ingredient combinations. Further studies including patient and prescriber perceptions of different combinations are recommended.
Introduction
Combination analgesics are widely prescribed and used in South Africa. It has been stated that South African doctors have the largest choice of combination analgesic preparations available for prescriptionCitation1 and that South Africa has the dubious distinction of having the largest number of compound analgesics available in the world.Citation2 Paracetamol-containing combination analgesics especially are widely prescribed. Different rationales for using compound or combination analgesics have been identified, but most combinations are formulated with two rationales in mind, namely enhancement of analgesia and reduction of adverse effects by combining two analgesics with different mechanisms of action.Citation3
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.Citation4 Pain is a leading cause of morbidity worldwide.Citation5 It is the most common reason patients seek medical care, and it is one of the most common complaints medical practitioners receive from patients.Citation6 There is a growing need for effective pain management that can eliminate pain or at least reduce it to a tolerable level so as to restore physical, psychological and social functioning.Citation5 Many patients, especially the elderly, have several co-morbid conditions and multiple sources of pain, including musculoskeletal and neuropathic pain.Citation5 When the pathophysiology of a medical condition is multi-modal, that is, related to multiple physiological causes or mediated by multiple pathways, a strategy can be to use a drug or a combination of drugs that contribute multiple mechanisms to the therapeutic endpoint.Citation7 Pain is such a condition. Diverse classes of medicines can therefore serve as an efficient complement to paracetamol (acetaminophen), non-steroidal anti-inflammatory drugs (NSAIDs) or opioids in the management of pain.Citation8 The success of a combination furthermore depends on the type of pain that is targeted (acute/chronic, inflammatory, neuropathic or cancer).Citation8 The value of combination (polycomponent or compound) analgesics should therefore not be overlooked.
Since pain is often associated with anxiety, on theoretical grounds it has been considered reasonable to add a sedative or tranquilliser such as meprobamate as a constituent to combination analgesics.Citation9 Meprobamate is currently a Schedule 5 medicine in South Africa.Citation10 In December 2013, the Medicines Control Council (MCC) in South Africa published a communication to industry regarding a proposal to re-schedule meprobamate (from Schedule 5 to Schedule 6) and interested persons were invited to submit comments, suggestions or recommendations to the Registrar of the MCC by not later than 31 January 2014.Citation11 Some of the reasons given wereCitation:11
| • | the addictive nature of meprobamate; | ||||
| • | the high abuse potential of meprobamate; | ||||
| • | to enable appropriate recording in line with Schedule 6 substances. | ||||
Previous studies have investigated combination analgesic prescribing in South Africa.Citation12–16 In 1993, there were more than 100 combination analgesics in tablet and capsule form available from retail pharmacies in South Africa.Citation12 A study conducted in 1992 and 1993 under dispensing doctors reported that one combination analgesic (paracetamol, meprobamate, codeine and caffeine) represented 49.2% of the combination analgesics dispensed.Citation12 In a later study conducted on the prescribing patterns in 200 medical practices in the four metropolitan areas of South Africa in 1995 (the study included 47 103 patients), it was found that meprobamate-containing analgesics constituted 12.1% of all prescriptions for central nervous system medicine and 29.6% of all analgesics that were prescribed.Citation13 More than half (52.7%) of all meprobamate-containing analgesics were prescribed to patients between the ages of 30 and 59 years.Citation13 The study further reported that 22 different brand names of meprobamate-containing analgesics were prescribed to patients, and that one brand name was responsible for 40.6% of all the prescriptions for meprobamate-containing analgesics.Citation13
Another South African studyCitation,15 conducted on a claims database, reported that combination analgesic drugs represented 8.87% of all medicines claimed during 2001; this decreased to 7.20% in 2004, after which it again increased to 7.92% in 2006. The study reported that generic substitution influenced the prescribing frequency of innovator combination analgesics.Citation15 A study conducted in 2007Citation16 identified 175 combination analgesic formulations with either paracetamol or aspirin as main ingredient (excluding combination products containing NSAIDs, such as ibuprofen). In the 2007 study, 21 Schedule 5 (prescription-only) meprobamate-containing tablet formulations (trade names) containing the identical active ingredients were prescribed.Citation16
The focus on the availability of meprobamate is in line with international developments. Meprobamate, which is a metabolite of carisoprodol, was banned in Sweden in January 1981 due to its potential for abuse and addiction, and lack of efficacy.Citation17,18 More recently the European Medicines Agency has investigated the safety and effectiveness of oral meprobamate-containing medicines, due to serious side effects seen with the medicine.Citation18 It was concluded that the benefits of meprobamate do not outweigh its risks, and that all marketing authorisations for oral medicines containing meprobamate should be suspended throughout the European Union.Citation18 In the United States of America it has been classified as C-IV and was the best-selling tranquilliser for a long time before being replaced with the benzodiazepines.Citation18 Meprobamate has therefore been withdrawn from the European market, and has limited availability on the American and Canadian market.
In South Africa, only stricter controls for meprobamate are considered. According to the Medicines and Related Substances Control Amendment Act 59 of 2002Citation,10 a Schedule 5 substance should not be repeated for longer than six months, and then only if the authorised prescriber has indicated on the prescription the number of times and the intervals at which it may be dispensed. In addition, the Act states that where a Schedule 5 substance is used for its analgesic properties it should not be prescribed for longer than six months unless the authorised prescriber has consulted another medical practitioner before issuing a new prescription.Citation10 A Schedule 6 substance may not be repeated without a new prescription being issued.Citation10 The intent with the re-scheduling is therefore stricter control over the availability of meprobamate and subsequently better control over its potential abuse or overuse.
The main indication for meprobamate (a carbamate, ATC code N05BC01) is short-term treatment of anxiety, in a dose of 400 mg 3 to 4 times daily.Citation19 It does not appear to be as effective in the management of anxiety as the benzodiazepines and has been superseded by them.Citation19 It displays a pronounced dependence potential.Citation19 According to the South African Acute Pain GuidelinesCitation,20 meprobamate is a weak analgesic with probable physical and emotional addiction after 10 days of use. Meprobamate is included in several combination analgesic preparations (classified under N02B in the Anatomical Therapeutic Chemical or ATC classification system)Citation,11 increasing the risk of its abuse. The Pain Society of South Africa (PainSA) has identified the two most dangerous constituents in compound analgesics as meprobamate and caffeine.Citation2 Meprobamate has been added for its sedative and tranquillising effect, whilst caffeine has traditionally been added to combination analgesics to reduce the sedative effects of the opioid component of these compounds.Citation2
Due to the lack of recent literature on the prescribing patterns of combination analgesics containing meprobamate and in the light of its possible higher re-scheduling, this study was conducted. The primary aim was to establish the extent of meprobamate-containing combination analgesic prescribing in South African using a large prescription claims database.
Methods
Study design and setting
A retrospective, cross-sectional drug utilisation study was conducted on prescription data of a medical insurance scheme administrator in South Africa for 2011. This study included prescriptions from general medical practices, pharmacies, private hospitals, day clinics and sub-acute facilities, to give a comprehensive overview of meprobamate prescribing patterns.
Data analysis
The database contained a total of 2 298 312 records for medicine, medical devices and procedures. All medication records for ATC category N02 were extracted and analysed (a total of 97 491 records). Each medication record contained information on the age and gender of the patient, with a unique number to identify each patient, the date of the prescription, detailed information on the dispensed drug (name, package size, formulation, strength and quantity) and sales value.
The ATC classification systemCitation,21 MIMSCitation22 and the South African Medicines FormularyCitation19 were used to identify the medicines that were prescribed. Microsoft Access® and Excel® (Microsoft Corp., Redmond, WA, USA) were used to analyse the data. Descriptive statistics were calculated. The costs indicated in the study are the amount that was paid by the respective medical insurance schemes.
Ethical approval
Ethical approval to conduct studies on prescription databases has been obtained from the Research Ethics Committee (Human) of the Nelson Mandela Metropolitan University (NMMU) (ethics clearance number: H08-HEA-PHA-005). No patient or prescriber in the study could be identified.
Limitations of the study
Limitations of the study were that no diagnoses or clinical information were available in the database. It was therefore not possible to determine the type or intensity of pain for which these analgesics were prescribed.
Results
General prescribing patterns of analgesics
A total of 31 854 patients (52.19% males) received one of more products from ATC category N02 during 2011. The average age of patients was 33.61 (SD = 19.19) years. The average age of female patients was 32.73 (SD = 19.45) years and of male patients 34.41 (SD = 18.91) years. More than half of the patients (54.68%) were between 30 and 59 years of age. Of the 31 854 patients, 30.74% (9 793 patients) were prescribed one or more opioids (N02A), 89.56% (28 530 patients) were prescribed other analgesics and antipyretics, and 2.31% (737 patients) were prescribed antimigraine preparations (N02C). The frequency of prescriptions in the different N02 ATC categories is given in Table for females and males.
Table 1: Frequency of prescriptions in the different ATC analgesic categories
Prescribing of meprobamate-containing analgesics
Within ATC category N02B, 62.10% (43 559 products) were analgesic combinations, of which 20 326 products were meprobamate-containing analgesics at a cost of R282 929.87. Three-quarters (76.29%; 15 506 products) of meprobamate-containing analgesics were dispensed by general medical practices or pharmacies. The rest were dispensed in private hospitals (23.49%), day clinics or sub-acute facilities.
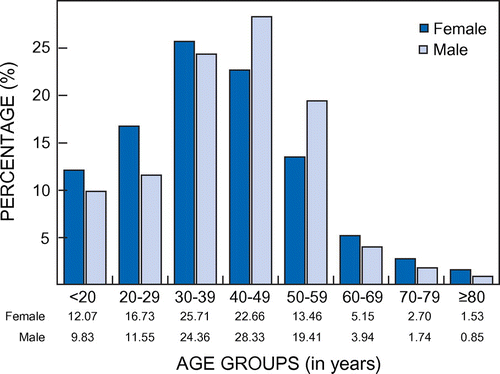
A third (32.66%; 10 404) of patients who were prescribed analgesics received one or more meprobamate-containing analgesics in 2011. More males than females were prescribed these products (53.00% males). The percentage age and gender distribution of patients is given in Figure . Prescribing differences were observed between females and males with regard to prescribing frequency (chi-square = 180.77; df = 7; p < 0.0001). In the age group 40 to 59 years, there were proportionally more male patients. The average age of patients who received meprobamate-containing analgesics was 39.52 (SD = 15.17) years (females: 38.64 (SD = 15.94) years; males: 40.31 (SD = 14.40) years).
Figure 1: Percentage age and gender distribution of patients prescribed meprobamate (n = 10 404).
The average number of meprobamate-containing analgesic prescriptions per patient over the year was 1.95 (SD = 1.96) (a prescription could contain a varying number of tablets or capsules). The average number of prescriptions for females was 1.96 (SD = 1.89) and for males 1.95 (SD = 2.02). Only 19.65% of these prescriptions were prescribed on the chronic plans of the respective medical insurance schemes (chronic prescriptions indicate that patients receive these products on a monthly basis for a period of at least three months). The number of prescriptions dispensed is compared with the number of patients according to age in Figure .
Figure 2: Number of prescriptions and number of patients according to age (nproducts = 20 326; npatients = 10 404).
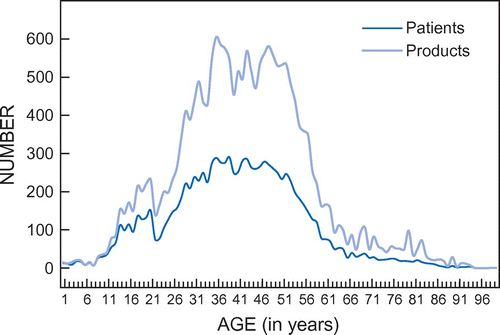
From Figure it is clear that patients aged 30 to 59 years received the most meprobamate-containing prescriptions (67.26% of patients and they were prescribed 69.57% of all meprobamate-containing analgesics). Only 301 prescriptions (1.48%) were dispensed to children younger than 12 years.
Trade name combination analgesics prescribed
Twenty-two trade names of meprobamate-containing analgesics were prescribed (41 different trade name products if different dosage strengths and package sizes are considered). Most prescriptions (86.35%) were for tablet formulations. The prescribing frequency according to gender is given in Table , together with the active ingredients. The most commonly prescribed combination analgesic was 320 mg paracetamol, 8 mg codeine phosphate, 32 mg caffeine and 150 mg meprobamate. Of the 20 326 products, one specific trade name product was responsible for approximately 70% of prescribing (Stilpane® capsules and tablets). This product is indicated as an analgesic in pain-tension states. The recommended dose is two tablets three or four times a day as required.Citation22 It is not recommended for children under 12 years of age.Citation22 Only one product (Tenston®) contained aspirin in combination with meprobamate.
Table 2: Prescribing frequency of meprobamate-containing combination analgesics according to gender
Cost of meprobamate-containing analgesics
The average cost per meprobamate-containing prescription was R13.92 (SD = R16.30). If the most commonly prescribed trade name product is considered, the average cost of the originator product was R30.42 per prescription (this product constituted 3.72% of prescribing frequency). The average cost of its most popular generic equivalent was R11.65 (this product constituted 70.63% of prescribing frequency).
Quantity prescribed
The number of tablets or capsules per prescription was analysed. The majority of prescriptions (70.86%) contained either 20 tablets or capsules (9 143 prescriptions; 44.98%) or 30 tablets or capsules (5 261 prescriptions; 25.88%) per prescription. Nearly all prescriptions (99.40%) contained 60 or fewer tablets or capsules. There were only 40 prescriptions with a quantity of 100 tablets or capsules per prescription. Of the prescriptions classified as chronic, only six prescriptions were for more than 60 tablets or capsules.
Of the 10 404 patients, 6 225 patients (59.83%) received only one prescription for a meprobamate-containing analgesic during the year, and 2 065 patients (19.85%) received two prescriptions during the year. Forty patients received more than 12 prescriptions during the year, and this was mostly for small quantities (for example, in a hospital setting where only 5, 7 or 10 tablets or capsules were dispensed, followed by further small quantities).
Discussion
Within ATC category N02B, 62.10% of prescriptions were for analgesic combinations, of which 20 326 prescriptions were for meprobamate-containing analgesics at a cost of R282 929.87. Overall therefore, a fifth (20.85%) of all analgesics prescribed were meprobamate-containing analgesics.
Females traditionally are prescribed more analgesics than males, as was the case in previous South African studies.Citation13,14 In a study conducted in 1995 that included patients from 50 medical practicesCitation,13 meprobamate-containing analgesics were prescribed nearly five times as often to female as to male patients. In this study, 52.79% of the patients were males. Two-thirds (67.26%) of patients who were prescribed meprobamate-containing analgesics were between 30 and 59 years of age (3 023 female and 3 975 male patients), and they received 69.57% of prescriptions. In the 1995 studyCitation,13 most patients using meprobamate-containing analgesics were females between the ages of 30 and 59 years (48.4%), whilst in this study the corresponding percentage of females was only 29.06%. It is not possible to explain the reason for this difference without also investigating the prescribing of other central nervous system medicine classes, especially sedatives and anxiolytics. It is possible that males in this study were prescribed a lower percentage of anxiolytic agents, and that they were using meprobamate-containing analgesics for their anxiolytic, rather than their analgesic, effect and hence the higher frequency of meprobamate-containing analgesic dispensing to male patients (there may be less stigma for a male patient to use an analgesic, than an anxiolytic or sedative, for anxiety). However, the dispensing patterns of all central nervous system classes will have to be interrogated before this statement can be verified. This aspect will be investigated in further studies.
The average age of patients using meprobamate-containing analgesics in the 1991 study was 35.8 years.Citation13 In this study, the average age was 39.52 years. Interestingly, patients who were prescribed meprobamate-containing analgesics in the 1995 studyCitation13 were slightly younger (35.8 years) than the average age of analgesic users in general (37.0 years). In this study, these patients were older (average age of 33.61 years for patients receiving analgesics; average age of 39.52 for patients receiving meprobamate-containing analgesics).
The paracetamol content of meprobamate-containing combination analgesics varied between 200 mg and 400 mg, the codeine phosphate dose was either 8 mg or 10 mg, caffeine varied between 32 mg and 50 mg (with only three trade name products not containing caffeine as a constituent), and except for one 200 mg dose, all trade name products contained meprobamate in a dose of 150 mg. Only one product contained meprobamate in combination with aspirin.
Twenty-two different trade names of meprobamate-containing analgesics were prescribed. Seventeen of these products contained exactly the same dosages of active ingredients, namely 320 mg paracetamol, 8 mg codeine phosphate, 32 mg caffeine and 150 mg meprobamate. Sixteen of the 17 products were tablet formulations. These 17 trade name products represented 84.25% of all the meprobamate-containing analgesics that were prescribed. In the 1995 studyCitation,13 14 brand-name products with the identical active ingredients were prescribed and they represented 49.2% of the total number of meprobamate-containing analgesics that were prescribed.
Generic substitution clearly played a role in how prescribing patterns have changed. In the 1995 studyCitation,13 four analgesic brand names (Stopayne®, Stilpane®, Tenston SA® and Antipyn Forte®) were responsible for 76.6% of the prescriptions for meprobamate-containing analgesics (three of these products were available as both tablets and capsules). In this study, one trade-name product (including both capsule and tablet formulations) constituted 70.63% of all prescriptions for meprobamate-containing combination analgesics. This product is a generic equivalent of the originator product (the originator product was the most frequently prescribed in previous studies). The originator product in this study constituted only 3.72% of the prescribing frequency of meprobamate-containing combination analgesics (compared with 70.63% for the most frequently prescribed generic product). Therefore, the specific formulation of analgesic ingredients (320 mg paracetamol, 8 mg codeine phosphate, 32 mg caffeine and 150 mg meprobamate) has strengthened its place as the dominant product on the market.
Legislation was passed in May 2003 under the Medicines and Related Substances Act, No. 101 of 1965 (90/95 as amended)Citation10 providing for mandatory generic substitution, which allows patients to request that the originator brand be substituted by a generic in order to allow for cost savings. Generic substitution clearly had an impact on the prescribing patterns of analgesics between the previous study and this study. It furthermore remains evident that medical practitioners have definite preferences for certain brand names in their prescribing of meprobamate-containing analgesics.
It is generally accepted that meprobamate has a high abuse potential due to its addictive nature, yet few studies have investigated the actual prescribing patterns of meprobamate. A combination analgesic is most effective when the individual agents act through different analgesic mechanisms and act synergistically. By activating multiple pain-inhibitory pathways, combination analgesics can provide more effective pain relief for a broader spectrum of pain, and might also reduce adverse drug reactions. Meprobamate is added for its anxiolytic and sedating effect. This study found that approximately a fifth of analgesics prescribed contained meprobamate and that, in general, results were reasonably similar to those of previous South African studies. It is not possible to conclusively state from this study that these products are overprescribed or abused. If the number of tablets or capsules dispensed per prescription is considered, as well as the number of prescriptions per patient, no clear indication of overprescribing or abuse could be detected. Most patients received one or two prescriptions during the year, and most prescriptions were in quantities of either 30 or 60 tablets or capsules per prescription.
It can be concluded that, first, generic substitution had an impact on prescribing patterns and, second, that more studies are needed to quantify combination analgesic and specifically meprobamate-containing analgesic prescribing. Also, that further studies should link prescribing to diagnoses. Prescribers should be conscious about the benefits and risks of the active ingredient combinations in the management of pain, and patients should be properly counselled on the appropriate and safe use of combination analgesics. Further studies including patient and prescriber perceptions on the effectiveness of meprobamate-containing combination analgesics are recommended.
Conflict of interest statement
The author declares that she has no financial or personal relationship(s) that may have inappropriately influenced her in writing this paper.
References
- Raff M. The usage and efficacy of a combination analgesic preparation. South Afr J Anaesth Analg. 2002;8(3):34–8.
- Hodgson E. Compound analgesics – so much inappropriate medicine in SA. Medical Chronicle. 2010. [cited 2015 Oct 6]. Available from: http://www.medicalchronicle.co.za/compound-analgesics-so-much-inappropriate-medicine-in-sa/.
- Beaver WT. Combination analgesics. Am J Med. 1984 Sep 10;77(3A):38–53.10.1016/S0002-9343(84)80101-1
- International Association for the Study of Pain (IASP). IASP Taxonomy. 2014. [cited 2015 Jan 6]. Available from: http://www.iasp-pain.org/Taxonomy?navitemNumber=576.
- Langford RM. Pain management today—what have we learned? Clin Rheumatol 2006;25(S1):2–8.10.1007/s10067-006-0311-5
- Taylor FA. Pain: A common complaint. JNECM 2008;4(2): 969–74.
- Raffa RB. The determination and application of fixed-dose analgesic combinations for treating multimodal pain. J Pain 2010;11(8): 701–9.10.1016/j.jpain.2009.12.010
- Ortiz MI, Molina MAR, Arai YP, Romano CL. Analgesic drugs combinations in the treatment of different types of pain. Pain Res Treat 2012;Article ID 612519 (2 p.). doi: 10.1155/2012/612519.
- Kantor TG, Laska E, Streem A. An apparent algesic effect of meprobamate. J Clin Pharmacol N D. 1973;13(4):152–9. doi:10.1002/j.1552-4604.1973.tb00076.
- South Africa. Medicines and Related Substances Control Amendment Act 59 of 2002. Government Printers: Pretoria; 2002.
- Hela M. Scheduling matters: proposal to reschedule meprobamate. Pretoria: Communication to Industry, Medicines Control Council (MCC); 2012 Dec.
- Truter I, Kotze TJW. An investigation into the prescribing of analgesics. S Afr Med J 1996;86(11):1394–7.
- Truter I. An investigation into compound analgesic prescribing in South Africa, with special emphasis on meprobamate-containing analgesics. Pharmacoepidemiol Drug Saf 1998;7:91–7.10.1002/(ISSN)1099-1557
- Truter I. Patterns of analgesic prescribing in a South African primary care setting. J Clin Phar Ther 1997;22:33–7.10.1046/j.1365-2710.1997.96175961.x
- Kruger H. A review of the prescribing patterns of combination analgesics in the private health care sector [Unpublished MPharm dissertation]. Potchefstroom: North-West University; 2008.
- Truter I, Knoesen BC. PMH73 potential abuse of combination analgesics: a database analysis. Value Health 2009;12(7): A364.10.1016/S1098-3015(10)74791-4
- “Consolidated List of Products”. World Health Organization. United Nations. 2005. [cited 2014 Jul 2]. Available from: http://apps.who.int/medicinedocs/documents/s16780e/s16780e.pdf.
- Ninan B, Wertheimer AI. Withdrawing drugs in the U.S. versus other countries. Inov Pharm. 2012;3(3, Article87):1–12.
- South African Medicines Formulary (SAMF), 11th ed. Rossiter D (ed.). Health and Medical Publishing Group of the South African Medical Association: Cape Town, 2014.
- South African Acute Pain Guidelines. The South African Society of Anaesthesiologists (SASA). 2009;15(6):1–120.
- ATC/DDD Index 2015. WHO Collaborating Centre for Drug Statistics Methodology: Oslo. 2015. [cited 2015 Oct 6]. Available from: http://www.whocc.no/atc_ddd_index/.
- MIMS Monthly Index of Medical Specialities (MIMS). Snyman JR(ed). MIMS: Saxonwold, 2011 May;51(5):61–72.
