Abstract
Background: HIV/HBV co-infection remains a global threat to HIV management despite the available effective hepatitis B vaccine and hepatitis B covering antiretroviral therapy. Many studies done in South Africa and internationally showed high prevalence of HIV/hepatitis B co-infection, which mandated routine screening for both infections before initiating HAART. Fewer studies have highlighted the prevalence of hepatitis B susceptibility in the general population starting HAART and most of them were limited to children and high-risk groups. The aim of this study was to demonstrate the extent of hepatitis B susceptibility, hepatitis B/HIV co-infections and hepatitis B immunity in general HIV-infected patients.
Method: This was a retrospective review of 1 066 randomly sampled files of patients initiated on HAART between January 2012 and December 2014 at two Durban hospitals. Data collection included demographic characteristic, CD4 counts and hepatitis B serology. Data were analysed for the prevalence of hepatitis B susceptibility, HIV/HBV co-infection and hepatitis B immunity, while correlations between age, CD4 count and these three groups were demonstrated. Statistical analysis was performed using SAS version 9.3.
Results: Total prevalence of HBV susceptibility was 69.7%, HBV immunity was 26.9% and true chronic HIV/HBV co-infection was 3.4%, while HBVsAg positivity accounted for 8.4% of the participants. Adults were more susceptible to HBV than children, with a median age of 36 years. Stratified for age, children were more immune (90%) to HBV than adults.
Conclusion: This study demonstrated a significantly high number of HIV-infected persons who were susceptible to hepatitis B infection in Durban, South Africa, where both HIV and HBV are endemic, co-infection is high, and safe and effective HBV vaccine is available. Hepatitis B vaccination of the hepatitis B susceptible patients initiating HAART in South Africa is recommended to prevent further HIV/HBV co-infection.
Introduction
Hepatitis B Virus (HBV) and human immunodeficiency virus (HIV) co-infection remains a major health concern in sub-Saharan Africa including South Africa,Citation1–6 where both diseases are endemic and share the same route of transmission. These studies showed a high prevalence of HBV/HIV co-infections ranging from 6% to 20% with geographic variation within the region. Two big studies done in Europe and North America showed a prevalence of 7–8%Citation7,Citation8 of HBV/HIV co-infection and as high as 20% prevalence was demonstrated in one province in South AfricaCitation6.
Co-infected patients have increased risk of fatal liver disease, specifically cirrhosis and death due to liver cancer.Citation2,Citation4,Citation5,Citation9 Although the current first-line highly active antiretroviral therapy (HAART) regimen covers HBV infection, drug resistance of both HIV and HBV is a threat to this treatment.Citation4,Citation10 Preventing HIV-infected patients from getting HBV infection therefore remains critical for the future management of HIV/HBV co-infection.
There is a safe and effective vaccine against hepatitis B infection that has been used since April 1995 in the children's Expanded Programme on Immunizations (EPI)Citation9,Citation11 and for non-immune medical staff in South Africa, but does not include the HBV susceptible (lack of HBV antibodies and surface antigen) HIV-infected patients. The potential delay in this regard could be due to the scarcity of local clinical data presented for clinical decision-making when interventions are planned.Citation5 South Africa has a vague idea of the prevalence of HBV susceptibility in the HIV- infected general population and this has potentially delayed the decision to vaccinate this susceptible group.
Some developed and developing countries that have included HBV vaccination for managing HIV-infected patients have reported good long-term public health benefits.Citation12–16 Studies done in sub-Saharan Africa reporting on HBV have focused mainly on the prevalence of acute and chronic infections, HBV/HIV co-infection in women or in pregnant women only,Citation17,Citation18 men having sex with men and children.Citation11 The few that were inclusive of the general HIV-positive population were limited by a small sample size.Citation5,Citation6 Larger studies that include adults and children are needed to inform South African decision-making in the prevention of HBV/HIV co-infection.
This study aimed to determine the extent of HBV susceptibility in HIV-infected patients in Durban, KwaZulu-Natal province in South Africa. The study showed the prevalence of HBV susceptibility, HBV/HIV co-infection and immunity but did not demonstrate the incidence rate of HBV in this population.
Methodology
This retrospective, descriptive and cross-sectional study was conducted at Masibambisane and Vusithemba Antiretroviral Clinics at Wentworth and Prince Mshiyeni hospitals respectively. The study sample consisted of HIV positive patients who were initiated on HAART between January 1, 2012 and December 31, 2014. The pilot audit, which included 66 patients at the Masibambisane clinic showed that 47% of the patients initiating HAART at Wentworth hospital in March 2013 were susceptible to hepatitis B infection (unpublished data). A sample size of 1 064 was required to estimate a proportion of 47% with a desired precision of 3% for a 95% confidence interval, with 1 066 files being reviewed. To eliminate selection bias, clinical files were systematically and randomly selected, with every second file being identified until 1 066 files were retrieved, half from each site. Neonates were excluded, as they were not fully vaccinated according to the South African EPI, as were pregnant women, as they were initiated on treatment in separate clinics from the general HIV-infected patients.
We reviewed the files for the routine hepatitis B serology, baseline characteristics, and included age, gender and CD4 count at the initiation of HAART. The missing data were searched using the TRAKCARE electronic system that is used to store data at both clinics. Hepatitis screening was done by the National Health Laboratory Services at Inkosi Albert Luthuli Central hospital, and no further phlebotomy was conducted to replace lost or missing data. The collected data were entered onto an approved MS Excel (Microsoft Corp, Redmond, WA, USA) spreadsheet, de-identified using a coding system and stored in a USB locked in a private cabinet to protect the patients’ confidentiality.
The statistical analysis was performed using SAS version 9.3 (SAS Institute, Cary, NC) and p-values of < 0.05 were considered significant. Simple mean and percentage calculations were used to analyse the baseline characteristics and the prevalence of hepatitis B susceptibility, HBV/HIV co-infection and immunity. The Wilcoxon rank sum test was used to compare differences in CD4 between adult males and females. Fisher's exact test was used to compare proportions of gender and age group across different HBVsAg categories. The Kruskal–Wallis test was used to compare CD4 count distribution across different HBVsAg categories
The study was approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee, KwaZulu-Natal Provincial Government Department of Health and the participating antiretroviral sites in Durban, South Africa.
Results
In this review of 1 066 files, the males and females were almost equally represented; there were more adults than children. An overall low median CD4 count of 167 cells/ml was demonstrated but, when separately analysed, children had higher CD4 counts than adults and males had a lower CD4 count median than females ().
Table 1: Baseline characteristics and CD4 count characteristics of HIV-infected patients (n = 1 066)
Of the selected 1 066 files, 144 had missing data, with 992 files being analysed for hepatitis B serology. A total of 69.8% of these patients were susceptible to HBV, 3.1% were HIV/HBV co-infected, and 25% were HBV immune. Adults (99.2%) showed a significantly higher susceptibility to HBV than children (0.8%), with more adults (96.8%) being co-infected than children (3.2%). Adults (71.8%) appeared more immunised that children (28.2%) but when stratified for age, children (92.1%) were more immunised than adults. There was no significant gender difference in the adults for susceptibility, co-infection and immunity ().
Table 2: HBV susceptibility, hepatitis B immunity and HIV/HBV co-infection by age, gender and CD4 count (n = 922)
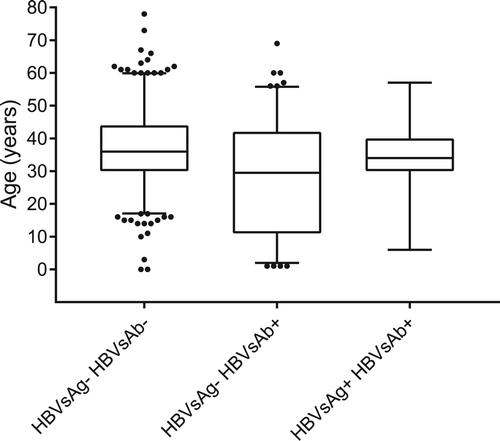
The box and whiskers plot in shows that adults were more susceptible and more likely to be co-infected with HBV, while children were more immune to HBV, although several outliers were also demonstrated.
Discussion
The high prevalence of hepatitis B susceptible patients (69.8%) with a higher prevalence in adults than children demonstrated in this cohort was due to lack of HBV B vaccination of people born before the inclusion of hepatitis B vaccine in the South African EPI.Citation9,Citation11 Even though routine screening is carried out in South Africa for hepatitis B at the initiation of HAART, vaccination of the susceptible HIV-infected patient is not routine.
The prevalence of HBV/HIV was higher (25%) than in most studies done in South Africa. This was even higher than the recent 20%Citation6 reported by a study done in Limpopo province. Both HBV and HBV are endemic (>8% prevalence) in South AfricaCitation2,Citation4 and they share a common route of transmission in adults, and this puts susceptible South Africans at high risk of acquiring both diseases.
Immunity was low (3.1%) because most adults did not receive HBV vaccine when they were children. When comparing adults with children, immunity appeared more prevalent in adults because there were more represented than children and the data was skewed in their favour. Stratified for age, children (92.1%) showed higher HBV immunity than adults because they were born after the inclusion of HBV immunisation for HBV.
Gender made no significant difference (p = 0.560) in prevalence of HBV susceptibility, co-infection and immunity because males and females of the same age were not vaccinated when born before 1995.
The study showed low CD4 at the initiation of HAART, which is a common trend with most studies done in South Africa.
Conclusion
The public health implication of the study result showed a significantly high hepatitis B susceptibility in HIV-positive patients initiating HAART in Durban, South Africa. These findings motivate for the inclusion of HBV vaccination in the management of susceptible patients initiating HAART in South Africa. The study results, supported by other similar studies, can be used to inform decision-making when planning for the prevention of HBV/HBV co-infection in South Africa. The study results will be made available to the KwaZulu-Natal and the South African Departments of Health for them to make informed clinical decisions concerning prevention of HBV/HIV co-infection. We recommend future cohort studies to explain the patterns that emerged from this cross-sectional study. This was one of the largest primary healthcare-based studies to describe hepatitis B serology amongst the general population, including females, males, adults and children, and not focusing only on pregnant women. As a retrospective study it could not explain the causes of the hepatitis B serology patterns demonstrated by the findings. Age was a confounder when analysing the data but was stratified for to account for the skewed results.
Authors’ contribution
F.C. was responsible for the project design, data collection and presentation of results. S.R. made major contributions and corrections during the project design and writing of the manuscript.
Acknowledgements
The authors would like to thank the participating sites, their staff for assisting in data collection and Ms Nonhlanhla Nyende for her statistical contribution.
Disclosure statement
There is no conflicting interest between the study and the funders.
Additional information
Funding
References
- Burnett R, François G, Kew MC, et al. hepatitis B virus and human immunodeficiency virus co-infection in sub-Saharan Africa: a call for further investigation. Liver Int 2005;25(2):201–13. http://doi.org/10.1111/j.1478-3231.2005.01054.x
- Firnhaber C, Reyneke A, Schulze D, et al. The prevalence of hepatitis B co-infection in a South African urban government HIV clinic. S Afr Med J. 2008;98:541–4.
- Mandiwana A, Tshitenge S. Prevalence of human immunodeficiency virus — hepatitis B virus co-infection amongst adult patients in Mahalapye, Ngami, Serowe, Botswana: a descriptive cross-sectional study. S Afr Fam Pract. 2017;59(3):94–7.
- Kew M. hepatitis B virus infection: the burden of disease in South Africa. South Afr J Epidemiol Infect. 2008;23(1):4–8. http://doi.org/10.1080/10158782.2008.11441293
- King J, Hagemeister D. hepatitis B co-infection in HIV-infected patients receiving antiretroviral therapy at the TC Newman Anti-Retroviral Treatment Clinic in Paarl, Western Cape. S Afr J HIV Med. 2016;17(1):541. http://doi.org/10.4102/sajhivmed.v17i1.336
- Ayuk J, Mphahlele J, Bessong P. hepatitis B virus in HIV-infected patients in Northeastern South Africa: prevalence, exposure, protection and response to HAART. S Afr Med J. 2013;103(5):330–3. http://doi.org/10.7196/SAMJ.6304
- Konopnicki, D, Mocroft A, de Wit S, et al. hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19(6):593–601. http://doi.org/10.1097/01.aids.0000163936.99401.fe
- Scott E, Kellerman D, Hanson A, et al. Prevalence of chronic hepatitis B and incidence of acute hepatitis B infection in human immunodeficiency virus–infected subjects. J Infect Dis. 2003;188(4):571–7. http://doi.org/10.1086/377135
- Hoffmann C, Thio LC. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7(6):402–9. http://doi.org/10.1016/S1473-3099(07)70135-4
- Núñez M, Soriano V. Management of patients co-infected with hepatitis B virus and HIV. Lancet Infect Dis. 2005;5:374–82. http://doi.org/10.1016/S1473-3099(05)70141-9
- Jooste P, van Zyl A, Adland E, et al. Screening, characterisation and prevention of hepatitis B virus (HBV) co-infection in HIV-positive children in South Africa. J Clin Virol. 2016;85:71–4. http://doi.org/10.1016/j.jcv.2016.10.017
- Abara W, Qaseem A, Schillie S, et al. hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167(11):794–804. http://doi.org/10.7326/M17-1106
- Martins S, do Livramento Ad, Andrigueti M, et al. Vaccination coverage and immunity against hepatitis B among HIV-infected patients in South Brazil. Braz J Infect Dis. 2015;19(2):181–6. http://doi.org/10.1016/j.bjid.2014.12.002
- Tedaldi EM, Baker RK, Moorman AC, et al. hepatitis A and B vaccination practices for ambulatory patients infected with HIV. Clin Infect Dis. 2004;38(10):1478–84. http://doi.org/10.1086/420740
- Phung BC, Launay O. Vaccination against viral hepatitis of HIV-1 infected patients. Hum Vaccin Immunother. 2012;8(5):554–9. http://doi.org/10.4161/hv.19105
- Tithiah N, Parboosing R, Singh L, et al. Human immunodeficiency virus and hepatitis B or C co-infection in KwaZulu- Natal: a retrospective analysis of a laboratory database.
- Coffie PA, Egger M, Vinikoor MJ, et al. Trends in hepatitis B virus testing practices and management in HIV clinics across sub-Saharan Africa. BMC Infect Dis. 2017;17(Suppl 1):706. http://doi.org/10.1186/s12879-017-2768-z
- Chambal LM, Gudo ES, Carimo A, et al. HBV infection in untreated HIV-infected adults in Maputo, Mozambique. PLOS ONE. 2017;12(7):e0181836. http://doi.org/10.1371/journal.pone.0181836