Abstract
Background: Prostate cancer is a leading cause of morbidity and mortality in our male population, thus screening initiatives will help to improve outcomes. The current screening marker, total prostate-specific antigen (PSA), is not prostate cancer specific. The development of percentage free PSA (%FPSA) has largely improved the detection of prostate cancer.
Objectives: To assess the performance of %FPSA ratio at the 25% cut-off and its ability to distinguish between prostate cancer and benign prostatic lesions.
Methods: This was a retrospective study conducted on male patients with total prostate-specific antigen values < 10 ng/ml and with prostate histology results. Male patients with total prostate-specific antigen between 4 and 10 ng/ml had their free prostate-specific antigen determined together with the calculation of the free prostate-specific antigen ratio. The ratio was then correlated with prostate histology results to determine the presence of prostate cancer at the cut-off ratio of 25%.
Results: Prostate cancer was detected in 28 (21.37%) patients out of the total population of 131. Ninety-two patients had a FPSA ratio of < 25%, 22 (22.8%) of whom were found to have prostate cancer. Notably the sensitivity and specificity were found to be 86% and 27% respectively, with a positive predictive of value of 21% at this cut-off.
Conclusions: The study demonstrates a %FPSA ratio of 25% not to be a good discriminator between prostatic cancerous and benign lesions. It is thus recommended that a prostate biopsy should be done based on clinical examination findings rather than the level of total prostate specific antigen from 0–10 ng/ml or %FPSA ratio.
Background
Prostate cancer (PCA) is a common male cancer globally and in South Africa (SA) the incidence has been reported to be 40.5 per 100 000 per year.1 The global incidence has been reported to vary substantially due to differences in the screening of cancer through prostate-specific antigen (PSA) testing. The incidence has been shown to increase with age and is common after the age of 50 with a peak at the age of 60–70 years.Citation2 Screening and diagnosing PCA early is crucial as these can prevent poor outcomes. PSA is the only useful tumour marker that has been approved by the Food and Drug Administration (FDA) for early detection of PCA.Citation3,4
Prostate-specific antigen is a proteolytic enzyme that belongs to the kallikrein family of serine proteases.Citation5 PSA is expressed in prostate tissue, breast tissue, salivary glands and skene glands.Citation2,6 In the prostate gland, PSA is produced as a proenzyme PSA (proPSA) by the epithelial cells of the acini and ducts of the prostate gland.Citation5,7 It is then secreted to the lumen where the propeptide is removed and active PSA is formed. Its major function is to regulate seminal fluid viscosity and it plays a major role in dissolving the cervical mucus cap, allowing sperm to enter the uterus. Due to efficient blood supply, small amounts (or concentrations) of PSA constantly pass to the bloodstream.Citation5
It is well known that PSA exists in multiple forms. The fraction that enters the circulation is intact and predominantly bound to proteins (alpha 2 macroglobulin and alpha1 antichymotrypsin) and is known to be active. The inactive enzymatic PSA form does not bind proteins and circulates freely as free PSA (FPSA).Citation2,8 The total PSA (TPSA) is a combination of complexed and free PSA. TPSA is used in monitoring treatment response in patients with prostate cancer, early detection of recurrence, assessment of tumour mass and is also useful in screening and diagnosing PCA.Citation2,7,9 Despite its useful clinical applications, TPSA lacks specificity because it can increase in patients with non-cancerous prostatic conditions, including benign prostatic hyperplasia, prostatitis, acute urinary retention , and on digital rectal examination, ejaculation and prostate biopsy.Citation7,10 Measures have been taken to improve the screening of PCA as the current TPSA screening shows poor sensitivity and specificity, especially in the TPSA grey-zone range (4–10 ng/ml). These measures include the introduction of different indices: %FPSA:TPSA ratio, %proPSA, PSA density and PSA velocity.Citation2,11
The most successful measure thus far is the use of FPSA:TPSA (FPSA/TPSA*100) ratio. This ratio is known to improve the specificity and sensitivity for detecting PCA, especially in patients with TPSA of 2/4–10 ng/ml.Citation2,12 Notably, the FPSA has three distinct forms: inactive PSA (iPSA), benign PSA (BPSA) and precursor isoforms PSA (pro-PSA). BPSA is found in the transition zone of the prostate gland and contributes to FPSA in the serum of patients with benign prostatic hypertrophy (BPH). The pro-PSA is a precursor form of PSA, localised to the peripheral zone of the prostate gland and contributes to FPSA in the serum of patients with cancer of the prostate. The iPSA is enzymatically inactive and is a minor variant of intact PSA.Citation2 The clearance of serum PSA is mainly through the liver (for both the complexed PSA and the FPSA). FPSA is also cleared by the kidneys. Their half-lives are 1.22 h and 22–333 h for FPSA and TPSA respectively.Citation2,13
Several studies done on the %FPSA:TPSA ratio have demonstrated lower ratios (or values) in males with PCA as compared with those without. The low ratio is attributed to the fact that PSA produced from malignant cells appears to escape proteolytic processes, resulting in more serum PSA complexed to alpha chymotrypsin (ACT) and a lower percentage of total PSA that is in free form. Notably PCA cells do not produce more PSA than benign prostate epithelium.Citation14 The ratio has assisted in determining the need for prostate biopsy in patients with a TPSA level between 4 and 10 ng/ml. A ratio of ≤ 25% indicates the need for a prostate biopsy whereas a ratio of > 25% requires monitoring with TPSA.Citation2 Sokoll et al. have shown that biopsied males with TPSA between 4 and 10 ng/ml and %FPSA ratio cut-off of < 25% showed sensitivity for PCA of 95%, whereas Catalona et al. showed that about 25% of patients will have a positive biopsy when their PSA level is between 2 and 10 ng/ml. Currently, %FPSA ratio is the marker that is used to predict PCA and is FDA approved.Citation2
Aim of the study
The study has been done to investigate whether the %FPSA ratio of 25% is able to distinguish between PCA and benign prostatic lesions and to determine the need for prostate biopsies in our population.
Methods
The study is a retrospective audit conducted on male patients above 18 years of age attending Dr George Mukhari Academic Hospital Urology clinic between June 2013 and March 2016. The study was conducted by the Department of Chemical Pathology, Sefako Makgatho Health Science University and study ethics approval was obtained from the Sefako Makgatho Health Science University Research ethics committee (SMUREC/P/106/2016). Patients who had TPSA values < 10 ng/ml, FPSA values, FPSA ratios (for TPSA 4–10 ng/ml) and prostate histology results were included in the study; patients who did not have FPSA (for TPSA between 4 and 10 ng/ml) and/or histology were excluded from the study.
In all selected patients the serum samples were collected in plain tubes and/or serum separator tubes for TPSA and routinely analysed at a South African National Accreditation System (SANAS) accredited laboratory. The measurement of serum TPSA and FPSA was performed on the Abbott Architect ci8200 immunoassay system (Abbott, Abbott Park, IL, USA). The TPSA assay is also a two-step chemiluminescent microparticle immunoassay and the calibrator is referenced against the World Health Organization (WHO) 1st International standard for PSA (90:10) 96/670 at each concentration level. The assay has a linear range of 0–50 ng/ml and a limit of detection of 0.008 ng/ml.
The FPSA assay is a two-step chemiluminescent microparticle immunoassay and the calibrator is referenced against the Stanford 90:10 PSA reference material at each concentration level. The assay has a linear range of 0–30 ng/ml and a limit of detection of 0.008 ng/ml. The %FPSA ratio was calculated by the Laboratory information system (LIS).
Prostate histology results were used as a gold standard to categorise patients as having prostate cancer or benign prostatic lesions. Histological samples were obtained by transrectal ultrasound (TRUS) guided biopsy and/or total prostatectomy. The biopsies were analysed using haematoxylin and eosin staining method at a SANAS accredited histopathological laboratory by histopathologists.
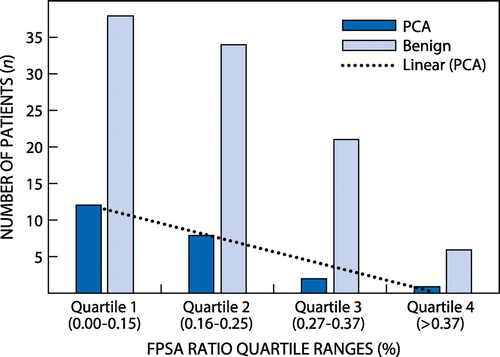
The patient population was divided into two groups, those with PCA and those with benign prostatic lesions and the group categories were based on histology reports and %FPSA ratio of 0.25. The two groups were further subdivided into quartiles based on %FPSA ratio 0.00–0.15, 0.16–0.25, 0.27–0.37 and > 0.37.
Statistical analysis
Data were analysed using SPSS®, version 24, statistical software (IBM Corp, Armonk, NY, USA). The Shapiro–Wilk test was used to establish if the data followed a Gaussian distribution. Since the data was non-parametric, Wilcoxon’s signed rank test was used to compare patients with prostate cancer and patients with benign prostatic lesions. A p-value < 0.05 was considered as statistically significant.
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated at different %FPSA ratio cut-offs (30%, 25%, 20%, 15% and 10%) to establish which cut-off will offer a better diagnostic utility using histology as a gold standard.
Results
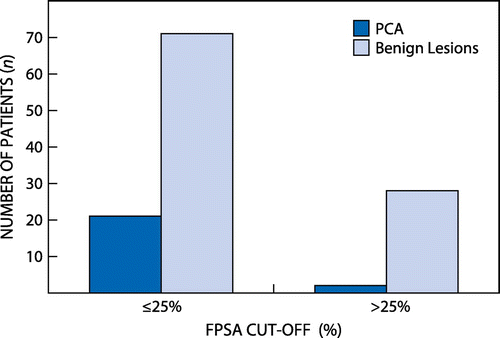
The total number of patients evaluated was 131 and their TPSA ranged from 0 to 10 ng/ml. The age of the cohort ranged from 47 to 90 years with a median age of 65 years. Twenty-eight out of 131 (21.37%) had PCA on biopsy. Nine out of 131 patients had TPSA < 4 ng/ml. In this group, five patients had invasive adenocarcinoma whereas four had benign prostatic lesions. The remaining 122 patients had TPSA 4–10 ng/ml with 23 having PCA and 99 having benign lesions. Of these 122 patients, using the %FPSA ratio and the TPSA:FPSA ratio quartiles. We established that more patients are classified as prostatic benign lesions as compared with PCA (Figure and ). The TPSA, FPSA and F/TPSA ratio did not show significant differences between the benign and cancerous histology (Table ), whereas age demonstrated a significant difference when Wilcoxon’s signed rank test was used. At the %FPSA ratio cut-off of 25%, PCA was found in 22.8% of patients (Figure ). The data demonstrated a sensitivity and specificity of 86% and 27% respectively at the 25% cut-off, with a PPV of 21%. At the 24% cut-off, the data showed a sensitivity and specificity of 83% and 47% respectively with the best NPV of 92%, but a poor PPV of 26% (Table ).
Table 1: Comparison between prostate cancer and benign prostatic lesion patients
Table 2: Diagnostic utility of F/TPSA ratio at different cut-offs
Discussion
Literature shows that PCA is the most common male cancer. In SA, the 2012 National Cancer Registry (NCR) recognises PCA as the second most common male cancer;Citation15 thus early detection is essential to reduce the high morbidity and mortality rates.
Most institutions do not recommend further testing when patients present with TPSA < 4 ng/ml, unless there is a clinical suspicion following a per-rectal examination. The prevalence of PCA in patients with TPSA < 4 ng/ml is estimated to be approximately 2–26%.Citation16 PCA with the PSA range of < 4 ng/ml was previously known to be indolent but has since been shown to be aggressive, with metastases being present at the time of diagnosis.Citation17 A study done by Ibrahaim demonstrated a detection rate of 13.1% for PCA with TPSA < 4 ng/ml and notably our study detected 55.55% (five out of nine patients).Citation18
The %FPSA has been recommended as the best strategy to improve the diagnostic sensitivity of TPSA for the grey-zone region (2 or 4–10 ng/ml). Sokoll et al. demonstrated that biopsied males with TPSA between 4 and 10 ng/ml and %FPSA ratio cut-off of < 25% showed sensitivity of 95%, whereas Catalona et al. showed that 25% of patients will have a positive biopsy when their PSA level is between 2 and 10 ng/ml. Using the 25% FPSA as a cut-off, our study demonstrated a 23% chance of detecting males with PCA while 77% of the patients would have been subjected to unnecessary biopsies as shown in Figures and .
Notably Figure demonstrates that at different cut-offs of %FPSA ratio there is poor stratification between the cancerous and the benign lesions. The study was able to demonstrate that more patients are diagnosed with PCA at lower %FPSA ratio cut-off as compared with higher %FPSA ratio cut-off value, which is in agreement with the findings of Catalona et al. The low PPV seen in our study with %FPSA ratio cut-off of < 25% is comparable to that of Agnihotri et al. who looked at Indian males and found a PPV of 15.95%.14 The study demonstrates that %FPSA at 25% does not have a high discriminating power between benign and cancerous lesions, as the sensitivity at the current cut-off is 86%, which is below the preferred 95% cut-off.Citation2,3 For our study the %FPSA at 25% could only detect 23% of the cancers. Notably there is no significant difference between benign and cancerous lesion as the TPSA mean was 6.46 ± 2.76 and 7.00 ± 2.06 for PCA and benign lesions respectively with p < 0.355. When using the %FPSA less than 25% as a cut-off, the difference between the mean ratios (0.16 ± 0.09 and 0.200 ± 0.105) for PCA and benign lesions, respectively, was still insignificant with p < 0.749.
Limitations
Not all patients with TPSA in the grey zone and available histology results could be included in the study due to the absence of FPSA levels. FPSA was initially not a reflexed test at the study laboratory.
In conclusion, the results of our study indicate that at 25% the %FPSA ratio is not a useful discriminator for PCA and benign lesions when TPSA is in the grey zone (4–10 ng/ml). The study demonstrates that the majority of patients are subjected to unnecessary biopsies based on the current 25% cut-off of the FPSA ratio; however, a few (23% of cases) patients benefited from the biopsies. Notably, we established that patients with low TPSA (0–3.9 ng/ml) are also at high risk of PCA, thus we recommend that the decision to refer for biopsy should be based on the presence of other risk factors and clinical findings.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Parkin DM . Cancer in indigenous Africans burden, distribution and trends. Lan Oncol 2008;9(7):683–692.10.1016/S1470-2045(08)70175-X
- Sokoll LJ . Tumour Markers. In: Wurm-Cutter E , editor. Tietz textbook of clinical chemistry and molecular diagnostics. Atlanta:Elsevier; 2005. p. 617–667.
- Hoffman RM . Using the free-to-total prostate-specific antigen ratio to detect prostate cancer in men with non-specific elevations of prostate-specific antigen level. J Gen Int Med. 2000;15: 739–748.10.1046/j.1525-1497.2000.90907.x
- Omar J . A pilot study on percent free prostate specific antigen as an additional tool in prostate cancer screening. Malaysian J Med Sci 2008;16(1): 44–47.
- Mccudden CR . Circulating tumour markers: Basic concepts and clinical application. In: Troy D , editor. Clinical Chemistry Principles, Techniques and Correlation. Philadelphia, PA : Lippincot and Williams; 2005. p. 664–679.
- Musrap W . Prostate specific antigen as a marker of hyperandrogenism in women and its clinical implications for antidoping. Clin Chem. 2016;62(8):1066–1074.10.1373/clinchem.2016.256198
- Adhyam M . A review on the clinical utility of PSA in cancer prostate. Indian J Surg Oncol 2012;3(2): 120–129.10.1007/s13193-012-0142-6
- Southwick PC . The role of free PSA in the detection of prostate cancer. J Lab Med 2001;32(5): 259–262.10.1309/06E3-4LG5-KYEG-FGAC
- Prcic A , Begic E , Hiros M . Usefulness of total PSA value in prostate diseases diagnosis. Acta Inform Med. 2016;24(3): 156–161. doi:10.5455/aim.2016.24.156-161.
- Carter HB . Prostate cancers in men with low PSA levels—must we find them? N Engl J Med. 2004;350: 2292.10.1056/NEJMe048003
- Loeb S . What to do with an abnormal PSA test. Oncol. 2008;13: 299–305.10.1634/theoncologist.2007-0139
- Catalona WJ . Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. J Am Med Assoc. 1998;279: 1542–1547.10.1001/jama.279.19.1542
- Kilic S . Determination of the site of metabolism of total, free and complexed prostate specific antigen. Urology 1998;52(3): 470–473.10.1016/S0090-4295(98)00208-8
- Agnihotri S , Mittal RD , Kapoor R , Mandhani A . Raising cut-off value of prostate specific antigen (PSA) for biopsy in symptomatic men in India to reduce unnecessary biopsy. Indian J Med Res. 2014;139(6):851–856.
- South African National Cancer Registry. Johannesburg: Cancer in South Africa; 2012 [cited 2017 Oct 25]. Available from: www.ncr.ac.zawww.ncr.ac.
- Thompson IM , Donna K . Prevalence of prostate cancer among men with prostate specific antigen level<4.0 ng/ml. N. Engl J Med. 2004;350: 2239–2246.10.1056/NEJMoa031918
- Nishio R . Metastatic prostate cancer with normal level of serum prostate specific antigen. Int Urol Nephrol J. 2003;35: 189–192.10.1023/B:UROL.0000020306.08275.49
- Ibrahim E . Incidence of carcinoma of the prostate in patients with normal prostate specific antigen following prostatectomy. Glob J Med Res Surg Cardiovas Syst. 2013;13(3).