Abstract
Background: Diabetes mellitus is a chronic metabolic disorder which leads to complications especially when not properly managed. The role of self-monitoring of blood glucose (SMBG) in type 2 diabetic patients using oral hypoglycaemic agents has been a source of controversy.
Objective: The objective was to study the effect of SMBG on glycaemic outcome among type 2 diabetics in a primary care setting.
Methodology: A randomised control study was conducted between March 2013 and November 2013 at the General Outpatient Clinic of the Family Medicine Department (FMD) in Lagos State University Teaching hospital. A total of 120 diabetic patients were randomised into intervention and control groups; 107 patients (55 in the intervention and 52 in the control group) completed the study. Intention-to-treat analysis was done. Chi-square, Students t- and paired t-test were used to determine variables significantly associated with SMBG.
Results: More than three-quarters (77.5%) of the participants were aware of SMBG prior to commencement of the study. Both the SMBG (8.7% vs. 7.2%; p-value < 0.001) and non-SMBG (8.7% vs 7.7%; p-value < 0.001) groups had a significant improvement in HbA1c at the end of the study. Similarly there was a significant improvement in FBG among both groups (SMBG 153 mg/dl vs. 123 mg/dl; p-value < 0.001 and non-SMBG (158 mg/dl vs. 137 mg/dl; p-value 0.022).
The HbA1c at the end of the study was 7.2% for the SMBG vs 7.7% for the non-SMBG group with no statistical difference (p-value 0.174).
Conclusion: The use of SMBG among type 2 DM patients did not result in better glycaemic control compared with patients who did not practise SMBG. It could be due to close follow-up and education of both groups.
Introduction
Diabetes mellitus (DM) can be defined as a syndrome of chronic hyperglycaemia characterised by insulin deficiency, insulin resistance or both.Citation1,2 In 2011 there were 366 million diabetics with a further 230 million at risk due to the evolving obesity pandemic and it is projected that by 2030, there will be 522 million diabetics worldwide and 398 million at risk.Citation2
Currently the global prevalence of DM is 2%.Citation2 In 2013, the International Diabetes Federation (IDF) reported a prevalence of 4.2% for the African region.Citation2 The burden of diabetes is increasing in low- and middle-income countries like Nigeria with more money being spent on complications due to poorly controlled diabetes.Citation3,4 Nigeria has a reported prevalence rate of 2.2% with regional differences.Citation4
Self-monitoring of blood glucose (SMBG) is defined as the collection by diabetic patients of detailed information about their blood glucose levels at many time points during the day on a day-to-day basis in order to aid adjustments in therapy and lifestyle activities and ultimately improve glycaemic control and prevent diabetes-related complications.Citation5 It is said to be structured SMBG when the blood glucose data are gathered according to a defined regimen, interpreted, and then utilised to make appropriate pharmacologic and/or lifestyle adjustments.Citation5
In Africa as in most developing countries, the practice of SMBG is poor.Citation6 SMBG is still not practised widely in Nigeria despite an increasing awareness.Citation7 A study in Port Harcourt reported only 27% of diabetic patients practised SMBG despite 96% of them being aware of its existence.Citation7 The multicentre diabetes care study, which was carried out across seven tertiary health centres in Nigeria to evaluate the quality of care of diabetics, reported that 72.8% of patients did not practise SMBG.Citation3
Other studies from Nigeria have also reported a variation in the practice of SMBG between urban and rural settings with rates of 3.4% in rural areas to 73% in urban areas in Nigeria.Citation3,7
Some of the reasons for the low use of glucometers includes cost, denial as patients do not want to know, doctors do not recommend or promote SMBG, results are not acted upon and pain.Citation8
Various studies have been carried out in Nigeria to determine the glycaemic control of DM patients using HbA1c. During more than 12 years of using HbA1c for monitoring of glycaemic control among patients at Nigerian hospitals, the mean glycosylated haemoglobin ranged from 7.9% to 8.3% with most patients (63% to 68%) having poor glycaemic control.Citation9
The reasons for poor glycaemic control among Nigerian diabetic patients are multi-factorial and include low level of literacy/health education, and poor drug and medication adherence amongst others.Citation10
The role of SMBG in assisting the achievement of glycaemic control by type 2 diabetic patients using oral hypoglycaemic agents has not been clearly defined.Citation1,11,12
Randomised control trials such as DiGEM, DINAMIC and ESMON reported no difference in levels of glycaemic control amongst type 2 DM patients who either practised or did not practise SMBG.Citation13–15 These trials did not use structured SMBG and only assessed HbA1c as treatment endpoint without checking for other benefits such as reduction in glycosylated haemoglobin.
Other randomised control trials which have used a study design incorporating structured SMBG testing have, however, showed benefit in greater mean reduction of glycosylated haemoglobin in type 2 DM patients who practised SMBG.Citation16
There are only a few studies in Africa, including Nigeria, on the efficacy of SMBG among type 2 DM patients. A cross-sectional study carried out in Sudan concluded that SMBG did not improve glycaemic control in type 2 DM patients.Citation17 A recent study in South Africa on the effect of SMBG, however, reported benefits regarding glycaemic control following the introduction of glucometers alongside patient education.Citation18
The aim of this study was to assess the effect of SMBG on glycaemic outcome among type 2 diabetics with a view to instituting its use to improve glycaemic control among type 2 DM patients.
Methods and materials
Study area
This study was carried out at the General Outpatient Clinic of the Family Medicine Department of Lagos State University Teaching Hospital, Ikeja, Lagos. The hospital is situated in the south-western part of Nigeria. Lagos is estimated to have a population of 21 million people.Citation19 The annual GOPD attendance is about 40 000 with a monthly average of 2 900 and daily attendance of 300–400 patients. There are about 15–20 DM patients who visit the clinic daily with an overwhelming majority being type 2 DM patients.
Study design
This was a randomised control trial of eight months’ duration conducted at the family medicine GOP clinic of the Lagos State University Teaching Hospital to assess the effect of self-monitoring of blood glucose on glycaemic outcome among Type 2 DM patients in a primary care setting.
Inclusion criteria
The study consisted of patients with type 2 DM who were on antidiabetic medications seeking medical care at the family medicine (GOP) clinic of the Lagos State University Teaching Hospital (LASUTH), Ikeja, Lagos State.
Exclusion criteria
Diabetic patients with emergencies, chronic complications such as nephropathy, neuropathy etc., those already using glucometer and < 18 years of age were excluded.
Sample size determination
The sample size was determined using the following formula for sample size with 2 means and normal distribution:Citation20
n is minimum sample size for each group; Zα is the probability of falsely rejecting a true null hypothesis (α) = 1.96; Z is the probability of failing to reject a false null hypothesis (β) = 0.84; σ1 is the standard deviation of glycosylated haemoglobin difference practising SMBG from a previous studyCitation21 = 1.1; σ2 is the standard deviation of glycosylated haemoglobin difference not practising SMBG from a previous studyCitation21 = 0.9; d is the size of the effect that is clinically worthwhile to detect clinical standardised difference = 0.9/1.1 = 0.819 = 0.82;
To adjust for attrition, an estimated dropout rate of 20% was assumed (because of duration of the study) and sample size calculated asCitation21:
Where N″ = corrected sample size; N = initial sample size; q = estimated attrition rate (20% = 0.2); ;
A sample size of 120 was used comprising 60 patients in each group (those practising SMBG and those not practising SMBG)
Recruitment and sampling technique
A total of 120 type 2 DM patients seeking medical care at the family medicine department who had met the inclusion criteria were consecutively recruited into the study after obtaining their consent, until the required sample size was attained. An average of 3–5 diabetic patients were recruited at every clinic visit.
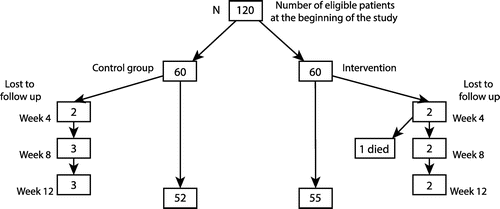
A simple randomisation scheme with the toss of a coin was used to allocate participants into the intervention group (given glucometers to practise SMBG) and the control group (not to practise SMBG). Figure shows the distribution of the study sample.
Study procedure
Patients in the intervention group were provided with a glucometer (which is a standardized autocoding blood glucose monitoring machine) and 25 fine test glucometer strips every month for three months. They were trained in the appropriate use of the glucometer. They were also required to write down their glucose reading, which was reviewed at their monthly clinic visits. Patients in both groups were given an initial structured education programme on diabetes and its complications by the researcher.
The participants in the intervention group (SMBG group) checked their blood glucose before and after meals (pre- and post-prandial) 3 days a week for 12 weeks in the following ‘structured’ manner:
Day 1: Breakfast (pre- and 2 hours post-prandial);
Day 2: Afternoon (pre- and 2 hours post-prandial);
Day 3: Night (pre- and 2 hours post-prandial).
Both groups were seen in the clinic by the researcher monthly and had fasting and two hours post-prandial glucose measurement taken at each visit with and appropriate treatment changes made based on the results. The patients were educated to have at least eight hours’ fast before the fasting blood glucose sample was taken. In a bid to prevent bias and reduce attrition among the study participants there was allocation concealment as each group was given separate days for its follow-up.
Participants were advised not to take medications apart from those prescribed by the researcher and also to present to the clinic if they had any complaints. Blood samples were taken for glycosylated haemoglobin at the beginning (initial visit) and the end of the study (12th week).
In total, each participant had 4 clinic visits (0, 4 weeks, 8 weeks and 12 weeks).
Instruments
A structured pretested questionnaire was used to obtain information on the patient’s biodata, average monthly income, knowledge of their care including the date of diagnosis, type of medication and adherence to medication use. Information on knowledge concerning self-monitoring of blood glucose and knowledge of glycaemic targets was also obtained.
Ethical considerations
Approval was obtained from the Health Research and Ethics Committee of LASUTH. Written informed consent was obtained from each participant after offering a detailed explanation of the purpose of the study.
Definition of outcome variables
| (1) | Glycaemic control was assessed as follows: good control was HbA1c of < 7% (< 53.3 mmol/mol) or FBG < 110mmg/dl (< 6.1 mmol/l) while poor control was HbA1c of ≥ 7% (53.3 mmol/mol) or FBG ≥ 110 mg/dl (> 6.1 mmol/l).Citation1 | ||||
| (2) | Medication adherence was assessed by asking the participants if they used their medications and checking the empty foils of used drugs. | ||||
| (3) | The glycaemic control was assessed using glycosylated haemoglobin and fasting blood glucose levels. | ||||
| (4) | The glycosylated haemoglobin was assessed using the DCCT aligned Clover A1c Analyser (Infopia Co. Ltd, Korea) with a test range of 4–14%. Daily calibration of the machine was done using check cartridges with lot number LK13G10. The Clover HbA1c machine is on the list of NGSP certified methods for assessing glycosylated haemoglobin.Citation22 | ||||
Data analysis
Data were entered and analysed using Epi info 6 (CDC, Atlanta, GA, USA). Percentages, mean and standard deviation of numerical variables were determined. Student’s t-test and paired t-test were used to compare numerical variables. A chi-square test and Fisher’s exact test were used to compare categorical variables as appropriate. For all statistical tests, the confidence interval was set at 95%. Intention-to-treat analysis was done. Statistical tests were considered significant if p-value was less than 0.05. Microsoft Excel (Microsoft Corp, Redmond, WA, USA) was used to draw charts.
Results
A total of 120 respondents divided into 2 equal groups were recruited for the study. In all, 107 respondents (52 in the control group and 55 in the intervention group) completed the study.
The age, gender, educational and other clinical parameters of the SMBG and non-SMBG groups were similar (p-value > 0.05) as shown in Table .
Table 1: Socio-demographic and clinical characteristics of the respondents (pre-intervention)
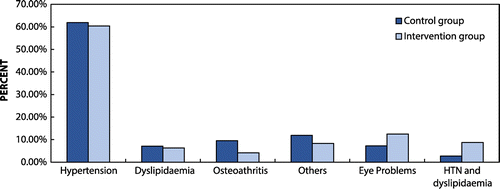
Three-quarters (90; 75.0%) of the respondents had co-morbid conditions with 48 (40.0%) respondents in the SMBG group and 42 (35.0%) in the non-SMBG group. Hypertension was the most common co-morbidity as shown in Figure .
The majority (93; 77.5%) of the respondents were aware of SMBG; there was no significant difference in the proportion of subjects that were aware in the SMBG and non-SMBG group at commencement of the study (p > 0.05), as shown in Table .
Table 2: Awareness of SMBG and glycaemic targets
About half (51; 42.5%) of the respondents were aware of their glycaemic targets with no difference in knowledge among the two groups of respondents at commencement of the study (p > 0.05) as shown in Table .
The HbA1c of the SMBG and non-SMBG groups (pre-intervention) was 8.7% (71.6 mmol/mol), which was similar. The FBG was 150 mg/dl (8.33 mmol/l) for the non-SMBG and 154 mg/dl (8.55 mmol/l) mg/dl for the SMBG group at the commencement of the study, which were similar (p-value 0.711) as shown in Table . The HbA1c at the end of the study was 7.2% (55.2 mmol/mol) for the SMBG and 7.7% (58.5 mmol/mol) for the non-SMBG group with no statistical difference (p-value 0.174). Similarly the FBG for the SMBG group and non-SMBG group at the end of the study was 123.2 mg/dl (6.83 mmol/l) and 137.6 mg/dl (7.64 mmol/l) respectively with no statistical difference (p-value 0.087) (see Table ).
Table 3: Change in HbA1c and FBG of the intervention and control groups
The reduction in HbA1c for SMBG and Non-SMBG groups at the end of the study was 1.5% and 1% respectively (see Table ). The reduction in FBG of the SMBG and non-SMBG groups at the end of the study was 31 mg/dl and 12.4 mg/dl respectively (see Table ).
At the end of the study, both the SMBG and non-SMBG groups had a statistically significant improvement in their HbA1c (p-value < 0.001) and FBG (intervention < 0.001, control p-value 0.022) as shown in Table .
Table 4: HbA1c and FBG before and after intervention in the two groups
Discussion
This study found that 77.5% of participants were aware of SMBG. A study in Port Harcourt on SMBG reported that 96% of diabetic patients were aware of SMBG.Citation7 Studies in Nigeria have reported knowledge and practice of SMBG ranging from 3.4–73% in rural and urban areas respectively.Citation8
The majority of the participants were not aware of their glycaemic targets prior to commencement of the study. A study among diabetics being managed in healthcare facilities in Imo state, Nigeria similarly reported that a majority of diabetics had poor knowledge about their management.Citation23 It is important that diabetic patients have a good knowledge of their care in order to utilise results obtained from SMBG in making decisions about their diet, exercise and medication use as well as to better manage their illness.
There was a significant improvement in HbA1c and FBG of the non-SMBG and SMBG groups, with no difference in glycaemic control between the two groups at the end of the study. This is in keeping with previous studies such as the ESMON, DiGEM, DINAMIC Swedish primary care studies, and King Drew Medical Centre trial amongst others, which reported no difference in glycaemic control among type 2 diabetics who were using oral antidiabetic agents, regardless of the practice of SMBG.Citation13–15,24,25 These studies instead highlighted education of patients and aggressive intensification of treatment as being more important than SMBG in achieving glycaemic control in type 2 DM.Citation13,14,25 The finding in this study differs from reports in trials such as the St Carlos study, Kaiser Permantle and a study in South Africa, which showed that type 2 diabetic patients using oral antidiabetic agents and who practised SMBG had better glycaemic control than those who did not.Citation26–29 The fact that both groups had education on diabetes mellitus and diet and were followed up closely with intensification could account for the finding in this study. The duration of follow-up being three months could also be responsible. Other factors such as diet and drug adherence amongst others could also have played a role.Citation11,16
The SMBG group had an HbA1c reduction of 1.5% while the non-SMBG group had a reduction of about 1%, though there was no statistical difference. A reduction in HbA1c of 1% leads to a 25% reduction in diabetes-related deaths, a 7% reduction in all-cause mortality, and an 18% reduction in combined fatal and non-fatal myocardial infarction.Citation1 The UKPDS study also reported that a 1.0% reduction in mean HbA1c was associated with a 37% decline in microvascular complications, as measured by reduced rates of laser therapy and cataract surgery.Citation1
Limitations of the study
The use of point-of-care machines for monitoring glycosylated haemoglobin could cause variability in the results. However, the machine is on the National Glycosylated Haemoglobin Standardisation Program (NGSP) list of validated point-of-care machines.Citation22 The packed cell volume and genotype of the patients were also not known, which could also affect the glycosylated haemoglobin, though the patients did not have any clinical features of anaemia or haemoglobinopathy.
Conclusion
In conclusion, this study highlights the importance of proper education of patients on diabetes mellitus medication intensification and close follow-up; the results cast doubt on the usefulness of SMBG among type 2 diabetics not using insulin. This is particularly important in resource-poor settings in Africa where the cost of glucometers and strips may be prohibitive for the average diabetic patient. There will, however, be a need for further studies of longer duration to assess the long-term benefits or otherwise of using glucometers.
References
- American Diabetes Association. Standard of medical care in diabetes mellitus 2014;35(Suppl 1):S11–S63.
- International Diabetes Federation Clinical Guidelines Task Force. Global guideline for type 2 diabetes. 2013. Available from: www.idf.org/diabetesatlas
- Chinenye S, Young E. Diabetes care in Nigeria. The Nigerian Health Journal. 2011;11(4):101–9.
- Oyebade OO, Abioye-Kuteyi EA, Kolawole BA. Screening for diabetes mellitus in a Nigerian family Practice population. S A Fam Pract. 2007;47(8):15.
- Leszek C, Laszlo B, Svetlana B, et al. Self-monitoring of blood glucose in diabetes: from evidence to clinical reality in central and eastern Europe recommendations from the international central-eastern European expert group. Diabetes Technol Therapeutics. 2014;16:7.
- Majikela-Dlangamandla B, Isiavwe A, Levitt N. Diabetes monitoring in developing countries. Diabetes Voice. 2006;51:28–31.
- Unachukwu CN, Young EE, Uchenna DI. Self monitoring of blood glucose among diabetic patients in Portharcourt, Nigeria. Afr J Diabetes Med. 2011;19(1):19–20.
- Ogbera AO, Ekpebegh C. Diabetes mellitus in Nigeria: the past, present and future. World J Diabetes. 2014; 15(6):905–91.
- Fischer, WA. Barriers and behaviors in blood glucose monitoring. US Endocrine Disease report. 2007;9–11.
- Oghagbon EK. Improving persistently elevated HbA1c in diabetes mellitus patients In Nigeria. Ethnicity & Disease. 2014;24:502–3.
- Self-Monitoring of Blood Glucose in Non-Insulin-Treated Type 2 Diabetes Recommendations based on a Workshop of the International Diabetes Federation Clinical Guidelines Taskforce in collaboration with the SMBG International Working Group. 2013 [cited 2013 Jul 20]. Available from: www.idf.org
- VA/DoD clinical practice guideline for the management of diabetes mellitus. 2013 [cited 2013 Aug 10]. Available from: http://www.healthquality.va.gov
- O’Kane MJ, Bunting B, Copeland M. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336:1174–7.10.1136/bmj.39534.571644.BE
- Barnett AH, Krentz AJ, Strojek K. The efficacy of self-monitoring of blood glucose in the management of patients with type 2 diabetes treated with a gliclazide modified release-based regimen. A multicentre, randomized, parallel-group, 6-month evaluation (DINAMIC 1 study). Diabetes Obes Metab. 2008;10:1239–47.
- Farmer A, Wade A, Goyder E. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335:132.10.1136/bmj.39247.447431.BE
- Parkin CG, Buskirk A, Hinnen DA, et al. Results that matter: structured vs. unstructured self- monitoring of blood glucose in type 2 diabetes. Diabetes Res Clin Pract. 2012;97:6–15.10.1016/j.diabres.2012.03.002
- Abdulquadir M, Elbagir M, Elton M. The Influence of glucose self monitoring on glycaemic control in patients with diabetes mellitus in Sudan. Diabetes Res Clin Pract. 2006;74(1):90–4.
- Pillay S, Aldous, C. Effects of self-monitoring of blood glucose on diabetes control in a resource-limited diabetic clinic. J Endocrinol Meta Diabetes S Afr. 2016;21(2):20–5. doi:10.1080/16089677.2016.1198112
- Lagos State Government, Population of Lagos State. 2017 [cited 2017 April]. Available from: www.lagosstate.gov.ng/pagelinks.php?p
- Parikh NM, Hazra A, Mukherjee J, et al. Research methodology simplified every clinician a researcher. Jaypee. 2010:108–9.
- Franciosi M, Lucisano G, Pellegrini F, et al. ROSES: role of self-monitoring of blood glucose and intensive education in patients with Type 2 diabetes not receiving insulin. A pilot randomized clinical trial. Diabet Med. 2011 Jul;28(7):789–96. doi:10.1111/j.1464-5491.2011.03268.x
- List of NGSP Certified Methods [Internet]. 2015 [cited 2015 Jun 10]. Available from: www.ngsp.org/docs/methods.pdf
- Nwankwo HC, Bikash N, Nwankwo B. Factors influencing management outcome among patients attending government health facilities in South East, Nigeria. Int J Trop Med. 2010;5(2):28–36.
- Anders T, Ewa G, Kjell LM, Sigvard ML. Self-monitoring of blood glucose and glycaemic control in type 2 diabetes. Scand J Prim Health Care 2007;25:140–6.
- Davidson MB, Castellanos M, Kain D. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial. Am J Med. 2005;118:422–5.10.1016/j.amjmed.2004.12.006
- Bonomo K, De Salve A, Fiora E, et al. Evaluation of a simple policy for pre- and post-prandial blood glucose self-monitoring in people with type 2 diabetes not on insulin. Diabetes Res Clin Pract. 2010;87(2):246–51.10.1016/j.diabres.2009.10.021
- Duran A, Martin P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset Type 2 diabetes mellitus: the St. Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2(3):203–11.10.1111/jdb.2010.2.issue-3
- Karter AJ, Parker MM, Moffet HH. Longitudinal study of new and prevalent use of self-monitoring of blood glucose. Diabetes Care. 2006;29:1757–63.10.2337/dc06-2073
- Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262–7.10.2337/dc10-1732