ABSTRACT
Introduction: Increasing use of extracorporeal membrane oxygenation (ECMO) for acute respiratory failure may increase resource requirements and hospital costs. Prediction of successful weaning in these patients may improve resource use and patients outcome. The Respiratory ECMO Survival Prediction (RESP) score has been proposed as an outcome prediction tool for patients undergoing venovenous (VV-ECMO). However, it was developed and validated on patients established on ECMO. This may limit its usefulness as an adjunct tool for decision-making process at the pre-ECMO stage.
Aim: The aim of the work was to assess the efficacy of RESP score as a tool to predict successful weaning in patients treated with VV-ECMO before initiation of treatment.
Patients and methods: The study was carried out on 23 adult patients who were admitted to the units of Critical Care Medicine Departments in Egyptian Armed Forces Hospitals within 1 year and were treated with VV-ECMO; all of them received the same treatment as recommended by ELSO guidelines for adult respiratory failure. They were classified into two groups according to ECMO weaning successfulness at the end of the study: group I (successful weaning) and group II (failed weaning). Complete physical assessment, laboratory investigations, and RESP score calculation were done before ECMO initiation.
Results: Pre-ECMO RESP score, in group I it ranged from −8 to 7 (mean 1.75 ± 3.65), while in group II it ranged from −11 to 1 (mean −6.38 ± 1.88), there was statistically significant difference between the two groups (p = 0. 003). The ROC curve of RESP score showed an AUC of 0.880 (95% CI 0.658–0.981) (p < 0.001). The best cutoff value was −1, at that level the sensitivity was 69.7%, specificity was 81.5%. Calculated positive predictive value of RESP score was 88.9% while negative predictive value was 63.6%.
Conclusion: RESP score may be effective tool to predict ECMO weaning successfulness before initiation of ECMO.
1. Introduction
Extracorporeal membrane oxygenation (ECMO), which is also named Extracorporeal life support (ECLS), is an evolution of the heart-lung machines used in cardiac surgeries. ECMO could be either venovenous (VV) or venoarterial (VA), it could be used for respiratory support, cardiac support, or both [Citation1].
ECMO is a bridge therapy, allowing either to healing of the natural organs or to long-term devices or transplantation. In fact, although ECMO has the capability to support cardiorespiratory function temporarily, it is not a cure for the underlying disease [Citation2].
The ECMO circuit for associate adult patient is typically very compact, with some flexibility for ICU patients transportation, mobilization, and general care. it always consists of an inflow cannula (drainage cannula), a circuit tubing which is made of polyvinyl chloride (PVC), a centrifugal pump, a polymethylpentene (PMP) membrane oxygenator, and a return cannula that transports arterialized blood (reemission cannula). Continuous renal replacement therapy could also be connected on the ECMO system [Citation3].
Two main types of ECMO are present which are described by the site of blood drainage and returns: VV-ECMO which provides only respiratory system support and VA-ECMO which provides cardiorespiratory system support [Citation4].
In VV-ECMO, blood from the patient is drained from the large central veins (via the “drainage cannula”) then it passed through an oxygenator and finally it returned to the venous system near the right atrium (via the “reemission cannula”). It provides a support for severe respiratory failure when there is no major cardiac impairment. It reduces the amount of blood that passes through the lung without being oxygenated and it removes CO2 from the patient’s blood. Allowing high ventilatory pressures to be reduced, this protects against ventilator-induced lung injury [Citation5].
The efficacy of the ECMO oxygenation depends on the pump flow relative to the patient’s cardiac output. Oxygenation should increase with increasing ECMO flow rate, if this does not occur, reasons should urgently investigated such as recirculation of blood between the inflow and outflow cannulae [Citation6].
VV-ECMO cannulation could be done using either two large bore cannulae or single double lumen cannula. If two cannulae are used, drainage cannula is inserted in the inferior (IVC) and superior (SVC) vena cava, usually through the femoral and internal jugular veins. Double lumen cannulas require only a single insertion site. In both techniques, cannulae are placed so that outflow ports are located in the inferior vena cava preferably near its intrahepatic portion, while the inflow port are located within the RA and is directed toward the tricuspid valve [Citation7].
Indications to VV-ECMO were prescribed by Extracorporeal Life Support Organization (ELSO), VV-ECMO may be considered in hypoxic respiratory failure due to any cause (primary or secondary) when the risk of mortality is 50% or greater when hypoxic index is lower than 150 on FiO2 more than 90% and/or Murray score 2–3. And is indicated when the risk of mortality is 80% or greater with a hypoxic index is lower than 100 on FiO2 more than 90% and/or Murray score 3–4 for 6 h or more despite of maximal conventional treatment [Citation8].
There are no absolute contraindications to ECMO, individually evaluated risks and benefits for each patient should be considered. However, conditions that are associated with a poor outcome despite ECMO treatment can be considered relative contraindications such as: mechanical ventilation at high settings for 7 days or more, recent or expanding CNS hemorrhage, nonrecoverable comorbidity such as major CNS damage or terminal malignancy, limited vascular access and any condition associated with contraindication for the use of anticoagulation [Citation8].
After initiation of VV-ECMO treatment, efforts are directed toward improving pulmonary function including diuresis to keep negative fluid balance, antibiotic therapy, bronchodilators, and bronchoscopy. Ventilator-associated lung injury should be prevented by keeping the rest settings. FiO2 is limited to 30%, PEEP to preserve recruitment, and limiting tidal volumes with low levels of support [Citation9].
Signs of improved pulmonary functions can be noticed while the patient is still on full ECMO support and resting ventilator settings is still unchanged. Increasing PaO2 or decreasing PaCO2 might be noticed. Increased levels of CO2 in capnometry also give a clue to improving alveolar gas exchange. Increasing tidal volumes despite unchanged pressure settings on ventilator reflects improved pulmonary compliance [Citation10].
As lung compliance is more than 20 cc/cmH2O, so adequate lung recovery is usually achieved and patient is usually ready to proceed to weaning. This is usually accompanied with radiological improvement in pulmonary aeration.
Once significant aeration is achieved, Cilley test should be performed on a daily basis. The test is done by increasing the ventilator FiO2 to 100%, with no other changes in ventilator settings. A positive test is marked by a rapid increase in oxygen saturation within a couple of minutes, this significant oxygen saturation increase was a result of native lung function and it is a marker for ECMO weaning [Citation11].
Although ARD on VV-ECMO are protected against hypoxia. However, ECMO therapy itself is a high-risk procedure and ECMO patients are in high risk of life-threatening complications such as bleeding complications or to infectious or noninfectious inflammation, leading to various degrees of organ dysfunction [Citation12].
In addition, these patients are vulnerable to significant long-term physical and neuropsychological impairments and still in high risk of mortality. In addition, the increased use of ECMO, with its associated needs for training expertise and resources, may also increase hospital costs. [Citation13]
Thus, in the modern era of ECMO, it is inevitable to define risk factors for death in these patients and to predict successful weaning prior to ECMO initiation, which will in turn allow institutions to appropriately allocate resources and estimate mortality outcomes. Predictive mortality scores have been recently proposed [Citation12].
Respiratory ECMO Survival Prediction (RESP) score is one of these scores which have been developed in 2014. It was validated as a tool to predict survival for patients receiving ECMO for respiratory failure after initiation of VV-ECMO after a study done by Schmidt et al., which was conducted on 2,355 adult patients with severe acute respiratory failure treated by ECMO from 2000 to 2012 who were extracted from the ELSO international registry [Citation11].
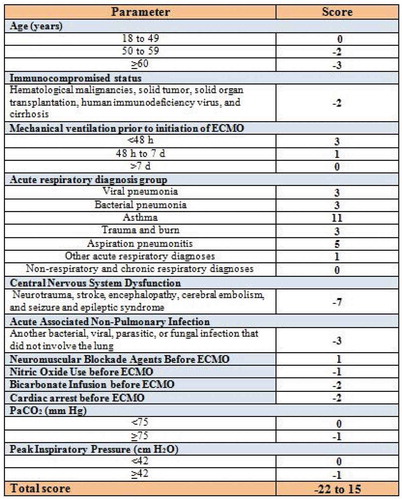
RESP score which does not include laboratory data consists of 12 items including: age, immunocompromised status, duration of mechanical ventilation before initiation of VV-ECMO, diagnosis and cause of acute respiratory failure, central nervous system dysfunction, acute associated nonpulmonary infection, neuromuscular blockade agents before ECMO, nitric oxide use before ECMO, bicarbonate infusion before ECMO, cardiac arrest before ECMO, the partial pressure of carbon dioxide (PaCO2), and peak inspiratory pressure (PIP). RESP score total score ranged from −22 to 15, and showed good prediction of survival for patient treated with VV-ECMO [Citation11].
RESP score classifies patients based on its pretherapeutic evaluation items into three groups. Higher RESP score is associated with better outcome for VV-ECMO patients. Higher RESP score calculation is associated with better survival. RESP score which is equal to or higher than 6 is associated with the best outcome with survival rate reaching 92% ().
2. Aim of the work
The aim of the work was to assess the efficacy of RESP score as a tool to predict successful weaning in patients treated with VV-ECMO before initiation of treatment.
3. Patients and methods
The study was carried out on 23 adult patients of both sexes; who were admitted to the units of Critical Care Medicine Departments in Egyptian Armed Forces Hospitals within 1 year and were treated with VV-ECMO. Approval of the medical ethics committee of Alexandria Faculty of Medicine, and an informed consent from next of kin were taken before conducting the study.
All of them received the same treatment as recommended by ELSO guidelines for adult respiratory failure.
Inclusion criteria:
(1) Adult patients above 18 years old
(2) Severe ARDS by the Berlin criteria and at least one of the following criteria:
● PaO2/FIO2 < 80 for ≥ 3 h despite VT of 6 mL/kg and PEEP of ≥ 5 cmH2O,
● pH < 7.25 for ≥ 3 h,
● pH < 7.20, PaCO2 > 80 mmHg,
● Static lung compliance < 0.5 mL cmH2O−1,
● PIP > 40 cmH2O with VT ≤ 6 mL kg−1,
● Oxygenation index (OI) = (MAP × FIO2 × 100)/PaO2 > 60 mmHg for 30 min or > 35 mmHg for 6 h,
● Murray lung injury score > 3.0.
Exclusion criteria:
(1) Pregnant females.
(2) Relative contraindication to VV-ECMO according to ELSO guidelines: mechanical ventilation at high settings (FiO2 > 0.9, P-plat > 30) for 7 days or more, major immunosuppression (absolute neutrophil count < 400/mm3), CNS hemorrhage that is recent or expanding, nonrecoverable comorbidity such as major CNS damage or terminal malignancy.
Patients were classified into two groups according to ECMO weaning successfulness; complete physical assessment, laboratory investigations and RESP score calculation were done before ECMO initiation.
4. Results
The 23 patients were classified into two groups according to successfulness of weaning from VV-ECMO:
Group I (Successful weaning): included 15 patients (65.2%) who were successfully weaned after VV-ECMO treatment.
Group II (Failed weaning): included eight patients (34.8%) who failed to be weaned from VV-ECMO treatment.
Demographic data characteristics of the study population are shown in . As regarding age, in group I it ranged from 20 to 59 years (mean 43.27 ± 10.45 years), while in group II it ranged from 35 to 57 years (mean 49.00 ± 7.03 years), there was no statistically significant difference between two groups (p = 0.180). As regarding sex, group I included 11 male (73.3%) and 4 female (26.7%), while group II included 4 male (50%) and 4 female (50%). There was no statistical significant difference between two groups as regarding the sex (p = 0.284)
Table 1. Demographic characteristics of the study population
As regarding comorbidities (), in group I, three patients were diabetics (20%), two patients were hypertensive (13.3%), eight patients were smokers (53.3%), one patient had chronic lung disease (6.6%), one patient had ischemic heart disease (6.6%), and one patient had chronic kidney disease (6.6%). While in group II, four patients were diabetics (50%), three patients were hypertensive (37.5%), four patients were smokers (50%), three patients had chronic lung disease (37.5%), two patients had ischemic heart disease (25%), and four patients had chronic kidney disease (50%). CKD is the only comorbidity that had a significant difference between two groups (p = 0.015). Otherwise DM, HTN, CLD, IHD, and smoking did not show statistically significant differences between two groups (p = 0.149, 0.197, 0068, 0.232, 0.886, respectively).
Table 2. Past medical history of the study population
As regarding pre-ECMO vital signs (), only mean arterial blood pressure showed significant difference between two groups (p = 0.032), in group I it ranged from 60 to 96.6 mmHg (mean 81.08 ± 7.18), while in group II it ranged from 43.3 to 93 mmHg (mean 66.60 ± 7.72). Other vital signs including heart rate, temperature, and respiratory rate did not show a significant difference between two groups (p = 0.149, 0.197, 0068, 0.232, 0.886, respectively).
Table 3. Vital signs of study population
Pre-ECMO laboratory results are presented in . As regarding complete blood picture only hematocrit was significantly different between two groups (p = 0.010) as it was 31.50–42.90% (mean 38.34 ± 1.97) in group I, while it was 30.58–39.62% (34.40 ± 1.34) in group II. Neither WBCs nor platelet count showed a significant difference between two groups (p = 0.315, 0.795, respectively).
Table 4. Pre-ECMO laboratory results
As regarding serum creatinine, it showed a significant difference between two groups (p = 0.010) as it was 0.70–2.10 mg/dL (mean 1.14 ± 0.24) in group I, while it was 0.70–3.40 mg/dL (mean 2.21 ± 0.40) in group II.
As regarding serum lactate, in group I it ranged 1.20–3.10 mmol/L (mean 1.94 ± 0.33), while in group II it ranged 1.80–3.80 mmol/L (mean 2.71 ± 0.32), there was a statistically significant difference between two groups (p = 0.11)
Otherwise, there was no statistically significant difference between two groups. As regards serum Na+ (p = 0.828), serum K+ (p = 0.425), bilirubin (p = 0.0.172), ALT (p = 0.546), AST (p = 0.638), PTT (p = 0.418), and INR (p = 0.625).
Pre-ECMO arterial blood gases and ventilator parameters are shown in . Except for HCO3 (p = 0.117), all arterial blood gases parameters showed significant difference between two groups. All ventilator parameters showed a statistically significant difference between two groups.
Table 5. Pre-ECMO arterial blood gases and ventilator parameters
Mechanical ventilation duration before ECMO showed a significant difference between two groups (p = 0.004), in group I it ranged from 20 to 96 h (mean 46.00 ± 27.21), while in group II it ranged from 4 to 144 h (mean 99.00 ± 45.69).
As regarding total pre-ECMO RESP score (), in group I it ranged from −8 to 7 (mean 1.75 ± 3.65), while in group II it ranged from −11 to 1 (mean −6.38 ± 1.88), there was statistically significant difference between the two groups as regarding total RESP score (p = 0.003).
Table 6. Pre-ECMO total RESP score
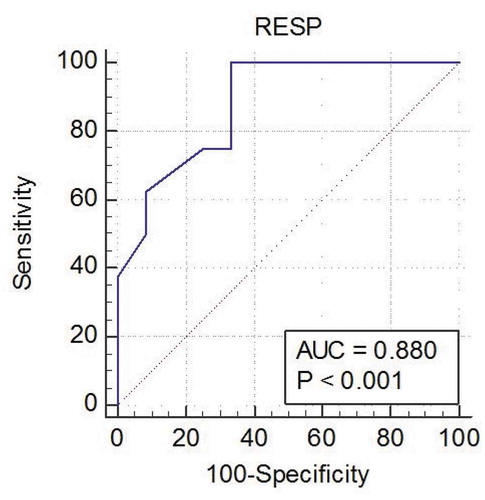
The ROC curve of RESP score (, ) showed an AUC of 0.880 (95% CI 0.658–0.981) (p < 0.001). The best cutoff value was −1, at that level the sensitivity was 69.7%, specificity was 81.5%. Calculated positive predictive value of RESP score was 88.9%, while negative predictive value was 63.6%.
Table 7. ROC curve analysis for RESP score in predicting successful ECMO weaning
As regarding ECMO duration and its effect on outcome (), ECMO duration showed a statistically significant difference between the two groups (p = 0.021), in group I it ranged from 72 to 192 h (mean 120.17 ± 23.18), while in group II it ranged from 96 to 216 h (mean 120.00 ± 19.69).
Table 8. Relation between ECMO duration and outcome
5. Discussion
Our study was carried out on 23 adult patients of both sex; admitted to the units of Critical Care Medicine Departments in Egyptian Armed Forces Hospitals within 1 year and were treated with VV-ECMO, all of them received the same treatment as recommended by the ELSO [Citation14]. They were classified into two groups according to successfulness of ECMO weaning: group I (successful weaning) and group II (failed weaning).
Our results showed that the group I included 15 patients (65.2%), while group II included eight patients (34.8%). This is in concordance with the ECLS registry international summary report which reported a worldwide successful weaning from adult respiratory ECLS mortality of 69% [Citation15].
The age of our patients ranged from 20 to 59 years. Patients in group II were older than patients in group I (49.00 ± 7.03 vs. 43.27 ± 10.45 years). Although age is a major predictor of outcome in VV-ECMO patients as reported by Schmidt et al. however, age difference between two groups was not significant (p = 0.180), this is can be explained that our patient were subjected to each hospital exclusion and inclusion criteria to initiate VV-ECMO treatment which favors young age of the treated patients and may exclude patient for their old age [Citation16].
As regarding sex, males represented 65.2% (15 patients) while females represented 34.8% (eight patients), this result agrees with most of epidemiological studies which show a greater incidence of acute respiratory distress syndrome in male sex, ranging from 52% to 66% [Citation17]. One potential explanation is that the rate of alveolar fluid clearance is faster in women with acute lung injury compared with men, which might lead to more rapid resolution of pulmonary edema [Citation18]. In addition, cigarette smoking has recently been shown to be a risk factor for ARDS [Citation19] and men are more likely to smoke than women. However, there was no statistical significant difference as regarding the outcome and sex (p = 0.284).
The preexisting comorbidities in our study included diabetes mellitus, hypertension, chronic lung disease, ischemic heart disease, smoking, and chronic kidney disease. The most common comorbidities were smoking and diabetes mellitus (52.1%, 30.4%, respectively). This is in concordance with study done by Calfee et al. where smoking was associated with increased risk of acute respiratory distress syndrome [Citation19]. There was no statistical significant difference between the two groups as regarding each comorbidity except for chronic kidney disease (p = 0.015). This is in concordance with study done by Rhee et al., which demonstrated that chronic kidney disease is associated with poor outcome during ECMO [Citation20].
There was significant difference between the number of comorbidities and the outcome (p = 0.002). This result agrees with study by Chang et al that showed that there is a positive correlation between the number of comorbidities and mortality in patients treated with ECMO [Citation21].
As regarding vital signs only mean arterial blood pressure showed significant difference between two groups (p = 0.032), in group I it ranged from 60 to 96.6 mmHg (mean 81.08 ± 7.18), while in group II it ranged from 43.3 to 93 mmHg (mean 66.60 ± 7.72). This is in concordance with study done by Hilder et al. in which mean arterial blood pressure was significantly higher in ARDS patients who were successfully weaned from ECMO [Citation22].
As regarding laboratory result, only hematocrit, serum creatinine, and serum lactate showed a significant difference between the two groups. Hematocrit (%) in group I ranged from 31.50 to 42.90 (mean 38.34 ± 1.97), while in group II it ranged from 30.58% to 39.62% (mean 34.40 ± 1.34) (p = 0.010). Cheng et al. conducted an epidemiological study in which they reported that hematocrit was significantly higher in patients who survived after VV-ECMO treatment compared to nonsurvivors [Citation23].
As regarding serum creatinine, in group I it ranged from 0.70 to 2.10 mg/dL (mean 1.14 ± 0.24), while in group II it ranged from 0.70 to 3.40 mg/dL (mean 2.21 ± 0.40), there was statistically significant difference between the two groups (p = 0.010), this result is in agreement with studies by Kielstein et al.., that reported that serum creatinine on admission was significantly higher in nonsurvivor patients who underwent ECMO therapy compared to survivors (p = 0.001) [Citation24].
As regarding serum lactate, in group I it ranged 1.20–3.10 mmol/L (mean 1.94 ± 0.33), while in group II it ranged 1.80–3.80 mmol/L (mean 2.71 ± 0.32), there was a statistically significant difference between two groups (p = 0.11). Although there was significant difference between the two groups, serum lactate levels were not high. This could be explained by early initiation of ECMO therapy before patient general condition was deteriorated and before development of sepsis-induced multiorgan failure. Hilder et al., reported similar results; they report that serum lactate was significantly higher in ARDS patient who did not survived after ECMO treatment compared to survivors (p = 0.001) as it ranged from 1.3 to 3.4 mmol/L in survivors, while it ranged from 1.6 to 8.5 mmol/L in nonsurvivors [Citation19].
As regarding arterial blood gases and ventilatory management, arterial pH, in group I ranged from 7.17 to 7.34 (mean 7.27 ± 0.05), while it ranged from 7.14 to 7.30 (mean 7.21 ± 0.06) in group II, showing statistically significant difference between two groups (p = 0.016), Hilder et al. reported similar results; they found that arterial pH was lower in nonsurvivors than survivors (7.24 ± 0.13 vs. 7.17 ± 0.11) which was statistically significant (p = 0.005) [Citation22].
PaCO2 showed significant difference between two groups (p = 0.021), as it ranged from 44 to 74 mmHg in group I (mean 59.00 ± 9.45), while it ranged from 55 to 79 mmHg (mean 69.25 ± 7.83). In agreement with our results, Schmidt et al., conducted a study on 140 patients who were treated with ECMO for severe acute respiratory distress syndrome which showed significant difference between survivors and nonsurvivors as regarding PaCO2 (p = 0.02) [Citation25].
Hypoxic index (PaO2/FiO2) was significantly higher in group I (p = 0.023). In group I, it ranged from 51 to 79 mmHg (mean 70.67 ± 7.75), while in group II it ranged from 48 to 74 mmHg (mean 61.62 ± 8.36). This result is in concordance with results of Kao et al., who conducted a prospective observational cohort study reported in which they reported that hypoxic index was significantly higher in patient who survived after ECMO for treatment of acute respiratory distress syndrome (p = 0.005) [Citation26].
Peak inspiratory pressure (PIP) was significantly high in group II (p = 0.022). In group I, it ranged from 31 to 43 cmH2O (mean 36.42 ± 3.63), while in group II it ranged from 32 to 45 cmH2O (mean 40.75 ± 4.06). In agreement with our results, Wu et al. found that lower PIP is associated with significant improvement in VV-ECMO patients as they reported that downgrading the median of PIP from 35 to 29 cmH2O was associated with better outcome (p < 0.001) [Citation27].
As regarding lung compliance, it was significantly higher in group I (p = 0.044), as it ranged from 18 to 46 mL/cmH2O (mean 34.42 ± 9.47) in group I, while it ranged from 12 to 42 mL/cmH2O (mean 25.12 ± 9.31) in group II. In agreement of our study, Schmidt et al., reported that higher compliance is associated with better outcome (p = 0.04), as it ranged from 15 to 21 mL/cmH2O (median 19) in the survivors, while it ranged from 12 to 20 mL/cmH2O (median 16) among nonsurvivors [Citation25]. Brunet et al also concluded similar results, compliance among survivors was 28.9 ± 4.7 mL/cmH2O while among nonsurvivors was 21.5 ± 6.8 mL/cmH2O, which was a statistically significant difference (p = 0.02) [Citation28].
Duration of mechanical ventilation before ECMO was significantly shorter among group I. It ranged from 20 to 96 h (mean 46.00 ± 27.21) in group I, while it ranged from 24 to 144 h (mean 99.00 ± 45.69) in group II. Similarly, Liu et al reported that mechanical ventilation duration in sever ARDS patients treated with ECMO was significantly shorter in successfully weaned patients (3.87 ± 4.64 days) compared to failed weaning patients (8.94 ± 9.21 days) (p = 0.036). [Citation29] In addition, Schmidt et al found that shorter mechanical ventilation time prior to initiation of ECMO was significantly associated with better outcome (p = 0.017) [Citation16].
Pre-ECMO RESP score was higher in group I, and this difference was statistically significant (p = 0.003). In group I it ranged from −8 to 7 (mean 1.75 ± 3.65), while in group II it ranged from −11 to 1 (mean −6.38 ± 1.88). Similarly, Schmidt et al reported same results as the reported that the RESP score offers, through 12 simple pre-ECMO items, a relevant and validated tool to predict survival for patients receiving ECMO for respiratory failure [Citation16]. In contrast, Gillon reported that RESP score underestimates survival in those with lower scores. And they advised that RESP score cannot be used as a means of predicting survival in those patients being considered for ECMO [Citation30].
Our results showed that the ROC curve of RESP score showed an AUC of 0.880 (95% CI 0.658–0.981) (p < 0.001). The best cutoff value was −1, at that level the sensitivity was 69.7%, specificity was 81.5%. Calculated positive predictive value of RESP score was 88.9%, while negative predictive value was 63.6%. In agreement of our results, Klinzing et al reported that AUC of the PESP score to predict survival in VV-ECMO patients is 0.81 (95% CI 0.67–0.95), it was only statistically significant (p = 0.035) [Citation31]. Similarly, Huang et al reported that the RESP score showed excellent discriminate performance in predicting survival in patient treated with VV-ECMO with AUC of 0.835 (95% CI 0.659–1.010, p = 0.007) [Citation32]. In contrary, Gillon et al, who conducted a single-center study reported that RESP underestimates survival in those with lower scores. This supports that RESP score cannot be used as a means of predicting survival in those patients being considered for ECMO [Citation30].
As regarding ECMO duration and its effect on outcome, longer ECMO duration was significantly associated with higher incidence of failed weaning from ECMO (p = 0.021). ECMO duration in group I ranged from 72 to 192 h (mean 120.17 ± 23.18), while in group II it ranged from 96 to 216 h (mean 120.00 ± 19.69). This is in concordance with study done by Liu et al, who reported that longer ECMO duration is associated with poor outcome (p = 0.039) [Citation29]. On the other hand Huang et al reported longer ECMO duration was noted among nonsurvivors but it was not statistically significant (p = 0.867) [Citation32].
The current study had some limitations. First, patients included in the study were subjected to each hospital inclusion criteria for ECMO treatment which may select patients who are predicted to have better outcome. Second, the small sample size did not allow in-depth analysis of the relationships between the studied score and outcome. The small sample size is a general problem in a lot of ECMO studies in the literature and this may be due to high costs of ECMO therapy. So, further multicenter studies with unify inclusion/exclusion criteria and including of larger number of patients should be included in future studies to maximize the accuracy of the statistical analysis of the results and make strong recommendations along with other variables used (steroids/antimicrobial use/level of CKD, etc.) that might help putting the (RESP) SCOR is good predictors and help in identify whom will benefit from ECMO intervention.
However, the present study might have strengths. First, the prospective nature of the study, all the data and variables used in the analysis were from the patients’ charts and direct clinical measurements. Second, transfusion records of the patients before and during admission were available. Third, all data are measured initially when the patients were admitted to the ICU, so the measurement time was uniform.
6. Conclusion
RESP score may be effective tool to predict successful weaning for patients receiving VV-ECMO for severe adult respiratory distress syndrome before initiation of ECMO therapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Tayseer M. Zayton
Tayseer M. Zayton is a Professor of Critical Care, Faculty of Medicine, Alexandria University, Alexandria, Egypt.
Ehab M. El-Reweny
Ehab M. El-Reweny is an Assistant Professor of Critical Care, Faculty of Medicine, Alexandria University, Alexandria, Egypt.
Haitham M. Tammam
Haitham M. Tammam is an Assistant Professor of Critical Care, Faculty of Medicine, Alexandria University, Alexandria, Egypt.
Kareem M. Gharbeya
Kareem M. Gharbeya is a Critical care specialist, Alexandria Armed Forces Hospital, Alexandria, Egypt.
References
- Zapol WM, Kitz RJ. Buying time with artificial lungs. N Engl J Med. 1972;286(12):657–658.
- Schmidt GA. Extracorporeal life support for adults. New York: Humana Press; 2016.
- Abrams D, Brodie D, Combes A. What is new in extracorporeal membrane oxygenation for ARDS in adults? Intensive Care Med. 2013;39(11):2028–2030.
- Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7(7):E166–76.
- Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med. 2011;37(9):1447–1457.
- Gaffney AM, Wildhirt SM, Griffin MJ, et al. Extracorporeal life support. BMJ. 2010;341:c5317.
- Combes A, Bacchetta M, Brodie D, et al. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr Opin Crit Care. 2012;18(1):99–104.
- Munoz J. Extracorporeal membrane oxygenation (ECMO). Current indications and infectious complications. Med Clin (Barc). 2017;149(10):439–440.
- Lopez Sanchez M. Mechanical ventilation in patients subjected to extracorporeal membrane oxygenation (ECMO). Med Intensiva. 2017;41(8):491–496.
- Mauri T, Bellani G, Grasselli G, et al. Patient-ventilator interaction in ARDS patients with extremely low compliance undergoing ECMO: a novel approach based on diaphragm electrical activity. Intensive Care Med. 2013;39(2):282–291.
- Lee YJ, Kim DJ, Kim JS, et al. Experience and results with VV-ECMO for severe acute respiratory failure: weaning versus nonweaning. Asaio J. 2015;61(2):184–189.
- Brogan TV, Thiagarajan RR, Rycus PT, et al. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med. 2009;35(12):2105–2114.
- Pappalardo F, Pieri M, Greco T, et al. Predicting mortality risk in patients undergoing venovenous ECMO for ARDS due to influenza A (H1N1) pneumonia: the ECMOnet score. Intensive Care Med. 2013;39(2):275–281.
- Extracorporeal Life Support Organization (ELSO). ELSO guidelines for cardiopulmonary extracorporeal life support [Internet]. Extracorporeal Life Support Organization (ELSO). 2017. Available from: https://www.elso.org/Portals/0/ELSO%20Guidelines%20For%20Adult%20Respiratory%20Failure%201_4.pdf.
- ECLS Registry Report, Internationl Summary [Internet]. Extracorporeal Life support organization. 2019 [cited Jul 2019]. Available from: https://www.elso.org/Registry/Statistics/InternationalSummary.aspx
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189(11):1374–1382.
- Chen W, Chen YY, Tsai CF, et al. Incidence and outcomes of acute respiratory distress syndrome: A nationwide registry-based study in Taiwan, 1997 to 2011. Medicine (Baltimore). 2015;94(43):e1849.
- Bastarache JA, Ong T, Matthay MA, et al. Alveolar fluid clearance is faster in women with acute lung injury compared to men. J Crit Care. 2011;26(3):249–256.
- Calfee CS, Matthay MA, Kangelaris KN, et al. Cigarette smoke exposure and the acute respiratory distress syndrome. Crit Care Med. 2015;43(9):1790–1797.
- Rhee JH, Bailey K, Siddique A. Who will survive Extracorporeal Membrane Oxygenation (ECMO)? CHEST. 2016;150(4):310A.
- Chang CH, Chen HC, Caffrey JL, et al. Survival analysis after extracorporeal membrane oxygenation in critically ill adults: A nationwide cohort study. Circulation. 2016;133(24):2423–2433.
- Hilder M, Herbstreit F, Adamzik M, et al. Comparison of mortality prediction models in acute respiratory distress syndrome undergoing extracorporeal membrane oxygenation and development of a novel prediction score: the prediction of survival on ECMO therapy-score (PRESET-score). Crit Care. 2017;21(1):301.
- Cheng YT, Wu MY, Chang YS, et al. Developing a simple preinterventional score to predict hospital mortality in adult venovenous extracorporeal membrane oxygenation: A pilot study. Medicine (Baltimore). 2016;95(30):e4380.
- Kielstein JT, Heiden AM, Beutel G, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant. 2013;28(1):86–90.
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1704–1713.
- Kao KC, Hsieh MJ, Lin SW, et al. Survival predictors in elderly patients with acute respiratory distress syndrome: a prospective observational cohort study. Sci Rep. 2018;8(1):13459.
- Wu MY, Huang CC, Wu TI, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome in adults: prognostic factors for outcomes. Medicine (Baltimore). 2016;95(8):e2870.
- Brunet J, Valette X, Buklas D, et al. Predicting survival after extracorporeal membrane oxygenation for ARDS: an external validation of RESP and PRESERVE scores. Respir Care. 2017;62(7):912–919.
- Liu X, Xu Y, Zhang R, et al. Survival predictors for severe ARDS patients treated with extracorporeal membrane oxygenation: a retrospective study in China. PLoS One. 2016;11(6):e0158061.
- Gillon S, Shankar-Hari M, Jones A, et al. Evaluation of the RESP score in patients at point of referral to a severe respiratory failure service: a single centre study. 2015.
- Klinzing S, Wenger U, Steiger P, et al. External validation of scores proposed for estimation of survival probability of patients with severe adult respiratory distress syndrome undergoing extracorporeal membrane oxygenation therapy: a retrospective study. Crit Care. 2015;19:142.
- Huang L, Li T, Xu L, et al. Performance of multiple risk assessment tools to predict mortality for adult respiratory distress syndrome with extracorporeal membrane oxygenation therapy: an external validation study based on chinese single-center data. Chin Med J (Engl). 2016;129(14):1688–1695.