Abstract
Aim: To determine the characteristics of patients with pulmonary tuberculosis registered in primary health care facilities in Moses Kotane region North West Province.
Method: A retrospective record review of pulmonary TB patients registered in five community health centres (CHCs) in 2010 was conducted.
Results: Of the 229 new patients diagnosed using sputum microscopy or culture, 176 were cured and 53 were not cured. The mean age for cured patients was 36.4 years and 34.0 years for not cured patients with standard deviations of 13.5 and 11.4 respectively (p-value 0.195). In total, 97 (55.1%) female patients and 79 (44.9%) male patients were cured while 24 (45.3%) female patients and 29 (54.7%) male patients were not cured (p-value 0.214). Among the 169 unemployed patients, 120 (68.2%) were cured and 40 (75.5%) were not cured. Of the 69 patients with employment, 56 (31.8%) were cured and 13 (24.5%) failed to cure (p-value 0.394). Of 176 cured patients, 130 had directly observed treatment (DOT) support while 31 of 53 not cured patients did not have DOT support (p-value 0.00002). Some 154 (67.2%) patients were HIV positive and among them 119 (67.6%) were cured and 35 (66.0%) were not cured while of the 75 who were HIV negative 57 (32.4%) were cured and 18 (33.9%) were not cured (p-value 0.8680).
Conclusion: DOT support was a strong predictive characteristic for the outcomes of these TB patients during their treatment with a statistically significant difference between cured and not cured patients; the majority of not cured patients did not have DOT support. Other characteristics like gender, age, HIV status, employment or other medical conditions did not show any statistically significant difference between cured and not cured patients.
Introduction
Globally, tuberculosis (TB) is a major public health problem. Almost a third of the world’s population (more than 2 billion people) is estimated to be infected with the TB bacillus, and one in every 10 infected people is estimated to become ill with active TB in their lifetime.Citation1 The largest numbers of new TB cases occur in the Southeast Asia, African and Western Pacific region (35%, 30% and 20% respectively), accounting for 58% of new cases globally. The top six countries with regard to TB caseload in 2014 were: India, Indonesia, Nigeria, Pakistan, China and South Africa.Citation2
Tuberculosis continues to cause considerable morbidity and mortality in Africa despite the availability of effective anti-microbial agents. Africa, which is home to 11% of the world’s population, carried an estimated 28% of the global burden of tuberculosis and 34% of related deaths in 2014.Citation3 According to the Ethiopian Ministry of Health, TB is the major cause of morbidity in Ethiopia and the second highest cause of mortality, superseded only by malaria.Citation4
Nigeria, the most populous country in Africa, has the third highest TB caseload globally after India and China. Every year Nigeria reports 590 000 new TB cases and 245 000 deaths. Tuberculosis accounts for more than 10% of all deaths in Nigeria.Citation5 Similar high TB death rates have been reported in the Democratic Republic of the Congo, Ethiopia and Malawi.Citation4,6,7
South Africa is ranked fourth among the 22 WHO-determined high-burden countries, with an estimated incidence of 450 000 cases of active TB in 2013.Citation3 The South African National TB Management Control data for 2006 show that North West Province (NWP) has the fifth highest incidence of smear-positive TB, behind KwaZulu-Natal, Eastern Cape, Western Cape and Gauteng.Citation8 TB continues to be the leading cause of death in South Africa. The World Health Organization (WHO) gives a figure of 25 000 deaths from TB in South Africa in 2011. This excludes people who had both TB and HIV infection when they died.Citation9 The number of TB deaths associated with HIV was 62 827 (11.6% of the total number of deaths) for 2010; 69 791 in 2009; and 75 281 in 2008.Citation10
Several factors including the HIV epidemic, weak healthcare systems, inadequate laboratory services with delayed diagnosis, ongoing transmission of drug-sensitive and drug-resistant TB and poor supply of drugs contribute to the rise in TB-related deaths.
TB that is not cured is a major cause of mortality, drug-resistant TB and indeed ongoing transmission. In 2010 TB data for the Moses Kotane region of the North West Province South Africa indicated a cure rate of 62.1%, a defaulter rate of 10.2%, a death rate of 6.7%, a treatment failure rate of 2.5%, and an MDR-TB rate of 0.3%.Citation11 These data raise concern regarding a cure rate that fell below the 85% WHO target and the high (35%) rate of TB that was not cured.
The aim of this study was to determine the characteristics of sputum-positive TB patients registered in 2010 who were not cured in five community health centres (CHCs) with a view to implementing targeted interventions to improve the TB treatment outcomes.
Method
A retrospective record review was conducted of pulmonary TB patients registered in five CHCs in Moses Kotane region, North West Province, South Africa in 2010. The 5 CHCs were selected out of the 49 primary health care facilities because TB care in this region is provided mainly at CHCs.
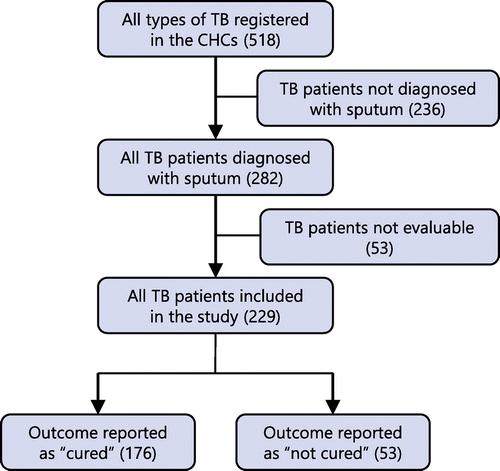
A total of 518 TB patients were registered in the five CHCs. Records of 282 patients in whom sputum was reported positive for TB were considered eligible for selection. Fifty-three records were excluded as there was no indication of the final outcome in 35, 14 were recorded as having been transferred out and 4 patients died. Records of patient in which diagnosis of tuberculosis was not based on sputum were also excluded. These included diagnostic criteria such as abnormalities in the chest X-ray, a history and clinical picture suggestive of TB, and histological and biological tests suggestive of TB. As a result, 229 patient files were analysed.
Data were collected from patient files and clinic registers for all 229 patients diagnosed with sputum-positive TB. Of the 229 records, 53 were for patients who were considered ‘not cured’. ‘Cure’ is described as a treatment outcome that depicts a negative TB smear/culture in the last month of treatment and on at least one previous occasion at least 30 days prior in a patient whose baseline smear or culture was positive (Tubercle bacilli) at the beginning of treatment. ‘Treatment completed’ is described as an outcome in which a patient who had a positive TB smear or culture completes the treatment but does not have a negative smear or culture in the last month of treatment. ‘Not cured’ describes treatment outcomes excluded from ‘cured’ and includes treatment failure, treatment completed, died, defaulted treatment, transfer out in which the treatment outcome is unknown. All patients had been on regimen 1 of the standard treatment protocol in which a fixed dose combination of rifampicin, isoniazide, pyrazinamaide and ethambutol is used in adults and children above 8 years for 6 months. Children below 8 years or weighing less than 30 kg were treated with rifampicin, pyrazinamide and isoniazid combinations.
Variables including demographic characteristics, concurrent medical conditions, use of directly observed treatment (DOT) and treatment outcome were extracted from the records and captured on a data collection sheet. Data were analysed using SAS System, version 9.2® (SAS Institute, Cary, NC, USA) (Figure ).
Permission for the study was obtained from Moses Kotane Health Sub-District Management Committee, and the North West Province Department of Health. The study was granted ethical approval by the Medunsa Research Ethics Committee (MREC) clearance certificate number MRREC/M/28/2013/PG.
Results
Of the 229 patient records, 53 recorded the TB outcome as ‘not cured’ while 176 were recorded as ‘cured’. The commonest age group of patients diagnosed with TB from sputum was 31–40 (37.8%), followed by the age group between 21 and 30. The extreme ages (less than 10 years and above 70) were the least affected (1.6% and 0.4% respectively). The mean age was 35.8 years, with a standard deviation of 13.0 years and range from 4 years to 91. Twelve patients were aged between 4 and 18, constituting 5.2% of the sample. The ages of those under 18 years were 4, 6, 7, 10, 11, 12 (two patients), 15, 16 (three patients) and 17 (two patients). Thirteen patients were aged between 18 and 20 years.
There was a marginal difference in proportions of females (52.8%) compared with males (47.2%). The majority of the patients were unemployed (160; 69.9%). DOT support had a ratio of 2:1 with 152 (66.4%) patients having had DOT support and 77 (33.6%) not having had DOT support during their treatment period (Table ).
Table 1: Baseline characteristic of participants
Tuberculosis and concurrent medical conditions
TB patients with HIV co-infection numbered 154 (67.3%) compared with 75 (32.7%) who were HIV-negative. Only 7 of the 229 patients had hypertension, 2 patients had diabetes mellitus, 1 had epilepsy and 1 patient suffered from a mental illness. All the patients with concurrent medical conditions were more than 18 years old except 6 who had HIV and were aged less than 18 (Tables and ).
Table 2: Tuberculosis and concurrent medical conditions
Table 3: Association between variables and cure
The mean age for cured patients was 36.4 years, which was marginally higher than that of 34 years for those not cured. The difference was statistically not significant (p-value 0.195).
The proportion of those cured among the employed (81.2%) was also not statistically different from that of the unemployed (68.2%), which included all children under 18. Among the 160 who were unemployed, 120 (68.2%) were cured, and 40 (31.8%) were not cured. Of the 69 patients with employment, 56 (81.2%) were cured, and 13 (18.8%) failed to be cured.
With regard to DOTS support, 73.9% cured patients had DOT support while 58.5% of those who were not cured did not have DOT support (p-value 0.00002).
With regard to other concurrent medical conditions, it was found that 154 (67.3%) were HIV-positive. Of the HIV-positive patients 119 (67.6%) were cured and 35 (66.0%) were not cured, while among 75 (32.8%) who were HIV-negative 57 (32.4%) were cured and 18 (34%) were not cured (p-value 0.8680).
There were also two patients with diabetes mellitus, seven patients with hypertension, one patient with epilepsy, and seven female patients were on family planning. These patients were all cured. Only one patient, who had a mental health problem, was not cured.
Discussion
This study investigated the characteristics of TB patients in the Community Health Centres of Moses Kotane. The majority of patients had DOT support (66.4%) that falls below the 100% target set by WHO.Citation12 Besides encouraging people to be screened for TB, DOTS supporters provide advice and support to TB patients and, crucially, ensure that they complete their course of medication. This intervention is vital, as many TB suffers do not complete the full course of their medication. Once they start feeling better they stop, which leads to the development of MDR-TB, which is extremely difficult to treat.
In a study assessing the impact of the DOTS strategy on tuberculosis case finding and treatment outcome in Gambella Regional State in Ethiopia from 2003 up to 2012, it was found that it was possible to achieve the recommended WHO target, which is 70% of case detection rate for smear-positive pulmonary TB, and 85% of treatment success rate (TSR) as they fulfilled the targets for TSR more than 85% from 2009 up to 2011 in that region.Citation13 These findings are similar those of the South Western Nigerian study.Citation14
The results of this study demonstrate that the majority (73.9%) of those who were cured had DOT support, compared with 41.5% of those who were not cured. This illustrates the importance of having all TB patients under DOT support during their treatment, so as to increase their cure rate, as this is supported by many studies done across the world. A meta-analysis done in China found that due to the implementation of DOTS, China had achieved significant success in the previous decade in tackling the TB epidemic.Citation15 Daniel and Alausa, commenting on a community-based TB programme, supported the use of volunteers or family members to supervise the administration of anti-TB drugs so as to ensure adherence and improve the treatment outcomes.Citation16 Tumbo and Ogunbanjo, in a study that evaluated the implementation of DOTS for TB in Bojanala Health District in North West Province, concluded that DOTS is an important strategy which, if appropriately implemented, will enhance TB treatment adherence, thereby reducing the default and failure rates, and prevent drug resistance.Citation17 In a similar study conducted in Nigeria, it was found that DOTS was an effective means of administering anti-TB drugs as the rate of cure/treatment completed was 86.1%, and the compliance rate was 93.8%.Citation18 Integration of the DOTS strategy for TB control with an existing HIV/AIDS home care programme in Ndola, Zambia led to improved TB programme performance.Citation19 George found that DOTS supervisors enhanced patients’ ability to comply with their TB treatment by providing enablers, education and supportive relationships.Citation20
The study found that the age group 31–40 years was the most affected, followed by the age groups 21–30 and 41–50 years. The mean age was 35.8 years, with a standard deviation of 13.0 years. These are age groups of people who are very active and mobile; they may be working or looking for work, travelling or migrating to different places, thus increasing their risks of contracting TB. These three age groups recorded 76% of the total number of sputum-positive TB patients in those CHCs. This is in line with an Indian study,Citation21 where 70% of their TB patients were under the age of 50.
In this study, unemployment was among the major risk factors for not curing TB (RR 2.3). Similar findings were reported in a study conducted in Russia, in which unemployment was associated with a substantially increased risk of poor TB outcomes.Citation22
The results of this study showed that two-thirds (67.3%) of TB patients were HIV-positive, and one-third or 32.8% were HIV-negative (see Table ). HIV drives TB incidence and in some African countries 70% of persons with TB also have HIV co-infection.Citation23 It is therefore critically important to improve the coordination and collaboration of TB and HIV healthcare services, to address the escalating rates of TB/HIV co-infection.Citation24
While several studies reported poor TB outcomes in advanced age (> 55 years),Citation25,26 this study showed the mean age of patients who were not cured to be 34.0 (SD ± 11.41) years. The male sex, HIV co-infection and lack of DOT support were contributing factors among the 53 patients who were not cured. These findings are similar to those of a study evaluating TB outcomes among prisoners in a correctional centre in North West Province South Africa.Citation27 Unemployment, which was another contributing factor to lack of cure, was similarly reported in the study done in Nigeria on the management outcome of pulmonary TB, and also in an Ethiopian study.Citation28,29
Limitations
As this study was limited to the Community Health Centres in one region of North West Province, the findings cannot be generalised.
Conclusion
DOT support was a strong predictive factor for cure of pulmonary TB with the majority of ‘not cured’ patients not having this intervention. This highlights the importance of having appropriate implementation of the DOT strategy to improve the rate of TB cure. Other characteristics such as gender, age, HIV status, employment or other medical conditions did not have any statistically significant difference between ‘cured’ and ‘not cured’ patients.
References
- World Health Organisation. Stop TB partnership. Tuberculosis facts. 2009. [cited 2015 Oct 15] Available from: http://www.who.int/tb/publications/2009update
- World Health Organisation. Global Tuberculosis report. 2015. [cited 2015 Nov 16] Available from: http://www.who.int/tb/publications/global_report/en/
- World Health Organisation. Global tuberculosis control. Global report. 2014. [cited 2015 Aug 24] Available from: http://www.tbfacts.org/tb-statistics-south-africa
- Gesesew H, Tsehaineh B, Massa D, et al. The role of social determinants on tuberculosis/HIV co-infection mortality in southwest Ethiopia: a retrospective cohort study. BMC Res Notes. 2016;9:144–11.10.1186/s13104-016-1905-x
- Anna V II. Nigeria perspectives: tuberculosis. Copenhagen: Copenhagen Consensus Center; 2015.
- Henegar C, Behets F, Vanden Driessche K, et al. Mortality among tuberculosis patients in the Democratic Republic of Congo. Int J Tuberc Lung Dis. 2012;16(9):1199–204.10.5588/ijtld.11.0613
- van Lettow M, Bedell R, Maosa S, et al. Outcomes and diagnostic processes in outpatients with presumptive tuberculosis in Zomba District, Malawi. PLOS ONE. 2015;10(11):e0141414–13.10.1371/journal.pone.0141414
- National Department of Health. National Tuberculosis management guidelines. NDOH. 2009; 1(1):9–74.
- World Health Organisation. Multi-resistant tuberculosis. 2013. [cited 2013 Dec 05] Available from: http://www.who.int/tb/challenges/mdr/en/.
- National Department of Health. National strategic plan HIV, STI and TB. 2012. [cited 2013 Jun] Available from: http://www.tbfacts.org/tb-statistiics-south-africa
- North West Province. Electronic tuberculosis register. NET report. Pretoria: Department of Health, South Africa; 2010.
- Foundation for Professional Development. Integrated Management of TB, HIV and STI in the primary Health-care setting. FPD. 2013;2(9):35–87.
- Sisay S, Mengistu B, Erku W, et al. Directly observed treatment short-course (DOTS) for tuberculosis control program in Gambella Regional State, Ethiopia: ten years experience. BMC Res Notes 2014;7:44–8.10.1186/1756-0500-7-44
- Sunday O, Oladimeji O, Ebenezer F, et al. Treatment outcome of tuberculosis patients registered at DOTS centre in Ogbomoso, Southwestern Nigeria: a 4-year restrospective study. Tuberc Res Treat. 2014;2014:1–5.
- Li X, Yang Y, Liu Jet al. Treatment outcomes of pulmonary tuberculosis in the past decade in the mainland of China: a meta-analysis. Front. Med. 2013;7(3):354–66.10.1007/s11684-013-0257-3
- Daniel OJ, Alausa OK. Treatment outcome of TB/HIV-positive and TB/HIV-negatiive patients on directly observed treatment, short course (DOTS) in Sagamu, Nigeria. Niger J Med. 2006;15(3):222–6.
- Tumbo JM, Ogunbanjo GA. Evaluation of DOT treatment for TB in the Bojanala health district, North West Province of South Africa. Afr J Prim Health Care Fam Med. 2011;3(1):191–4.
- Erhabor GE, Adewole O, Adisa AO, et al. Directly observed short course therapy for tuberculosis - a preliminary report of a three-year experience in a teaching hospital. J Natl Med Assoc. 2003; 95(11):1082–8.
- Miti S, Mfungwe V, Reijer P, et al. Integration of tuberculosis treatment in a community-based home care programmme for persons living with HIV/AIDS in Ndola, Zambia. Int J Tuberc Lung Dis. 2003;7(9):92–8.
- George LJ. Self-determination and compliance in directly observed therapy of tuberculosis treatment in the kingdom of Lesotho. Soc Work in Health Care. 2008;46(4):81–99.10.1300/J010v46n04_05
- Sinha AK. 70% of TB patients in India are under 50. The Times of India. 2011. [cited 2013 Mar 09] Available from: http://timesofindia.indiatimes.com/india/
- Coker R. Risk factors for pulmonary tuberculosis in Russia: case-control study. BMJ .2006;332(7533):85–7.10.1136/bmj.38684.687940.80
- Granich R, Akolo C, Gunneberg C, et al. Prevention of Tuberculosis in People Living with HIV. Clin Infect Dis. 2010;50(s3):S215–22.10.1086/651527
- Daftary A, Padayatchi N. Social constraints to TB/HIV healthcare: Accounts from coinfected patients in South Africa. AIDS Care. 2012;24(12):1480–6.10.1080/09540121.2012.672719
- Vasankari T, Holmström P, Ollgren J, et al. Risk factors for poor tuberculosis treatment outcome in Finland: a cohort study. BMC Public Health. 2007;7:291–9.10.1186/1471-2458-7-291
- Munoz-Sellart M, Cuevas LE, Tumato M, et al. Factors associated with poor tuberculosis treatment outcome in the Southern Region of Ethiopia. Int J Tuberc Lung Dis. 2010;14(8):973–9.
- Mnisi T, Tumbo J, Govender I. Factors associated with pulmonary tuberculosis outcomes among inmates in Potchesfstroom Prison in North West Province. South Afr J Epidemiol Infect. 2013;8(2):96–100.
- Salami AK, Oluboyo PO. Management outcome of Tuberculosis: a nine year review in Ilorin. West Afr J Med. 2003;22(2):114–9.
- Berhe G, Enquselassie F, Aseffa A. Treatment outcome of smear-positive pulmonary tuberculosis patients in Tigray Region, Northern Ethiopia. BMC Public Health. 2012;12:537–9.10.1186/1471-2458-12-537