ABSTRACT
Brucellosis is an infectious disease caused by different Brucella species. The outer membrane proteins 25 and 31 play a significant role in stimulation of immunity against Brucella. Herein, the humoral and cellular immune responses of selected recombinant proteins emulsified in chitosan nanoparticles as individual (univalent) and simultaneous (divalent) injections were assessed. The humoral and cellular immune responses were measured by enzyme-linked immunosorbent assay in 12 groups (individual and simultaneous injections of rOMP25 and rOMP31 in different protein concentrations) and lymphocyte proliferation was measured using 3–4,5-dimethylthiazol-2-yl2,5-diphenyltetrazolium bromide (MTT) assay based on average Optical density (OD) of stimulated cells/average OD of unstimulated cells. Finally, data were analyzed using one-way analysis of variance. rOMP25 + rOMP31 group stimulated higher titer of INF-γ than other groups, whilst there were no statistically significant differences between all uni/divalent immunized groups. Tumor necrosis factor alpha titer showed no significant difference between divalent immunized groups (except rOMP31 + rOMP25(1) and rOMP31 + rOMP25(3)) and positive control group. Interleukin-4 analysis results demonstrated that there were no significant differences between positive control and uni/divalent vaccinated groups. In addition, analysis of antibody responses revealed rOMP25 + rOMP31, rOMP25 + rOMP31(2), and rOMP25 + rOMP31(3) groups induced higher level of total antibody compared to other immunized groups, although both univalent and divalent immunized mice induced higher IgG2a titer than IgG1 with the mean of IgG2a/IgG1 ratio ~1.01 indicating strong bent of Th1 immune response. The cell proliferation assay demonstrated the vaccination with rOMP25 + rOMP3, rOMP25 + rOMP31(2), and rOMP25 + rOMP31(3) elicited vigorous antigen-specific cell proliferative. rOMP25 + rOMP3, rOMP25 + rOMP31(2), and rOMP25 + rOMP31(3) treatments could be used as potential candidates for developing new subunit vaccines.
KEYWORDS:
1. Introduction
Brucellosis is one of the important zoonotic diseases causing the death of 500,000 people around the world [Citation1,Citation2]. Brucella melitensis is the most frequent pathogenic Brucella species infecting both animal and human [Citation3,Citation4]. This disease is usually characterized by abortion and reduced fertility in animal and by undulant fever and arthritis in human [Citation5,Citation6].
At present, there is no safe and protective human vaccine against brucellosis, and the only preventive strategy is animal vaccination using the live attenuated vaccines which harbors significant disadvantages such as abortion in immunized pregnant animals and interfere in the serological tests [Citation7,Citation8]. Therefore, subunit vaccines conferring protection against brucellosis are being developed [Citation9]. The Brucella OMP25 and OMP31 are cell-specific surface antigens having remarkable immunogenicity characteristics [Citation10,Citation11]. OMP25 is one of the virulent factors and highly conserved antigens among different Brucella species that play an important role in survival of Brucella [Citation10]. Several studies have shown that OMP25 and OMP31 could be used as vaccine candidates to confer protection against B. melitensis infection by eliciting Th1 response [Citation12,Citation13]. In the current study, the immune responses stimulated by various concentrations of OMP25 and OMP31 recombinant proteins formulated in nanoparticle were assessed, to find whether the simultaneous injections of OMP25 and OMP31 recombinant proteins induce suitable immunity rather than individual ones and which protein concentrations could be more efficient as well.
2. Methods
2.1. Experimental groups and immunization
Six-week-old female BALB/c mice (obtained from Razi Vaccine and Serum Research Institute, Iran, and kept according to institutional policies for animal health and welfare) were randomly classified into 12 experimental groups (5 mice/group) and immunized intraperitoneally three times (days 0, 15, and 30) by univalent (rOMP25 or rOMP31) and divalent (rOMP25 and rOMP31) vaccines (the rOMP25 and rOMP31 recombinant proteins were produced as described previously contain different dosages of proteins) () [Citation14,Citation15]. The dose of first protein in divalent vaccines was constant and equal to the dose of univalent injections in each injection time, but the dose of second recombinant protein in each strategy was variable (). To remove any interference effect, negative control groups were injected by phosphate buffered saline (PBS) and PBS contained self-expressed pET-32a(+) vector (). A dose of live attenuated vaccine B. melitensis Rev1 (106 CFU/mice) was injected as positive control (). A volume of 50 μl of CS-NPs (Sigma, USA) was added to each treatment.
Table 1. Vaccination schedule of mice using different immunization schemes.
2.2. Immunogenicity assessment
Antibody responses, cytokines determination, and lymphocyte proliferation assay were performed as described previously [Citation16]. Briefly, the mice were slaughtered 2 weeks after lasted injection for serum collection by centrifugation at 3000 g for 20 min and the supernatant was stored at −80°C. The purified rOMP25, rOMP31, and rOMP25 + rOMP31 (1 µg/ml) proteins were coated in 96-well plates (Nunc, Naperville, IL) and incubated for 24 h at 37°C. Wells were washed with PBS containing 0.05% Tween 20 (TPBS) and blocked for 1 h at 37°C with 5% skimmed milk in PBS. Plates were incubated with serial dilutions of mouse sera (1/100–1/10,000) for 2 h at room temperature and washed three times. Subsequently, they were incubated with 100 µl of 1/10,000 dilution of anti-mouse IgG–horseradish peroxidase (HRP) conjugate antibody (Sigma, USA) to determine humoral response using indirect enzyme-linked immunosorbent assay (ELISA). Moreover, IgG isotyping was performed under the same condition using 100 µl of 1/4000 dilution of goat anti-mouse IgG1–HRP and IgG2a–HRP conjugated antibodies (Sigma, USA). The results for IgG and its isotypes were represented as the mean of triplicates ± SE of the OD405 nm from five samples. Cytokines determination was demonstrated by homogenizing of mice spleens with 10 ml PBS containing 5 mM ethylenediamine-tetraacetic acid (PBS-EDTA) on ice. The cells were washed twice by PBS-EDTA and mononuclear cells were isolated followed by culturing in RPMI 1640 (supplemented with 4 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% heat inactivated fetal bovine serum [FBS]). Splenocytes were then counted and a total number of 4 × 106 cells were seeded in a 24-well plate and stimulated in vitro with 10 μg/ml of recombinant proteins for 48 h at 37°C in 5% CO2. Cell culture supernatants were collected at the end of the incubation and centrifuged at 300 g for 10 min. Interferon gamma (INF-γ), tumor necrosis factor alpha (TNF-α), and interleukin-4 (IL-4) levels were measured by a sandwich ELISA according to the manufacturer’s instructions (Mabtech, Nacka, Sweden) [Citation16]. To lymphocyte proliferation assay, spleens were dissected from the mice and suspended in sterile cold PBS containing 2% FBS. Red blood cells were lysed with lysis buffer and the single-cell suspension was adjusted to 3 × 106 cells/ml and dispensed into a 96-well plate in triplicate and incubated with 10 µg/ml of the recombinant proteins for 48 h. Incubation was continued by 20 µl 3–4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide (MTT) (Sigma), followed by 100 µl of dimethyl sulfoxide (Sigma). Absorbance was measured using a spectrophotometric plate reader at 590 nm [Citation16].
One-way analysis of variance, followed by Tukey’s post hoc test, was used for data analysis. Differences with p < 0.05 were considered statistically significant [Citation16].
3. Results
3.1. Both univalent and divalent immunization-induced humoral responses
Total antibody results showed immunized groups with recombinant proteins induced higher and lower level of antibody titer compared to the negative and positive control groups, respectively (from 1/100 to 1/1000; ). As represented in , most divalent immunized groups particularly rOMP25 + rOMP31, rOMP25 + rOMP31(2), and rOMP25 + rOMP31(3) showed higher total antibody titer than univalent groups (from 1/100 to 1/1000 IgG titer), while rOMP25 + rOMP31 injection induced the highest titer of IgG among all immunized groups with recombinant protein ().
Figure 1. Kinetics production of antibody. (a) Total antibody titer for each treatment in different concentrations. (b) IgG1 and IgG2a responses in immunized mice. (c) The ratio of IgG2a to IgG1 in immunized mice. Levels of each antibody were measured at OD405 nm with an ELISA reader. Each value represents the mean of triplicates ± SD of antibody responses from five samples. Different letters indicate statistically significant differences between experimental groups (p < 0.05). PBS and PBS-pET-32a(+) refer to negative control groups. Live attenuated vaccine B. melitensis Rev1 refers to positive control group.
IgG1 and IgG2a are considered as Th2 and Th1 response markers, respectively. Results illustrated both univalent and divalent immunization elicited high levels of IgG1 as well as IgG2a antibodies in comparison to negative control groups (p < 0.05, ). Generally, IgG1 isotype results showed that there were no statistically significant differences between univalent and divalent immunized groups. Also, there was no statistically significant difference between positive control group and other experimental groups, except rOMP25, rOMP25 + rOMP31, and rOMP25 + rOMP31(2) (). Furthermore, the titer of IgG2a antibody in both univalent and divalent immunization groups was statistically similar, although rOMP25 + rOMP31(1) immunized group with the highest amount of IgG2a titer among all univalent and divalent injections showed no significant difference compared to positive control (). In addition, the antibody titers demonstrated skew from IgG1 to IgG2a (IgG2a:IgG1 ratio 1.01) in both univalent and divalent immunized groups, in case the rOMP25 + rOMP31(1) shows the highest switch from IgG1 to IgG2a compared to all injected groups with recombinant protein.
3.2. Divalent immunization induced mixed Th1–Th2 cytokines response
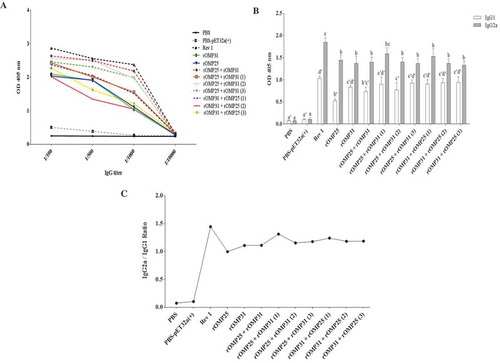
Cellular immune response results revealed no significant difference between univalent and divalent injections in all three cytokine types (). While INF-γ cytokine response was significantly higher in positive control group than other groups (p < 0.05), three treatments (rOMP25 + rOMP31(2), rOMP31 + rOMP25(2), and rOMP31 + rOMP25(3)) had no difference with negative groups. Moreover, rOMP25 + rOMP31, rOMP25 + rOMP31(3), and rOMP31 + rOMP25(1) groups showed relatively higher titer of INF-γ cytokine among all univalent and divalent injections (). TNF-α analysis revealed that although there were no statistically significant differences between both univalent and divalent treatments, lower TNF-α titer was allocated to rOMP31 + rOMP25(1) and rOMP31 + rOMP25(3) which had no significant difference with negative control groups (). In addition, positive control group induced statistically similar TNF-α cytokine titer compared to rOMP25 + rOMP31, rOMP25 + rOMP31(1), rOMP25 + rOMP31(2), rOMP25 + rOMP31(3), and rOMP31 + rOMP25(2) treatments which induced higher titer of TNF-α among all univalent and divalent injections (). IL-4 cytokine was assessed and results indicated that all immunized mice showed significantly higher titer than negative control groups (p < 0.05), while all those groups showed significantly similar IL-4 titer with positive control (). These results suggested that all immunized groups induced the same type of Th response; however, some of the divalent injections induced higher Th1–Th2 cytokine responses than univalent ones.
Figure 2. Determination of cytokine responses in spleen cells from immunized mice. (a) INF-γ response from immunized mice with different doses. (b) TNF-α response from immunized mice with different doses. (c) IL-4 response from immunized mice with different doses. Levels of each cytokine were quantified (pg/ml) by ELISA at OD405 nm. Each value represents the mean of triplicates ± SD of antibody responses from five samples. Different letters indicate statistically significant difference between experimental groups (p < 0.05). PBS and PBS-pET-32a(+) refer to negative control groups. Live attenuated vaccine B. melitensis Rev1 refers to positive control group.
3.3. Lymphocyte proliferation
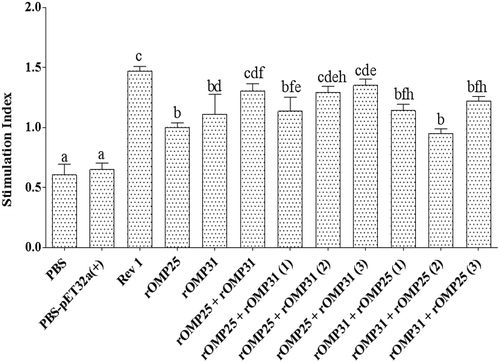
The results showed that all injected groups had significantly higher lymphocyte proliferation than negative control groups (p < 0.05, ). Generally, there were no statistically significant differences between divalent and univalent injections (). However, rOMP25 + rOMP31, rOMP25 + rOMP31(2), and rOMP25 + rOMP31(3) injections not only stimulated higher lymphocyte proliferation among all recombinant treatments but also they had no significant differences compared to positive control group ().
Figure 3. Lymphocyte proliferation responses of the experimental groups after in vitro antigen recall (average OD of stimulated cells/average OD of unstimulated cells). The stimulation indexes of the experimental groups are shown as mean of triplicates ± SD from five samples. Different letters indicate statistically significant difference between experimental groups (p < 0.05). PBS and PBS-pET-32a(+) refer to negative control groups. Live attenuated vaccine B. melitensis Rev1 refers to positive control group.
4. Discussion
Nowadays, brucellosis vaccines based on attenuated strains have been used in animal for prevention. Although these vaccines have reduced virulence in animals, they are usually pathogenic for humans [Citation17,Citation18]. In this regard, several studies have facilitated the identification of immunogenic proteins in Brucella, but only few antigens have shown significant protective activities in vivo such as L7/L12, Omp16, TF, Omp31, and P39 [Citation7,Citation19–Citation21]. Also, some recent studies demonstrated that divalent vaccines induced better immune responses and protection than univalent vaccines [Citation1,Citation3,Citation22]. Therefore, based on previous studies, we hypothesized that recombinant OMP25 and OMP31 proteins together might be efficient candidates for subunit vaccines.
Results showed that some divalent vaccines induced higher titer of lymphocyte proliferation than univalent groups. This consequence was confirmed by cytokine response results as some protein concentrations of divalent vaccines induced higher titer of INF-γ, TNF-α, and IL-4 than univalent immunized groups. INF-γ and TNF-α are two important components of Th1 immune response which play a key role in disease control, followed by activating macrophages and changing antibody responses toward IgG2a [Citation11,Citation20]. Moreover, the humoral immune response showed the higher titers of IgG2a over IgG1 indicate the strong Th1 bent of immune response. IgG2a isotype is important because binding of antibody’s Fc to its receptor on the surface of phagocytes stimulates a wide range of antimicrobial responses. Totally, results represented constant concentration of rOMP25 (20 µg) in combination with increasing concentrations of rOMP31 (20, 30, 40 µg) as variable recombinant protein inducing higher immune responses, as rOMP25 + rOMP31 and rOMP25 + rOMP31(3) injections exposed higher performance than other formulated vaccines.
These results are in agreement with some other reports that have shown efficient immune responses against Brucella associated with high levels of Th1 cytokines and IgG2a [Citation7,Citation13]. Luo et al. [Citation20] and Luo et al. [Citation23] showed divalent genetic vaccines elicit stronger cellular immune response and better protection against Brucella abortus than univalent vaccines. Also, Tadepalli et al. [Citation1] studied immunogenicity efficacy of rOmp19, rP39, and rOmp19 + rP39 injections. They found rOmp19 + rP39 immunized mice induced significantly higher proliferative response with considerable cytokines expression, higher IgG2a antibody titer than univalent injected groups, as well. In another study, the simultaneous injection of HSP60 and chimeric BLS-OMP25 antigen showed better immunity than univalent injections of each recombinant protein [Citation20]. However, Abbassi-Daloii et al. [Citation24] found that the simultaneous injection of GroEL with OMP31 and OMP25 did not improve immune responses rather than individual injections of OMP31 and OMP25.
5. Conclusion
Although no statistically significant difference was observed between univalent and divalent injections, rOMP25 + rOMP3, rOMP25 + rOMP31(2), and rOMP25 + rOMP31(3) formulated vaccines which showed better immune responses could be potential candidates for developing new subunit vaccines against B. melitensis Rev 1. However, further experiments include that protection efficiently and test on animal should be performed and it is ongoing in our lab.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Tooba Abbassi-Daloii
Tooba Abbassi-daloii is a PhD graduated in the field of Animal Molecular Genetics at Ferdowsi University of Mashhad and she is working in the field of Bioinformatics at Leiden University Medical center in the Netherlands.
Soheil Yousefi
Soheil Yousefi is a PhD graduated in the filed of Animal Molecular Genetics at Ferdowsi University of Mashhad and he is working in the filed of Bioinformatics at Erasmus Medical Center in the Netherlands.
Mojtaba Tahmoorespur
Mojtaba Tahmoorespur is a professor in the filed of Animal sciences and biotechnology at Ferdowsi University of Mashhad. He is specialist in recombination protein, molecular genetics and population genetics.
Mohammad Hadi Sekhavati
Mohammad Hadi Sekhavati is a Assistant professor in the filed of Animal sciences and biotechnology at Ferdowsi University of Mashhad. He is specialist in recombination protein, molecular genetics, antibody study and structural protein.
References
- Tadepalli G, Singh AK, Balakrishna K, et al. Immunogenicity and protective efficacy of Brucella abortus recombinant protein cocktail (rOmp19+rP39) against B. abortus 544 and B. melitensis 16M infection in murine model. Mol Immunol. 2016;71:34–41.
- Singh D, Goel D, Bhatnagar R. Recombinant L7/L12 protein entrapping PLGA (poly lactide-co-glycolide) micro particles protect BALB/c mice against the virulent B. abortus 544 infection. Vaccine. 2015;33(24):2786–2792.
- Golshani M, Rafati S, Dashti A, et al. Vaccination with recombinant L7/L12-truncated Omp31 protein induces protection against Brucella infection in BALB/c mice. Mol Immunol. 2015;65(2):287–292.
- Franco MP, Mulder M, Gilman RH, et al. Human brucellosis. Lancet Infect Dis. 2007;7(12):775–786.
- Pappas G. The changing Brucella ecology: novel reservoirs, new threats. Int J Antimicrob Agents. 2010;36(Suppl 1):S8–11.
- Pappas G, Papadimitriou P, Christou L, et al. Future trends in human brucellosis treatment. Expert Opin Investig Drugs. 2006;15(10):1141–1149.
- Cassataro J, Velikovsky CA, de la Barrera S, et al. A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect Immun. 2005;73(10):6537–6546.
- Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol. 2010;140(3–4):392–398.
- Ghasemi A, Jeddi-Tehrani M, Mautner J, et al. Immunization of mice with a novel recombinant molecular chaperon confers protection against Brucella melitensis infection. Vaccine. 2014;32(49):6659–6666.
- Edmonds MD, Cloeckaert A, Elzer PH. Brucella species lacking the major outer membrane protein Omp25 are attenuated in mice and protect against Brucella melitensis and Brucella ovis. Vet Microbiol. 2002;88(3):205–221.
- Gupta VK, Radhakrishnan G, Harms J, et al. Invasive Escherichia coli vaccines expressing Brucella melitensis outer membrane proteins 31 or 16 or periplasmic protein BP26 confer protection in mice challenged with B. melitensis. Vaccine. 2012;30(27):4017–4022.
- Bowden RA, Cloeckaert A, Zygmunt MS, et al. Evaluation of immunogenicity and protective activity in BALB/c mice of the 25-kDa major outer-membrane protein of Brucella melitensis (Omp25) expressed in Escherichia coli. J Med Microbiol. 1998;47(1):39–48.
- Commander NJ, Spencer SA, Wren BW, et al. The identification of two protective DNA vaccines from a panel of five plasmid constructs encoding Brucella melitensis 16M genes. Vaccine. 2007;25(1):43–54.
- Yousefi S, Sekhavati MH, Tahmoorespur M, et al. Cloning and molecular characterization of Omp31 gene from Brucella melitensis Rev 1 strain. Arch Razi Inst. 2016;71(2):117–124.
- Yousefi S, Tahmoorespur M, Sekhavati MH. Cloning, expression and molecular analysis of Iranian Brucella melitensis Omp25 gene for designing a subunit vaccine. Res Pharm Sci. 2016;11(5):412–418.
- Yousefi S, Abbassi-Daloii T, Sekhavati MH, et al. Evaluation of immune responses induced by polymeric OMP25-BLS Brucella antigen. Microb Pathog. 2018;115:50–56.
- Nicoletti P. Vaccination against Brucella. Adv Biotechnol Processes. 1990;13:147–168.
- Schurig GG, Sriranganathan N, Corbel MJ. Brucellosis vaccines: past, present and future. Vet Microbiol. 2002;90(1–4):479–496.
- Al-Mariri A, Tibor A, Mertens P, et al. Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxynucleotides as adjuvant. Infect Immun. 2001;69(8):4816–4822.
- Luo D, Ni B, Li P, et al. Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp16 genes of Brucella abortus in BALB/c mice. Infect Immun. 2006;74(5):2734–2741.
- Pasquevich KA, Estein SM, Garcia Samartino C, et al. Immunization with recombinant Brucella species outer membrane protein Omp16 or Omp19 in adjuvant induces specific CD4+ and CD8+ T cells as well as systemic and oral protection against Brucella abortus infection. Infect Immun. 2009;77(1):436–445.
- Yang X, Walters N, Robison A, et al. Nasal immunization with recombinant Brucella melitensis bp26 and trigger factor with cholera toxin reduces B. melitensis colonization. Vaccine. 2007;25(12):2261–2268.
- Luo DY, Li P, Xing L, et al. DNA vaccine encoding L7/L12-P39 of Brucella abortus induces protective immunity in BALB/c mice. Chin Med J (Engl). 2006;119(4):331–334.
- Abbassi-Daloii T, Yousefi S, Sekhavati MH, et al. Impact of heat shock protein 60KD in combination with outer membrane proteins on immune response against Brucella melitensis. Apmis. 2018;126(1):65–75.
