ABSTRACT
Helicobacter pylori (H. pylori)
is a microbe with wide genetic diversity that infects the stomach of most people in developing countries, leading to several clinical outcomes among different individuals such as gastritis, ulcers, or gastric cancer. Outer inflammatory protein A (oipA) and duodenal ulcer promoting (dupA) genes are among the possible virulence factors which determine the patient outcome.
Aim
To detect oipA and dupA genes of H. pylori among dyspeptic Egyptian patients, and to investigate their correlation with the varying degrees of the associated chronic gastritis.
Methods
The study enrolled 50 patients with dyspepsia, attending the Gastrointestinal Endoscopy unit of the Gastroenterology and Tropical Departments at Ain Shams University Hospital for upper gastrointestinal endoscopy, in the period between, June and, December 2019. Four antral gastric biopsies were taken from each patient for polymerase chain reaction assay to detect the virulence genes oipA, dupA, and cagA and for histopathological assessment.
Results
Forty patients were H. pylori positive by histopathology and PCR. cagA, oipA, and dupA were identified in 6 (15%), 13 (32.5%), 9 (22.5%) of biopsies, respectively. Both cagA and oipA genes were highly significantly associated with increasing the severity of gastritis. Only oipA virulence gene showed a highly significant association with gastroduodenitis. There was a highly significant moderate association between cagA and oipA genes.
Conclusion
oipA
could be a virulence biomarker that serves a great value in predicting the progress of gastric mucosal damage in patients with chronic gastritis, and targeting antimicrobial therapy in those patients to prevent severe gastroduodenal diseases.
1. Introduction
H. pylori colonizes the stomach of around 50% of the world’s population, leading to acute and chronic gastritis, gastric, and duodenal ulcers when patients are not treated appropriately [Citation1]. It has been classified by the World Health Organization as Class I Carcinogen and a predisposing factor for gastric cancer, which is recognized worldwide as the second among cancers causing deaths [Citation2].
Variable clinical outcomes among different individuals can be attributed to host, environmental, dietary factors, as well as virulence factors of different strains [Citation3]. Certain virulence factors possessed by strains might be recognized as a selective advantage for these strains, and a biomarker that aids in predicting the outcome of infection and preventing the resulting damage to gastric mucosa [Citation4].
The cytotoxin-associated gene (cagA) exists in nearly half of H. pylori strains and is a common marker for the presence of the cag-pathogenicity island [Citation5]. The strains positive for this gene are frequently associated with the pathogenesis of gastric cancer as a result of excessive mucosal inflammation and production of interleukin-8 [Citation6].
Outer inflammatory protein A (oipA) is one of the outer membrane proteins, its presence results in a higher probability of gastric cancer and peptic ulcers than gastritis and functional dyspepsia [Citation7]. The possible mechanism of action of oipA is through accomplishing H. pylori adherence, potentiating the activity of the cag-pathogenicity island, as well as inducing proinflammatory immune responses [Citation8].
The duodenal ulcer promoting gene (dupA) can result in duodenal ulceration and/or reduction of risk of gastric cancer in some populations. The dupA protein induces the secretion of IL-8 and −12 by antral gastric mucosa in vivo as well as by gastric epithelial cells in vitro [Citation9]. In addition, a significant association was observed with treatment failure [Citation10].
In Egypt, previous studies were conducted to determine some virulence genes such as cagA, vac A, iceA1, and babA2 and assess their relation to clinical presentation [Citation11–Citation13]; however, no enough reports are available about oipA and dupA genes. Therefore, this study aimed to detect oipA and dupA genes among dyspeptic Egyptian patients and to investigate their correlation with the varying degrees of the associated chronic gastritis.
2. Materials and methods
2.1. Study population
The present cross-sectional study was conducted on 50 dyspeptic patients, referred for upper gastro-duodenoscopy at the Gastrointestinal Endoscopy unit of the Gastroenterology and Tropical Departments at Ain Shams University Hospital, in the period between, June and, December 2019. Patients were selected according to the following criteria: being more than 18 years, of either sex, suffering from various dyspepsia symptoms, e.g. nausea, vomiting, epigastric pain, heartburn, and hematemesis, and not responding to proton pump inhibitors. Patients were excluded from the study if they received nonsteroidal anti-inflammatory drugs, H2 receptor antagonists, proton pump inhibitors, or antibiotics 2 to 4 weeks before the procedure, also if they suffered inflammation caused by other bacteria or GIT cancers.
2.2. Specimen collection and processing
Four antral gastric biopsies were taken from each patient, divided into two tubes. One of them contained 0.9% of sterile saline solution and was stored in −80°C for DNA extraction and polymerase chain reaction (PCR) for detection of H. pylori and the virulence genes cagA, oipA and dupA. The other one was sent for histopathological assessment. An informed consent was taken from the patients or their relatives for sample collection, according to the regulations of the ethical committee of scientific research (Faculty of Medicine – Ain Shams University). Diagnosis of H. pylori was done according to histopathological findings and then confirmed by PCR amplification of the ureC gene in gastric biopsies.
2.3. Histopathological examination of H. pylori
Pathologic sections stained with Hematoxylin-Eosin were examined and assessed for the following criteria according to updated Sydney Classification: presence of H. pylori, degree of gastritis, and presence of gastric atrophy, intestinal metaplasia, dysplasia, or malignancy [Citation14].
2.4. DNA extraction and H. pylori detection
DNA was extracted from biopsy specimens, using Qiagen DNA tissue kit (Qiagen, Germany) according to the manufacturer’s instructions. Eluted DNA was stored at −20°C until used. Samples positive for H. pylori by histopathology were examined for the presence of ureC gene by PCR assay according to Kargar et al. [Citation15].
The target genes cagA, oipA, and dupA were analyzed through PCR, using one set of oligonucleotides for each gene fragment. summarizes the primers’ sequences, PCR conditions, and amplicon sizes according to Oktem-Okullu et al. [Citation16]. DNA samples from H. pylori strain D0008 (Genekam, Germany) were used as a positive control, and sterile distilled water was used as a negative control. Detection of amplicons was done by gel electrophoresis with ethidium bromide 2% agarose gel.
Table 1. Primers’ sequences, PCR conditions, and amplicon sizes for detection of target genes.
2.5. Statistical analysis
All statistical procedures were carried out using SPSS version 20 for Windows (SPSS Inc, Chicago, IL, USA). Fisher’s exact test was used to examine the relationship between H. pylori genotypes and disease severity. Kappa statistics was determined to measure the degree of agreement between two genes.
3. Results
Among 50 dyspeptic patients, H. pylori was detected in 40 (80%) antral gastric biopsies by histopathological assessment and by PCR amplification of the ureC gene. H. pylori infected patients were 19 (47.5%) males and 21 (52.2%) females with mean age ±SD (36.20 ± 13.78).
Based on the histopathological examination, the enrolled H. pylori positive patients had varying degrees of non-ulcerative chronic gastritis, 22 (55%) had mild gastritis, 13 (32.5%), and 5 (12.5%) showed moderate and severe gastritis, respectively. Gastroduodenitis was observed in 8 (20%) cases. No ulcerative or neoplastic lesions were diagnosed in our patient group. The main complaint was epigastric pain (55%) followed by heartburn and hematemesis (20%).
3.1. Detection of virulence genes and their relation to histopathological intensity of gastritis
The virulence genes were recognized in the gastric biopsy samples with different rates and their PCR products are shown in –. & summarize the relation between the target genes and histopathological intensity of gastritis as well as gastroduodenitis among the studied patients. CagA gene was identified in 6(15%) cases. Its presence was highly significantly associated with increasing intensity of gastritis (p = 0.001); as it was positive in 4 out of 5 cases of severe gastritis (80%), as well as 2 out of 13 cases of moderate gastritis.
Table 2. Relation between studied virulence genes and histopathological intensity of gastritis among cases.
Table 3. Relation between studied genes and gastroduodenitis among cases.
The oipA gene was found in 13 (32.5%) cases. This gene was highly significantly associated with increasing intensity of gastritis (p = 0.001); as it was positive in all cases of severe gastritis (100%) and 8 (61.5%) out of 13 cases of moderate gastritis. However, both cagA and oipA genes were not detected in any case of mild gastritis. Also, OipA gene showed a highly significant association with gastroduodenitis being positive in 6(75%) out of 8 cases.
The dupA gene was positive in 9 (22.5%) cases distributed as; 3 mild cases, 3 moderate, as well as 3 cases of severe gastritis. However, no significant association was observed for dupA and intensity of gastritis. Neither the presence of cagA nor dupA genes had a statistically significant association with gastroduodenitis.
3.2. Association between cagA, oipA, and dupA genes
shows there was a highly significant moderate association between cagA and oipA genes (kappa = 0.536); oipA was present in all cagA positive cases. On the other hand, oipA was present in 7 cases out of 34 cagA negative cases (20.6%). There was a non-significant fair association (kappa = 0.268) between both cagA and dupA, and non-significant slight association between dupA and oipA (kappa = 0.133).
Figure 1. PCR products for H. pylori with cagA gene-based primers. Lanes 2–4 and 23, 25 and 35 are patients’ positive biopsy samples. Lanes Pc & W are positive (strain D0008) & negative (sterile distilled water) control, respectively.
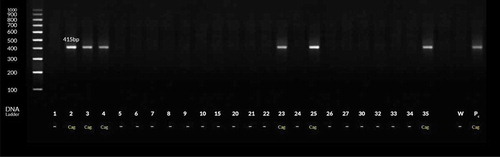
Figure 2. PCR products for H. pylori with oipA gene-based primers. Lanes 1–4 and 23, 25–26, 31–32, 34–35 and 38–39 are patients’ positive biopsy samples. Lanes Pc & W are positive (strain D0008) & negative (sterile distilled water) control, respectively.
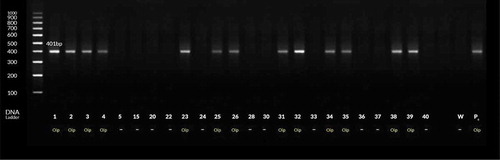
Figure 3. PCR products for H. pylori with dupA gene-based primers. Lanes 1–4 and 15, 20 and 24–26 are patients’ positive biopsy samples. Lanes Pc & W are positive (strain D0008) & negative (sterile distilled water) control, respectively.
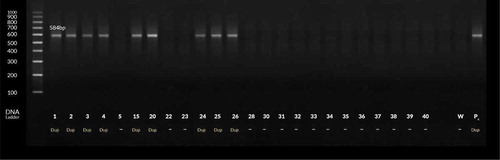
Table 4. Association between cagA, oipA, and dupA genes among cases.
4. Discussion
All patients participated in this study suffered from varying degree of chronic gastritis; 87.5% had mild and moderate degree, while 12.5% had severe degree of gastritis. Nearly similar to our results, Diab et al. [Citation3] in their study reported the predominance of mild and moderate gastritis (82.4%) over severe degree (17.7%).
The cagA gene was identified in 15% of the cases; 4 cases with severe gastritis and 2 cases with moderate gastritis; however, the gene did not exist in any of mild gastritis cases. Our results agree with those reported by Ezzat et al. [Citation17], they detected cagA gene in 13.3% of gastritis patients. Similarly, El Shenawy et al. [Citation13] and Diab et al. [Citation18] found the gene in 18.2% and 18.5% of H. pylori strains isolated from gastritis cases, respectively. Other studies reported prevalence about 33–38% [Citation10,Citation12,Citation19]. The variation between different studies could be explained by different number of participants in addition to their living conditions and socioeconomic status [Citation10]. We observed a highly significant association of cagA gene with increasing the severity of gastritis but not with the presence of gastroduodenitis. Our study is consistent with previous studies [Citation3,Citation10]. Others [Citation19,Citation20] found no statistically significant association between cagA genotype and gastroduodenal diseases.
In the present study oipA gene was detected in 32.5% of the cases. A higher prevalence of the gene 52.6% and 57% was found by Saeidi et al. [Citation21] and Dadashzadeh [Citation9], respectively. We found a highly significant association between the oipA gene and the intensity of gastritis and presence of gastroduodenitis; being positive in all cases and 61.5%, of severe and moderate gastritis, respectively, as well as 75% of patients with gastroduodenitis. Similar results were reported by Ben Mansour et al. [Citation22] in Tunisia, Souod et al. [Citation23] in the west of Iran, and Sallas et al. [Citation24] in Brazil. Other studies reported no correlation between oipA and disease outcome or increased gastroduodenal damage [Citation25,Citation26].
In this work, dupA gene was found positive in 22.5% of cases. The prevalence of dupA ranged from 6% to 92% as reported by different studies around the world [Citation27]. Our results are consistent with Lu et al. [Citation28], they detected the gene in 21% of the gastritis cases from East Asia and South America, and its presence was associated with more density of neutrophil infiltrate and IL-8 levels. Also, Arachchi et al. [Citation29] showed that dupA was present in 22.8% of dyspepsia patients, from north India. A higher prevalence was reported by Nguyen et al. [Citation30], who found 29.5% of H. pylori strains isolated from chronic gastritis patients in Japan were positive for this gene. Lower prevalence 12.2% was reported in south-east India [Citation31], 18.8% in Northern Iraq [Citation32], and 13.6% in Okinawan subpopulation, Japan [Citation33]. Differences in results reported by mentioned studies may result from the characteristics of studied dyspeptic population, in addition to regional variability and study methods [Citation9]. In the present study, no significant association was observed between dupA gene and gastroduodenal diseases. Our results agree with previous reports [Citation30,Citation34]. On the other hand, another systematic review confirmed the association of dupA with gastroduodenal diseases [Citation35]. The conflicting reports of dupA association with disease outcomes might be attributed to the location of this gene in the plasticity region of H. pylori resulting in variability of its expression or the limitation of applied PCR technique to detect the different forms of the gene [Citation36].
Regarding the association pattern between the H. pylori studied virulence genes; we found a highly significant moderate association between cagA and oipA genes. Previous studies showed a close association of both genes [Citation37]. They may act in a synergistic manner regulating the signaling pathways that mediate inflammation [Citation38]. On the other hand, we found a non-significant fair association between both cagA and dupA, and a non-significant slight association between dupA and oipA genes. Similar findings were reported by Zhang et al. [Citation39], they showed that the presence of dupA was not associated with any other virulence factors (cagA, vacA, iceA2, babA2) for all patient groups enrolled in their study. Also, Matteo et al. [Citation4] reported that dupA and oipA genes were present together in a limited number of strains. Other reports found a statistically significant relationship between the presence of dupA and cagA genes [Citation24], as well as between dupA and oipA genes in isolated strains [Citation9,Citation24].
Several studies have evaluated the association between different virulence factors of H. pylori and clinical manifestations in Egyptian patients. To our knowledge, this study is the first to detect oipA and dupA genes among dyspeptic Egyptian patients infected with H. pylori and investigate their relationship with the degree of associated chronic gastritis. Our results highlight the role of oipA as a virulence biomarker and a candidate that could be used for future preparation of vaccines against such pathogen. The limitations of our work were the relatively small sample size of studied patients and they were all from a single endoscopy unit.
5. Conclusion
OipA could be a virulence biomarker that serves a great value in predicting the progress of gastric mucosal damage in patients with chronic gastritis and targeting antimicrobial therapy in those patients to prevent severe gastroduodenal diseases.
6. Recommendations
Extended large-scale studies over longer periods include patients with other H. pylori related complications such as peptic ulcer disease and gastric cancer are required to confirm these results and distinguish the role of oipA and dupA genes in the pathogenesis of H. pylori related diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Nevine Musa
Nevine Musa is a Professor of Internal medicine, Ain Shams University Hospitals, Faculty of Medicine since 30/9/2019.Master of internal medicine in 2003(Ain Shams university).MD degree in Internal Medicine in 2009 (Ain Shams university).Specialist at Endoscopy unit Ain Shams university hospitals. JAG accredited from Hull University in 2012, UK.Master Colonoscopy Class from Hull University, 2014 UK.Professional Diploma in Video Capsule endoscopy, in 2016 from China.One of Committee team of VCE at Kafrawy unit at Endoscopy unit, Ain Shams university hospitals.Member of the Arab society of Infectious diseases and antimicrobials ASIDA since 2005.Member of the Egyptian society of endoscopy and hepatogastroenterology since 2004.Member of the Scientific Society of faculty of medicine, Ain Shams University.Participated in a number of national and international publications in the field of GIT and Hepatology.
Mohamed Eltabbakh
Mohamed Eltabbakh is a Lecturer of Tropical Medicine, hepatology, gastroenterology and liver transplantation, Ain Shams University, Faculty of Medicine.Fellowship in Advanced Endoscopic Resection (EMR-POEM-ESD) at Zhongshan hospital-Shanghai-China.I am interested in advanced therapeutic endoscopic techniques. I have many research interests in the field of HCV, HBV, HCC, IBD, H.pylori , liver transplantation and advanced endoscopic techniques. I have many international publications in the last 3 years.
Dalia Hosni Abdelhamid
Dalia Hosni Abdelhamid is a Assistant professor of Clinical Pathology (subspecialty microbiology), Ain Shams Faculty of Medicine since 2017, MD degree in Clinical Pathology 2010 (Ain Shams university), Professional Diploma in Infection control, Jan 2014.
Member of The Society for practitioner of Infection Control Egypt (SPICE) and Egyptian Society of Laboratory Medicine (ESLM). Participated in a number of national and international publications. participating in several conferences , training courses and workshops.
Shimaa Mostafa Ismail Mostafa
Shimaa Mostafa Ismail Mostafa is a Lecturer of Clinical pathology, Faculty of Medicine, Ain Shams University since 2018, MD degree in Clinical pathology in 2018 (Ain Shams university).Clinical pathology consultant at main labs in Eldemerdash hospital and Ain Shams university specialized hospital.
Member of the Egyptian society of Laboratory Medicine. Member of quality control unit of clinical pathology department, Ain shams university. Participated in a number of national conferences in the field of Clinical and chemical pathology.
Manar Mohamed Salah
Manar Mohamed Salah is a Lecturer of Tropical Medicine, Gastroenterology and Liver Diseases Faculty of medicine – Ain shams universityConsultant of Hepatology, Gastroenterology & Liver transplantation at Ain Shams Center for Organ Transplantation” ASCOT”.Member of ESLC, ETS& EASLGDGastrointestinal Endoscopy Consultant
Heba Ahmed Faheem Faheem
Heba Ahmed Faheem Faheem is a Lecturer of Internal Medicine, Gastroenterology and Hepatology, Ain Shams University, Faculty of Medicine since 2017, MD degree in Internal Medicine in 2017 (Ain Shams university).
Member of ASCOT (Ain Shams Center for Organ Transplantation), and The Egyptian Society of Liver Disease. Participated in a number of national and international publications in the field of Internal Medicine, Hepatology and Gastroenterology
Rania Ahmed Hassan
Rania AhmedHassan is a Assistant professor of Medical Microbiology and Immunology, Ain Shams Faculty of Medicine since 2016, MD degree in Medical Microbiology and Immunology in 2009 (Ain Shams university), Professional Diploma in Infection control, 2018.
Member of the Arab society of Infectious diseases and antimicrobials, the Egyptian Society of Virology, the Egyptian Society for Medical Microbiology, and the Clinical & Scientific Society of faculty of medicine, Ain Shams University. Participated in a number of national and international publications in the field of Medical Microbiology and Immunology
References
- Malnick SD, Melzer E, Attali M, et al. Helicobacter pylori: friend or foe? World J Gastroenterol. 2014;20:8979–8985.
- Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta analysis. Gastroenterology. 2017;153(2):420–429.
- Diab M, Saad El-Dine S, Aboul-Fadl L, et al. Helicobacter pylori cag pathogenicity Island genes among dyspeptic patients with chronic gastritis. Egypt J Med Microbiol. 2009;18(1):43–53.
- Matteo MJ, Armitano RI, Granados G, et al. Helicobacter pylori oipA, vacA and dupA genetic diversity in individual hosts. J Med Microbiol. 2010;59:89–95.
- Amer FA, El-Sokkary RH, Elahmady M, et al. Helicobacter pylori genotypes among patients in a university hospital in Egypt: identifying the determinants of disease severity. J Microbiol Infect Dis. 2013;3(3):109–115.
- Bibi F, Alvi SA, Sawan SA, et al. Detection and genotyping of Helicobacter pylori among gastric ulcer and cancer patients from Saudi Arabia. Pak J Med Sci. 2017;33(2):320–324.
- Liu J, He C, Chen M, et al. Association of presence/absence and on/off patterns of Helicobacter pylori oipA gene with peptic ulcer disease and gastric cancer risks: a meta-analysis. BMC Infect Dis. 2013;13:555.
- Imkamp F, Lauener FN, Pohl D, et al. Rapid characterization of virulence determinants in Helicobacter pylori isolated from non-atrophic gastritis patients by next-generation sequencing. J Clin Med. 2019;8(7):1030.
- Dadashzadeh K. Relevance of Helicobacter pylori dupA and oipA genotypes and development of gastric disease. Biomed Res. 2017;28(19):8179–8183.
- Abu-Taleb AM, Abdelattef RS, Abdel-Hady AA, et al. Prevalence of Helicobacter pylori cagA and iceA genes and their association with gastrointestinal diseases. Int J Microbiol. 2018;Article ID 4809093:7.
- Zaki ME, Elewa A, Ali MA, et al. Study of virulence genes Cag A and Vac A in Helicobacter pylori Isolated from Mansoura University hospital patients by multiplex PCR. Int J Curr Microbiol App Sci. 2016;5(2):154–160.
- Azeem EM, Abdel-Ghaffar AB, Shokaeir MH, et al. Genotyping of Helicobacter pylori isolates from Egyptian patients. Int J Biosci. 2017;10(4):121–128.
- El-Shenawy A, Diab M, Shemis M, et al. Detection of Helicobacter pylori vacA, cagA and iceA1 virulence genes associated with gastric diseases in Egyptian patients. Egypt J Med Hum Genet. 2017;18(4):365–371.
- Stolte M, Meining A. The updated sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15:591–598.
- Kargar M, Baghernejad M, Doosti A. Role of NADPH-insensitive nitroreductase gene to metronidazole resistance of Helicobacter pylori strains. Daru. 2010;18(2):137–140.
- Oktem-Okullu S, Tiftikci A, Saruc M, et al. Multiplex-PCR-based screening and computational modeling of virulence factors and T-cell mediated immunity in Helicobacter pylori infections for accurate clinical diagnosis. PLoS ONE. 2015;10(8):e0136212.
- Ezzat AH, Ali MH, El-Seidi EA, et al. Genotypic characterization of Helicobacter pylori isolates among Egyptian patients with upper gastrointestinal diseases. Chin Ger J Clin Oncol. 2011. DOI:10.1007/s10330-011-0880-x
- Diab M, Shemis M, Gamal D, et al. Helicobacter pylori Western cagA genotype in Egyptian patients with upper gastrointestinal disease. Egypt J Med Hum Genet. 2018;19(19):297–300.
- Habib AM, Alam MJ, Rudra B, et al. PCR-based analysis of Helicobacter pylori virulent genotypes among dyspeptic patients from Chittagong, Bangladesh. Malaysian J Microbiol. 2017;13(1):33–40.
- El-Toukhy N, Saeed AM, Emara NM. Clinical relevance of the cagA, vacA and babA2 virulence factors of Helicobacter pylori in Egyptian patients with gastroduodenal diseases. Int J Adv Res. 2016;4(4):1002–1020.
- Saeidi Y, Pournajaf A, Gholami M, et al. Determination of Helicobacter pylori virulence-associated genes in duodenal ulcer and gastric biopsies. Med J Islam Repub Iran. 2017;31:95.
- Ben Mansour K, Fendri C, Zribi M, et al. Prevalence of Helicobacter pylori vacA, cagA, iceA and oipA genotypes in Tunisian patients. Ann Clin Microbiol Antimicrob. 2010;9:article 10.
- Souod N, Sarshar M, Dabiri H, et al. The study of the oipA and dupA genes in Helicobacter pylori strains and their relationship with different gastroduodenal diseases. Gastroenterol Hepatol Bed Bench. 2015;8:S47–S53.
- Sallas ML, Melchiades JL, Zabaglia LM, et al. Prevalence of Helicobacter pylori vacA, cagA, dupA and oipA genotypes in patients with gastric disease. Adv Microbiol. 2017;7:1–9.
- Torres K, Valderrama E, Sayegh M, et al. Study of the oipA genetic diversity and EPIYA motif patterns in cagA-positive Helicobacter pylori strains from Venezuelan patients with chronic gastritis. Microb Pathog. 2014;76:26–32.
- Farzi N, Yadegar A, Aghdaei HA, et al. Genetic diversity and functional analysis of oipA gene in association with other virulence factors among Helicobacter pylori isolates from Iranian patients with different gastric diseases. Infect Genet Evol. 2018;60:26–34.
- Shiota S, Matsunari O, Watada M, et al. Systematic review and meta-analysis: the relationship between the Helicobacter pylori dupA gene and clinical outcomes. Gut Pathol. 2009;2:1–6.
- Lu H, Hsu PI, Graham DY, et al. Duodenal ulcer promoting gene of Helicobacter pylori. Gastroenterology. 2005;128:833–848.
- Arachchi HS, Kalra V, Lal B, et al. Prevalence of duodenal ulcer-promoting gene (dupA) of Helicobacter pylori in patients with duodenal ulcer in North Indian population. Helicobacter. 2007;12:591–597.
- Nguyen LT, Uchida T, Tsukamoto Y, et al. Helicobacter pylori dupA gene is not associated with clinical outcomes in the Japanese population. Clin Microbiol Infect. 2010;16:1264–1269.
- Alam J, Maiti S, Ghosh P, et al. Significant association of the dupA gene of Helicobacter pylori with duodenal ulcer development in a South-east Indian population. J Med Microbiol. 2012;61:1295–1302.
- Salih AM, Goreal A, Hussein NR, et al. The distribution of cagA and dupA genes in Helicobacter pylori strains in Kurdistan region, northern Iraq. Ann Saudi Med. 2013;33(3):290–293.
- Takahashi A, Shiota S, Matsunari O, et al. Intact long-type dupA as a marker for gastroduodenal diseases in Okinawan subpopulation, Japan. Helicobacter. 2013;18(1):66–72.
- Jung SW, Sugimoto M, Shiot S, et al. The intact dupa cluster is a more reliable Helicobacter pylori virulence marker than dupA alone. Infect Immun. 2012;80(1):381–387.
- Hussein NR. The association of dupA and Helicobacter pylori-related gastroduodenal diseases. Eur J Clin Microbiol Infect Dis. 2010;29:817–821.
- Alam J, Ghosh P, Ganguly M, et al. Association of intact dupA (dupA1) rather than dupA1 cluster with duodenal ulcer in Indian population. Gut Pathog. 2015;7:9. PMID: 25829953.
- Markovska R, Boyanova L, Yordanov D, et al. Helicobacter pylori oipA genetic diversity and its associations with both disease and cagA, vacA s, m, and i alleles among Bulgarian patients. Diagn Microbiol Infect Dis. 2011;71:335–340.
- Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629–641.
- Zhang Z, Zheng Q, Chen X, et al. The Helicobacter pylori duodenal ulcer promoting gene, dupA in China. BMC Gastroenterol. 2008;8:49.
