ABSTRACT
Background: Adipose tissue (AT) is a rich source of mesenchymal stem cells (MSCs), however, there is no standardized protocol for stem cell isolation and culture. This leads to inconsistency of the results and limits the comparison of the data from different laboratories. Our aim was to provide an applied protocol for ASCS isolation and expansion, study the cell behavior and define their cellular surface markers. ASCs were cultured from both resected adipose tissue (RAT) obtained following abdominoplasty or breast reduction and lipoaspirates (LPA) following laser-free liposuction.
Method: the protocol entailed coculturing of stromal vascular fraction (SVF) with RAT as raw pieces using DMEM medium with varying glucose concentration. The coculture protocol aimed to mimic the normal physiological conditions required for cell growth. ASCs were immunophenotyped to define their MSCs surface markers by flowcytometry.
Results: ASCs were isolated from coculturing RAT with SVF with fibroblast-like adherent cells morphology. The ASCs yield isolated from LPA was significantly greater than from RAT on day 14 and 28 (p = 0.002, <0.001, respectively). Significant increase in ASCs proliferation rate was detected when ASCs were cultured under high glucose (4.5 g/L) compared to low glucose (1 g/L) condition on day 7 and 14 (p = 0.04, 0.015, respectively). ASCs isolated from both protocols were positive for CD34, CD49d, CD73, CD90 and CD105 and negative for CD3, CD14, CD19, CD45 and HLA-DR.
Conclusion: We concluded that the cells harvested by our protocol were ASCs. Hence, our method can be an efficient isolation tool to obtain primary ASCs under culture conditions mimicking normal physiological status. This will help in providing ASCs which can be similar to cells in human tissue for further study.
1. Introduction
Mesenchymal stem cells (MSCs) is a valuable source in cell-based therapy strategies owing to their distinctive characteristics for multilineage differentiation [Citation1,Citation2]. MSCs have immunoregulatory potentials as they can interact with the immune cells and alter the immune response [Citation1,Citation2]. Additionally, MSCs have anti-inflammatory and regenerative capabilities [Citation3–Citation5].
Bone marrow (BM) is the source of BM-derived MSCs and is obtained by an invasive procedure with the impeding problem of a significant decline in differentiation potentials of the progenitor cells with increasing age [Citation3]. A favorable alternative reliable source to MSCs is adipose tissue (AT) and known as adipose-derived mesenchymal stem cells (ASCs). AT can be easily obtained with less invasive procedures and patient morbidity than other tissues [Citation4].The estimated frequency of isolated stem and precursor cells from AT is approximately 2,500-fold greater than that in bone marrow [Citation5].
ASCs originate from the human fat stromal vascular fraction (SVF), which is a heterogeneous mixture of cells [Citation6]. Culturing of SVF resulted in the developing of a relatively homogenous ASCs population. However, many factors have impact on the cellular and molecular profiles of ASCs as; age, tissue depot, isolation and culture conditions. The International federation for adipose therapeutics and science (IFATS) and the international society for cellular therapy (ISCT) proposed the criteria to identify MSCs based on plastic adherence and expression of cell surface markers [Citation7,Citation8]. IFATS proposed that a foundational ASCs phenotyping should include at least two negative markers (e.g CD3, CD11b or CD14, CD31, CD45, CD79a or CD19, CD106, CD235a and HLA-DR) and two positive markers in the same analysis (e.g CD13, CD29, CD44, CD49d, CD73, CD90, and CD105) [Citation8]. Meanwhile, the debatable expression of CD34 was referred to by IFATS as an unstable positive marker which presents at variable levels [Citation8]. However, considerable sections of these guidelines are under study for further elucidation.
Although many scientists have successfully isolated ASCs, there is no well-defined protocol for in vitro ASCs isolation and culture. Lack of standardized method to culture ASCs is a main hurdle in comparing results from different laboratories as the composition and phenotype of ASCs subpopulations change due to culture conditions. Currently, many protocols are applied to obtain AT in clinical practice, and the commonest AT source for fat harvest is liposuction [Citation9–Citation11]. There are different approaches for liposuction as; suction-assisted; a mechanical suction of AT through suction canula, laser-assisted and ultrasound-assisted liposuction. Several studies demonstrated that ASCs yield, viability and regenerative characteristics were affected according to the liposuction technique of fat harvesting [Citation12,Citation13].
The current body of ASCs literature is inconsistent, partly due to an incomplete understanding of how various harvesting and processing methods affect ASCs biology. AT microenvironment is comprised mainly of mature adipocytes, ASCs, blood-derived cells, endothelial (progenitor/mature) cells, smooth muscle cells, and pericytes [Citation14]. This study is based on the hypothesis that culturing ASCs in conditions mimicking the in vivo microenvironment, where the cells physiologically stimulated for growth and proliferation, could help in accomplishing a better understanding of ASCs biology. The aim of our study was to use a simple isolation and culture protocol for ASCs from resected adipose tissues (RAT) that may simulate the physiological microenvironment and help to obtain adequate yield of primary ASCs for further study. We compared the ASCs isolated from our method to the common source of fat isolation; lipoaspirate (LPA) and demonstrated the profile of MSCs surface markers.
2. Methods
2.1. Subjects
Human AT was obtained from 12 healthy subjects (18–60 years) following abdominoplasty (n = 4), breast reduction (n = 2) or liposuction (n = 6) that were conducted in the plastic surgery department, Alexandria Main University Hospital. AT samples were obtained either as 2–4 grams of subcutaneous resected adipose tissues (RAT) from patients undergoing abdominoplasty or breast reduction ()) or 40 ml of lipoaspirate (LPA) material collected in a sterile syringe (emptied and delivered in sterile cups) from patients undergoing liposuction ()). Informed consents were obtained from subjects enrolled in the study before participation. The study was approved by the Medical Ethics Committee of the Faculty of Medicine, Alexandria University and the practical work was carried in accordance with Declaration of Helsinki. Patients on regular insulin or corticosteroids therapy were excluded as these drugs may influence ASCs profile [Citation15]. Breast cancer patients were excluded as malignancy may be associated with genetic aberrations which may alter the behavior of isolated ASCs.
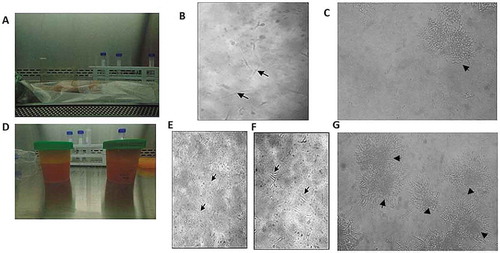
Figure 1. Morphological characteristics of ASCs isolated from RAT and LPA. ASCs isolated from RAT (IA-C) and LPA (II D-G). (I) ASCs isolated from RAT shows (a) unmanipulated RAT delivered post operatively. (b) culture on day 7 where few spindle-like cells started to appear (black arrows). (c) culture on day 28, colonies of fibroblast-like cells were formed (black arrowhead). (II) ADSCs isolated from LPA shows (d) LPA material emptied in sterile cups. (e) Culture on day 0 with numerous rounded non-adherent cells (black arrows). (f) Culture on day 3 shows few single adherent spindle fibroblast-like cells (black arrows). (g) culture on day 28 shows several colonies of fibroblast-like cells (black arrowheads). Images were examined by Olympus IMT-2 inverted light microscopy with 10× and 20× magnification
2.2. Isolation and primary culture of ASCs from resected adipose tissue (RAT) vs lipoaspirates (LPA)
RAT was cultured according to Zeng et al. (2013) protocol but with introduced modifications. The RAT into small pieces and plated them directly in T-25 cm2 culture flasks followed by turning of culture flasks every 12 hours for 48–72 hours to allow the plastic adherent cells to crawl from pieces of AT [Citation16]. The modifications introduced to Zeng et al (2013) included dividing RAT into two section; one was cut into small pieces by scissors and gently dissociated with a syringe plunger against cell strainer and the other section was treated with collagenase to isolate SVF pellet. The SVF pellet was cocultured with the retrieved RAT pieces in T-25 cm2 culture flasks. Briefly, the RAT was immediately washed with 20 mL sterile PBS (Sigma-Aldrich, Germany) in sterile 10 cm2 culture plates at room temperature (rt) to clean the tissues from blood clots, red blood cells, contaminating debris and anesthetics drug. In a petri dish, the RAT pieces were cut into small pieces (1–5 mm3) using ophthalmic sterile scissors and forceps. Section of RAT pieces were minced to allow exposure of the internal contents of the tissue. The other RAT section was treated in the same way as LPA to obtain SVF (further in the method). The minced RAT and suspended SVF pellet were transferred into T-25cm2 Nunc culture flasks where the RAT pieces were aligned to 0.5–1 cm distance intervals in 2 ml culture medium. The used culture medium was DMEM-high glucose (4.5 g/l) with 2%L-glutamine (Lonza, Belgium)- 10%FBS (Fetal bovine serum) (Biochrom, Germany)-P/S 1% (Euroclone, Italy).
LPA delivered in syringes were emptied in 50 mL falcon tubes and washed with equal volume of PBS at rt before use. The samples were left standing for 2 minutes in PBS till an upper layer (lipoaspirate mass) and a lower layer (red-tinged PBS) were formed. The upper layer was treated with and equal volume of 0.075% collagenase solution (type I; Sigma-Aldrich, Germany) for 1 hour in shaking water bath at 37°C, 500 rpm. The collagenase enzyme was deactivated with equal volume of culturing medium and the cells were washed 3000 rpm, 5 min at 4° C. Red cell lysis buffer was added for 2–3 minutes, then the cells were washed with culture medium at 2000 rpm for 5 min. The cell pellet was resuspended and passed through a 100-um cell strainer (Corning, sigma Aldrich) and these cells represented the initial SVF fraction. SVF cells were seeded at a density of 2.6x105/10cm2 in T-25 cm2 Nunc culture flasks. ASCs were incubated in 95% O2, 5%CO2 at 37° C. After 48 hours, the culture flasks were washed with PBS to remove non-adherent cells. Cell cultures were maintained until fibroblast-like adherent cells reached 80–90% confluence with media being changed every 48–72 hours. ASCs were harvested using 0.05% trypsin/1.0 mM EDTA (Euroclone, Italy) and cultures were expanded for further passages. DMEM-high glucose (4.5 g/l) with 2% L-glutamine was the regular culture medium used for both protocols. In the experiments were glucose concentration was tested, ASCs isolated from LPA were cultured in two media; DMEM-Low glucose (1 g/l) and DMEM-high glucose (4.5 g/l) and the cell proliferation was tested at day 7and 14.
Evaluation of each applied protocol was assessed by observing the cell morphology and proliferation with an Olympus IMT-2 inverted light microscopy. Cell count was assessed using hemocytometer and cell viability was assessed using trypan blue stain and calculated as total unstained (viable) cells/total (stained and unstained) cells x100. Fold increase of cells was determined by total number of the harvested cells divided by the initial number of the plated cells. Confluence was estimated according to the occupied surface of the tissue culture flask.
2.3. Characterization of ASCs by immunophenotypic analysis
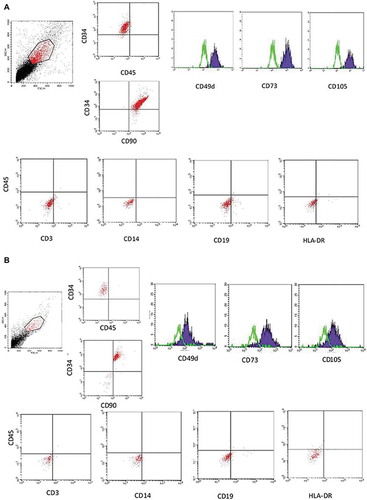
ASCs isolated from RAT and LPA were immunophenotyped for the expression of MSCs cell surface markers by flowcytometry in passage one and after reaching confluence. The dynamic of MSCs surface markers expression by ASCs isolated from LPA was analyzed at different time points; day 0, 7, 14, 21 and 28. Cell suspension of ASCs were harvested using 0.05% trypsin/EDTA, washed and incubated for 30 min with FITC- or PE-conjugated monoclonal antibodies: anti-CD3-FITC, anti-CD14- FITC, anti-CD19-FITC, anti-CD34-PE, anti-CD45–FITC and -PE, anti-CD49d-FITC, CD73-FITC, anti-CD90-FITC, anti-CD105-FITC and anti-HLA-DR-FITC. A nonspecific FITC/PE- conjugated IgG was used as an isotype control to assess the background fluorescence. Analysis was done by BD FACS Calibur flow cytometer (BD biosciences, California, USA) using the BD CellQuest Pro software.
2.4. Statistical analysis
All data are presented as mean ± standard deviation (SD). Mann-Whitney U test (non-parametric two-tailed t-test) was used to calculate statistical differences using GraphPad Prism version 5.00 for Windows. A value of p < 0.05 was regarded as statistically significant.
3. Results
Isolation and in vitro culture of ASCs from RAT pieces showed that the plated RAT pieces attached to the culture flasks wall to allow the stem cells to grow out ((b, c)). In the first 3 days, the cell culture showed few spot-like cells of ASCs that started to grow from the edges of RAT pieces. The culture showed spindle-like cells by day 7 of primary culture (). After 28 days of incubation, few sphere-like colonies of fibroblast-like cells were observed under an inverted light microscopy ()). Passaging for ASCs to expand in vitro was done by day 35–40 of primary culture. Generally, an average of 2 × 106 ASCs were obtained from about 4 grams of AT after 5 weeks of culture using our method.
Isolation and in vitro culture of ASCs from LPA expressed morphological changes ((e–g)). On day zero, SVF started as rounded non-adherent cells () and turned to adherent spindle fibroblast-like cells (ASCs) by day 3 (). Several colonies of fibroblast-like adherent ASCs reached >60% confluency by day 28 (). An average of 1 × 107 ASCs were obtained from about 50 ml of LPA after 4 weeks of culture. The cultured cells retained their fibroblast-like morphology and were successfully propagated in vitro by repeated 2–5 passaging rounds.
ASCs yields from both LPA and RAT were compared after 14 and 28 days of cell culture. The results showed significant differences between LPA and RAT cell yields, where on day 14; ASCs average counts were 90 ± 18.2x105 and 4.5 ± 2.5x105 (p 0.002), and on day 28; ASCs average counts were 100 ± 16.4x 105 and 20 ± 8.2x105 (p < 0.001) ()
Figure 2. ASCs proliferation study. ASCs proliferation study: (a) ASCs yield was compared between ASCs isolated from LPA (blue histogram) vs RAT (brown histogram) on day 14 and day 28. (b) ASCs fold increase was compared between ASCs isolated from LPA (blue line) vs RAT (brown line) on day 7, 14 and day 28. Data are presented as mean ± SD **p < 0.01, ***p < 0.001
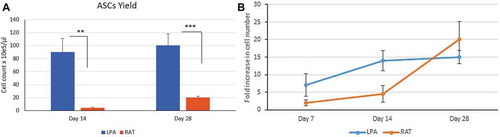
Fold increase of ASCs isolated from LPA or RAT were compared on day 7, 14 and 28 as determined by total cell number harvested at certain time-point divided by the initial cell number plated. The results showed no significant difference in cell fold increase between ASCs isolated from LPA or RAT. The ASCs cultured from RAT showed a longer lag period with little expansion than LPA in the first 2 weeks. ASCs isolated from LPA expanded rapidly in a log phase by the first week, while it took 2 weeks for ASCs isolated from RAT. ASCs isolated from LPA entered to stationary phase where they showed decrease in growth rate by day 28, while ASCs isolated from RAT showed increase in growth rate during the corresponding period ().
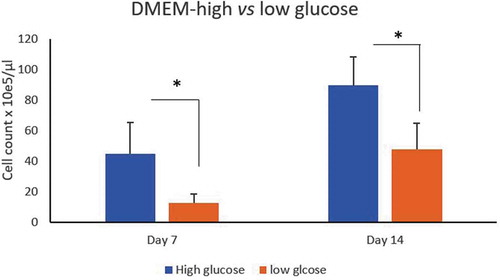
ASCs counts isolated from LPA cultured in DMEM with high glucose (4.5/L) vs low glucose (1 g/L) concentrations showed significant increased proliferation rate under high glucose condition as the cell counts were compared at day 7and 14 (p = 0.04, 0.015, respectively) ().
Figure 3. Effect of glucose concentration on ASCs culture. Count of ASCs isolated from LPA cultured in DMEM-high glucose (4.5/L)(blue histogram) vs low glucose (1 g/L) (brown histogram) concentrations at day 7 and 14. *p < 0.05
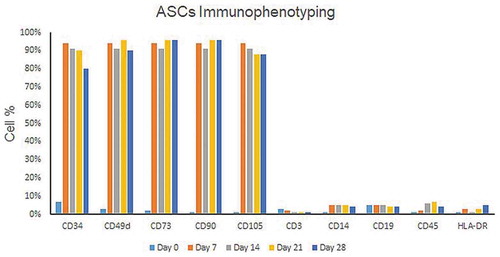
The harvested fibroblast-like adherent cells were immunophenotyped to assess the expression of MSCs surface markers by flow cytometry. The results showed that ASCs isolated from RAT and LPA were positive for CD34, CD49d, CD73, CD90 and CD105 and negative for CD3, CD14, CD19, CD45 and HLA-DR (). Expression of MSCs surface markers by SVF isolated from LPA showed, on day zero, that 5% of the population were CD34+ and negative for the MSCs positive markers. Specific surface markers as CD34 decreased gradually over different timepoints ().
4. Discussion
The phenotype, proliferation, immunogenicity and genetics of ASCs vary between different isolations and culture conditions. However, there is no standardized method for stem cell isolation and culture, which limits the comparison of the results from different laboratories. This summons the need to develop a unanimous efficient replicable method for ASCs isolation and culture that would be cost-effective, clinically practical and with no excessive handling processes to preserve the physiological characteristics and avoid cell changes.
Several attempts have been conducted to culture stem cells under conditions simulating physiological conditions e.g. human serum in place of FBS or human platelets as a source of growth factors [Citation17]. Researches have proposed explanting RAT as source of ASCs [Citation16,Citation18,Citation19]. In the current study, ASCs were isolated following coculturing of SVF with autogenic resected AT pieces without using external growth factors. We hypothesized that coculturing SVF along with RAT explant will simulate the physiological microenvironment and favor cell-extracellular matrix and cell-cell interactions, hence promote the survival of ASCs. Endothelial cells have been shown to endorse the growth of adipogenic cells, hence, the two cell types communicate by either surface markers, growth factors or extracellular matrix proteins [Citation20–Citation22].
In the current study, we isolated ASCs from RAT explants, following abdominoplasty or breast reduction, and compared them with cells isolated from LPA obtained by liposuction. Explant culture of ASCs from RAT was conducted where AT were cut into pieces and cultured directly with no enzymatic treatment. However, the isolated cells following the explant culture protocol were scanty and the proliferation rate was poor. We introduced modifications to that protocol, as we treated part of RAT with collagenase to isolate SVF and cocultured them with the other part of untreated RAT that were cut into small pieces and gently dissociated with a syringe plunger. These modifications improved the ASCs yield, where more spindle cells started to be observed. Generally, an average of 2 × 106 ASCs were obtained from about 4 grams of RAT after 28 days of culture using this method. Several protocols used for ASCs culture depended on isolating SVF and discarding the remaining AT [Citation23–Citation25]. We believe that keeping RAT in the culture along with SVF pellet may mimic the normal microenvironment where the cells were initially stimulated. However, this protocol needed further optimization regarding the density of initial plating of the AT pieces and ratio of SVF to be seeded.
For the in vitro culture of ASCs from LPA, we applied the protocol adopted by Zuk et al. (2001) except we prolonged the incubation time of SVF pellet with collagenase to 1 hour with continuous gentle agitation in shaking water bath at 37° C. Applying this protocol provided a yield of 19 × 106 cells/ml DMEM medium from 40 ml LPA. ASCs yield is affected by many factors as duration of collagenase digestion [Citation26]. In Zuk et al. (2001) study, they showed that the optimal viability of SVF was obtained after 2 hours of collagenase digestion, while others applied 30 mins digestion [Citation27]. Optimization of the duration for collagenase digestion will be tried in our protocol for ASCs isolation from LPA and RAT to determine the optimal condition.
Our data showed significant difference in ASCs yield between cells isolated from LPA vs RAT. The average counts of isolated ASCs from RAT were significantly fewer than that from LPA as compared on day 14 and 28 of primary cell culture. It is difficult to compare our data with others due to differences in culture conditions; however, our observation was not in accordance to that observed by Duscher et al. (2016) as they showed that fewer ASCs were obtained from LPA compared to excised fat tissue. Meanwhile, Yoshimura et al. (2006) tried isolation of ASCs from the fluid portion of LPA and compared it to the adipose portion and observed that more ASCs were obtained from fluid portion than adipose one. ASCs are distributed in the adipose connective tissue between adipocytes or around blood vessels [Citation22]. Additionally, the procedure used for AT harvesting and the extent of mechanical injury affected the yield and growth characteristics of ASCs [Citation28,Citation29]
Our data showed that the cell yield and growth rate of ASCs isolated and cultured from LPA were better than RAT, and ASCs isolated from RAT resected from abdominoplasty were better than that isolated from breast reduction. This finding is in accordance with the common consensus that ASCs depot account for the differences in morphology, function and response to metabolic challenges detected in different AT depots. These differences could be ascribed to cell intrinsic differences as gene expression, adipogenic and angiogenic potentials controlled by microenvironment of adipose progenitors cells within a distinct depot [Citation28,Citation30].
Our study showed significant increase in ASCs proliferation rate under high glucose (4 g/L) as compared to low glucose (1 g/L) culture condition. Our data is in accordance with Mischen et al. (2008) as they showed that the metabolism of ASCs was dependent on the access to glucose and oxygen, while L-glutamine played much lesser role [Citation31]. They demonstrated that ASCs differentiation was hindered in hypoxic and low-glucose conditions and was completely inhibited in conditions lacking glucose. On the contrary, other published studies propagated ASCs in either glucose free- [Citation22] or low glucose- DMEM medium [Citation16].This highlighted the importance of standardizing the glucose content as an important variable in ASCs culture conditions.
In the current study, characterization of isolated ASCs by immunophenotype analysis using flow cytometry showed that ASCs isolated from RAT and LPA were positive for MSCs surface markers; CD34, CD49d, CD73, CD90 and CD105. It was proposed by IFATS that a foundational ASCs phenotyping should include at least two negative markers (in our study; CD45, CD3, CD14) and two positive markers in the same analysis (in our study; CD34, CD49d CD73, CD90, and CD105) [Citation8]. The percentage of CD34 expression by SVF cells has been reported with great inconsistency among authors. Our study showed that 5% of SVF population isolated from LPA were CD34+ and negative for the MSCs positive markers. This is in accordance with a study which showed that only 7% of the SVF cells expressed CD34 [Citation32]. In contrast to our findings, a study used immunomagnetic method to identify SVF and demonstrated about 40% of SVF as CD34+ that increased to 80% in cultured cells [Citation33]. Generally, different subpopulations can be detected in ASCs culture as they change their surface marker profiles depending on the isolation methods. Our data showed that the dynamic of ASCs phenotype changes during cell culture, which is in accordance with the published data. Studies showed that, early in culture, ASCs do not consistently express all characteristic MSC markers, where some specific surface markers (e.g.CD105) increased during culture, while others decreased (e.g.CD34) [Citation24].
We must recognize several limitations in our study, as direct explanting of RAT pieces may not allow proper optimization of initial plating densities, which is an important factor impacting ASCs behavior [Citation34]. We planned to optimize the ratio between SVF initial count to RAT pieces; size, numbers and distribution to study the optimum ratio for culturing. Furtherly, we will apply our suggested protocol on AT derived from the same depot and analyze the effect on the yield and viability of ASCs. The immune-modulatory properties of MSCs were attributed to the expression of immune markers and variety of adhesion molecules that mediate MSCs interaction with immune cells [Citation2]. Hence, we planned to study culturing ASCs under our modified protocol using autogenic and allogenic donors and study the immune profile of ASCs in the respective protocol. Moreover, biochemical analysis of the culture supernatant can give an idea about the important regulatory cytokines that govern the culture conditions.
It can be concluded from the current study that the cells harvested from coculturing of SVF with RAT were ASCs that were positive for CD34, CD49d, CD73, CD90 and CD105. We believe that the suggested culturing modification is based mainly on simulating the physiological microenvironment where ASCs reside and can be an efficient isolation tool to obtain primary ASCs for further studies.
Availability of data and material
The data used or analysed during the study are available from the corresponding author on reasonable request
Disclosure statement
The authors declare that they have no conflict of interests.
Additional information
Funding
Notes on contributors
Myriam A. Helmy
Myriam A. Helmy PhD: Professor clinical and chemical pathology. Faculty of medicine, Alexandria University, Egypt. Former head of Clinical Immunology and Histocompatibility laboratories.
Adham F. Mohamed
Adham F. Mohamed, MD. Assistant professor Plastic surgery department. Faculty of medicine, Alexandria University, Egypt.
Hadeer M. Rasheed
Hadeer M. Rasheed, MScs. Assistant lecturer clinical and chemical pathology. Faculty of medicine, Alexandria University, Egypt.
Amira I. Fayad
Amira I. Fayad, PhD Imperial college, UK. Lecturer clinical and chemical pathology. Faculty of medicine, Alexandria University, Egypt. Technical manager of Clinical Immunology and Histocompatibility laboratories.
References
- McIntosh K, Zvonic S, Garrett S, et al. The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells (Dayton, Ohio) 2006 May;24(5):1246–1253.
- Ryan JM, Barry FP, Murphy JM, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflammation. 2005 July;26;2(1):8.
- Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells (Dayton, OH). 2006 May;24(5):1294–1301.
- Gentile P, Orlandi A, Scioli MG, et al. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem cells Transl Med. 2012 Mar;1(3):230–236.
- Fraser JK, Zhu M, Wulur I, et al. Adipose-derived stem cells. Methods Mol Biol (Clifton, NJ).2008;449:59–67.
- Zimmerlin L, Donnenberg VS, Pfeifer ME, et al. Stromal vascular progenitors in adult human adipose tissue. Cytometry. 2010 Jan;77(1):22–30.
- Dominici M, Le Blanc K, Mueller I. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006;8(4):315–317.
- Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013 Jun;15(6):641–648.
- Scuderi N, Tenna S, Spalvieri C, et al. Power-assisted lipoplasty versus traditional suction-assisted lipoplasty: comparative evaluation and analysis of output. Aesthetic Plastic Surgery. 2005 Jan-Feb;29(1):49–52.
- Raposio E, Simonacci F, Perrotta RE. Adipose-derived stem cells: comparison between two methods of isolation for clinical applications. Ann Med Surgery. 2017 Aug; 20:87–91.
- Raposio E, Bertozzi N. Isolation of ready-to-use Adipose-Derived Stem Cell (ASC) pellet for clinical applications and a comparative overview of alternate methods for ASC isolation. Curr Protoc Stem Cell Biol. 2017 May 16;41(1):1F 17 1–1F 17 12.
- Chung MT, Zimmermann AS, Paik KJ, et al. Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine. Stem Cells Transl Med. 2013 Oct;2(10):808–817.
- Duscher D, Maan ZN, Luan A, et al. Ultrasound-assisted liposuction provides a source for functional adipose-derived stromal cells. Cytotherapy. 2017 Dec;19(12):1491–1500.
- Baer PC, Geiger H. Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int. 2012;2012:812693.
- Cervelli V, Scioli MG, Gentile P, et al. Platelet-rich plasma greatly potentiates insulin-induced adipogenic differentiation of human adipose-derived stem cells through a serine/threonine kinase Akt-dependent mechanism and promotes clinical fat graft maintenance. Stem Cells Transl Med. 2012 Mar;1(3):206–220.
- Zeng G, Lai K, Li J, et al. A rapid and efficient method for primary culture of human adipose-derived stem cells. Organogenesis. 2013 Oct 1;9(4):287–295.
- Doucet C, Ernou I, Zhang Y, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005 Nov;205(2):228–236.
- Priya N, Sarcar S, Majumdar AS, et al. Explant culture: a simple, reproducible, efficient and economic technique for isolation of mesenchymal stromal cells from human adipose tissue and lipoaspirate. J Tissue Eng Regen Med. 2014 Sep;8(9):706–716.
- Jing W, Xiao J, Xiong Z, et al. Explant culture: an efficient method to isolate adipose-derived stromal cells for tissue engineering. Artif Organs. 2011 Feb;35(2):105–112.
- Frye CA, Wu X, Patrick CW. Microvascular endothelial cells sustain preadipocyte viability under hypoxic conditions. In Vitro Cell Dev Biol Anim. 2005 May-Jun;41(5):160–164.
- Hutley LJ, Herington AC, Shurety W, et al. Human adipose tissue endothelial cells promote preadipocyte proliferation. Am J Physiol-Endocrinol Metab. 2001 Nov;281(5):E1037–E1044.
- Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008 Jan 4;102(1):77–85.
- Bochev I, Elmadjian G, Kyurkchiev D, et al. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol Int. 2008 Apr;32(4):384–393.
- Duscher D, Luan A, Rennert RC, et al. Suction assisted liposuction does not impair the regenerative potential of adipose derived stem cells. J Transl Med. 2016 May 6;14(1):126.
- Lin C-S, Xin Z-C, Deng C-H, et al. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010 Jun;25(6):807–815.
- Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7–14.
- Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001 Apr;7(2):211–228.
- Jeffery E, Wing A, Holtrup B, et al. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab. 2016 Jul 12;24(1):142–150.
- Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8(2):166–177.
- Tchoukalova YD, Koutsari C, Votruba SB, et al. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity. 2010 Oct;18(10):1875–1880.
- Mischen BT, Follmar KE, Moyer KE, et al. Metabolic and functional characterization of human adipose-derived stem cells in tissue engineering. Plast Reconstr Surg. 2008 Sep;122(3):725–738.
- Astori G, Vignati F, Bardelli S, et al. “In vitro“ and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med. 2007 Oct;5(1):55.
- Maumus M, Peyrafitte J-A, D’Angelo R, et al. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obesity. 2011 Sep;35(9):1141–1153.
- Kim DS, Lee MW, Yoo KH, et al. Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PloS One. 2014;9(1):e83363.