ABSTRACT
Introduction
Uremic pruritus is one of the most common and complicated symptom affecting hemodialysis patients, it can be persistent, distressing and having a significant impact on the quality of life. We aimed to study the relationship between high sensitive C reactive protein and adequacy of dialysis with uremic pruritus in hemodialysis patients.
Patients and Methods
In this Case control study one hundred prevalent hemodialysis patients were enrolled from hemodialysis units in Ain Shams University hospitals, fifty of them had pruritus and the other fifty patients had no pruritis, all of them underwent urea reduction ratio for dialysis adequacy assessment, pruritus assessment by using visual analog scale and high sensitive C reactive protein serum level.
Results
Uremic pruritus has statistically significant negative correlation with urea reduction ratio and consequently with dialysis adequacy. However, uremic pruritus has statistically significant positive correlation with highly sensitive C reactive protein serum levels in hemodialysis patients.
Conclusion
Inadequate hemodialysis and increased hsCRP serum level play an important role in aggravating intensity and severity of uremic pruritus.
1. Introduction
Hemodialysis is one of the most important methods of treatment in patients with chronic kidney disease. Removal of the excess materials and maintaining the stability of the body’s internal environment, are the goals of hemodialysis. It is also a process of removing the toxic and poisonous substances that cause permanent or fatal damages [Citation1]. Inadequate dialysis is responsible for the high mortality of patients with end stage renal disease. Apart from duration of dialysis and blood flow rates, body surface area of the patient, composition of diet and nutritional status may also influence the adequacy of dialysis [Citation2]. Urea reduction ratio greater than 60% and a Kt/V greater than 1.2 are recommended for adequate hemodialysis. Inadequate dialysis is associated with uremia, anemia, fluid overload, hyperkalemia, hyperphosphatemia, pruritus, restless leg syndrome, dialysis-related B2 microglobulin, and Aluminum toxicity [Citation3]. The pathogenesis of chronic kidney disease-associated pruritus remains obscure. A variety of patient characteristics and dialysis parameters have been associated, with the increased burden of pruritus. These include lower dialysis adequacy, use of low- (versus high-)flux dialyzer [Citation4], hepatitis C positivity, high serum high sensitive c reactive protein, higher serum calcium and/or phosphorus levels, low serum albumin level, increased ferritin level, older age, male sex, and underlying depression [Citation5]. Newer hypotheses are focusing on opioid-receptor derangements and microinflammation as possible causes of chronic kidney disease-associated pruritus. Pruritus may be extremely difficult to control, as therapeutic options are limited. The most consequential approaches to treatment are: topical treatment with or without anti-inflammatory compounds or systemic treatment with (a) gabapentin, (b) μ-opioid receptor antagonists and κ-agonists, (c) drugs with an anti-inflammatory action, (d) phototherapy, or (e) acupuncture. A stepwise approach is suggested starting with emollients and gabapentin or phototherapy as first-line treatments. In refractory cases, more experimental options as μ-opioid-receptor-antagonists (i.e., naltrexone) or κ-opioid-receptor agonist (nalfurafine) may be chosen [Citation6]. It must be noted that uremic pruritus may be difficult to be differentiated from pruritus caused by non-renal diseases frequently associated with chronic kidney disease, such as liver diseases (hepatitis B and C infections) [Citation7].
Table 1. Comparison between pruritic and nonpruritic group as regard demographic data, etiology of end stage renal disease.
Table 2. Comparison between pruritic and nonpruritic group as regard high sensitive C reactive protein serum level, urea reduction ratio, and other laboratory tests.
Table 3. Descriptive data of pruritus parameters measured by visual analog scale and pruritus scoring system.
Table 4. Correlation between high sensitive C reactive protein, urea reduction ratio, phosphorus, and pruritus parameters among pruritic patients.
Table 5. Correlation between high sensitive C reactive protein and urea reduction ratio among patients with pruritus.
2. Aim of the study
The present work aimed to study the relationship between high sensitive C reactive protein and adequacy of dialysis with uremic pruritus in hemodialysis patients.
3. Patients and methods
This Case control study was conducted on one hundred end stage renal disease (ESRD) patients on maintenance hemodialysis for more than three months. All patients were older than 18 years old and used Arteriovenous fistula and synthetic graft as a vascular access.
They were selected from hemodialysis units in Ain Shams University hospitals and subdivided into 2 groups: Fifty pruritic patients and Fifty non- pruritic patients.
In all patients, hemodialysis was performed three times a week for 4 hours on average, using the same low flux dialyzer. A bicarbonate dialysis solution was used during hemodialysis sessions with an average flow rate of 300 ml/h. Periodic follow up visits were done for patients in their dialysis units.
Patients with active infection, recent hospitalization within 3 months, psychotic illness or other communication problems, primary skin disorders, active malignancy, cholestatic liver disease, or active hepatitis or patients with positive hepatitis C virus antibody were excluded from the study.
All patients were subjected to: full history including history of diabetes, hypertension, and etiology of end stage renal disease, clinical examination and laboratory investigations as calcium (Ca), parathyroid hormone (PTH), phosphorus, hemoglobin, serum creatinine, sodium (Na), potassium (K), and liver enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST), also albumin, total protein, bilirubin, and high sensitive C reactive protein serum level (were detected by ELISA which intended for the quantitative determination of C-reactive protein (CRP) in human serum. Enhanced sensitivity measurements of CRP can be useful for the detection and evaluation of infection, tissue injury, inflammatory disorders) [Citation8]. Urea reduction ratio (URR) was done for dialysis adequacy assessment, (urea pre – urea post)/urea pre) 100%. Dialysis was adequate if Urea reduction ratio more than 60% [Citation9], and pruritus assessment by using visual analog scale (VAS) [Citation10] which assess intensity of pruritus and pruritus scoring system [Citation11], which assess severity, distribution of pruritus, and sleep disturbance).
3.1. Statistical analysis
Data were collected, revised, coded, and entered to the Statistical Package for Social Science (IBM SPSS) version 23. The quantitative data were presented as mean, standard deviations and ranges when their distribution found parametric and median with interquartile range (IQR) when their distribution found nonparametric. In addition, qualitative variables were presented as number and percentages.
The comparison between groups regarding qualitative data was done by using Chi-square test.
The comparison between two independent groups with quantitative data and parametric distribution were done by using Independent t-test while data with nonparametric distribution were done by using Mann-Whitney test.
4. Results
Regarding demographic data and the etiology of ESRD, this table shows no significant difference between the two groups, also as regard body mass index there was no significant difference between the two groups.
The comparison between high sensitive C reactive protein levels in the two groups showed statistically significant increase in high sensitive C reactive protein in pruritic group than in non-pruritic (p value <0.001).
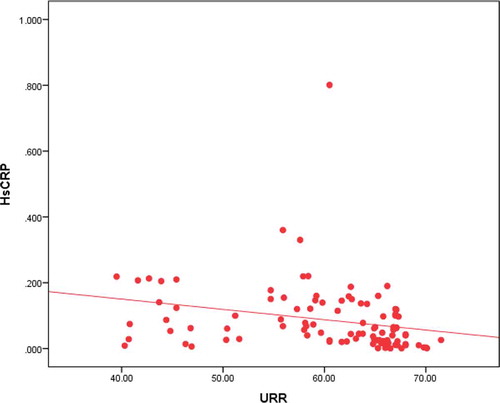
Also, as regard dialysis adequacy assessed by urea reduction ratio in the two groups showed statistically significant decrease in pruritic group than in nonpruritic group (p value 0.017).
In addition, there was a statistically significant increase in serum phosphorus (p value 0.037) and serum bilirubin (p value 0.019) in pruritic group rather than in nonpruritic group.
VAS which measure intensity of pruritus range from 0 to 10 with a mean 2.41 ± 2.99 and median 0.5 (0.0–5.0).
Regarding VAS score, we used a parametric scale as VAS possesses interval properties, therefore, it can be treated as numerical data. For this reason, we did not classify the patients into qualitative categories.
Pruritus scoring system showed:
Severity of pruritus: range from 0 to 10 with a mean 2.75 ± 3.08 and median 1.5 (0.0–6.0).
Distribution of pruritus: range from 0 to 6 with a mean 2.09 ± 2.23 and median 1(0.0–4.0).
Sleep disturbance: range from 0 to 15 with a mean 1.17 ± 2.72and median 0 (0.0–0.0).
Total range of Pruritus scoring system was 0–45 with mean 7.43 ± 9.83 and median 2.5 (0.0–12.0).
It shows statistically significant positive correlation between high sensitive C reactive protein and intensity of uremic pruritus measured by visual analog scale (p value 0.001) (). And as regard pruritus scoring system parameters it was statistically significant positive correlated with severity of pruritus (p value 0.001), distribution of pruritus (p value 0.001), and with sleep disturbance (p value 0.010).
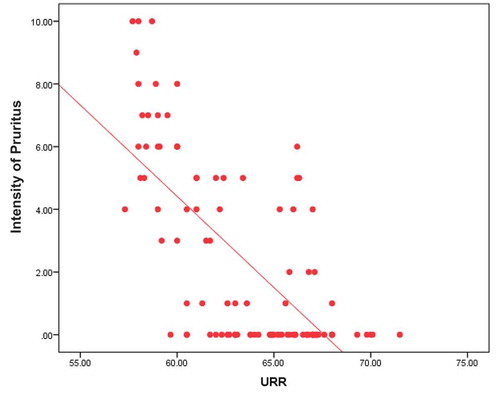
In addition, it shows statistically significant negative correlation between dialysis adequacy assessed by urea reduction ratio and intensity of uremic pruritus measured by visual analog scale (p value <0.001) (). In addition, as regard pruritus scoring system parameters it was statistically significant negative when correlated with severity of pruritus (p value <0.001), distribution of pruritus (p value <0.001), and with sleep disturbance (p value <0.001).
Phosphorus level was directly correlated with intensity of UP measured by VAS (p value 0.006). Also as regard pruritus scoring system parameters it was directly correlated with severity, sleep disturbance (p value 0.032), and was not correlated with distribution (p value 0.384).
Figure 1. Correlation between high sensitive C reactive protein and intensity of uremic pruritus measured by visual analog scale.
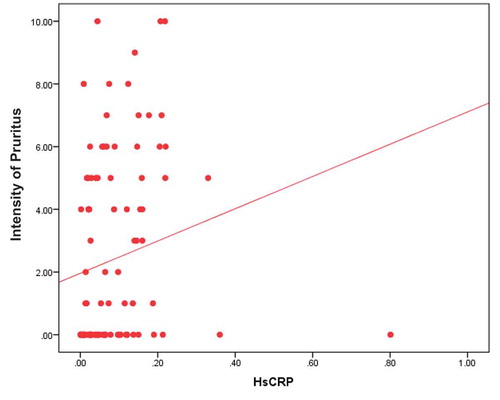
Figure 2. Correlation between urea reduction ratio and intensity of uremic pruritus by visual analog scale.
It shows statistically significant negative correlation between high sensitive C reactive protein and dialysis adequacy which was assessed by using urea reduction ratio with (p value 0.011) ().
5. Discussion
In our study, we found statistically highly significant positive correlation between high levels of high sensitive C reactive protein and uremic pruritus (p value <0.001). Similarly, another study showed that uremic pruritus is associated with high levels of high sensitive C reactive protein in agreement with the thought that inflammation is principally associated with uremic pruritus in the hemodialysis patients and the patients with severe skin pruritus may have higher high sensitive C reactive protein levels. High sensitive C reactive protein elevation predicts a worse outcome and high mortality in the hemodialysis patients [Citation4]. It appears that clinicians need to resolve the underlying inflammatory status to improve the patients’ survival on dialysis. In general, the skin of the end-stage renal disease patients with uremic pruritus has an increased number of mast cells, and they release some substances that can initiate the inflammatory processes [Citation12]. In addition to skin inflammation is further complicated by the release of interleukin 2 and tumor necrosis factor α secondary to pruritus. Impairment of T helper (TH) cells balance with T helper 1 predominance can also support systemic inflammation in uremic pruritus [Citation13].
High sensitive C reactive protein was statistically positive correlated with intensity of uremic pruritus which was measured by visual analog scale (p value = 0.001) and statistically positive correlated with severity of pruritus (p value = 0.001), distribution of pruritus (p value = 0.001), and sleep disturbance (p value = 0.010), which were measured by pruritus scoring system. Patterns Study a large-scale observational study of hemodialysis patient outcomes in 12 different countries. They have previously found a strong association between inflammation, measured by high sensitive C reactive protein or inflammatory cytokines, and sleep disturbance in dialysis patients. In this study, being a poor sleeper was only mortality predictor after adjusting for pruritus and (high sensitive C reactive protein) (moderate/severe uremic pruritus) [Citation14]. Nevertheless, other researchers have not confirmed the positive correlation between high sensitive C reactive protein concentration and pruritus [Citation15].
Our study results also showed that there was statistically significant negative correlation between adequacy of dialysis assessed by urea reduction ratio and uremic pruritus (p value = 0.024). Interestingly, the two pruritic scales we used in our study visual analog scale (p value < 0.001) and pruritic scoring system (p value < 0.001) were higher in patients with low urea reduction ratio. In addition, statistically negative correlation with severity of pruritus (p value <0.001), distribution of pruritus (p value <0.001), and sleep disturbance (p value <0.001). These findings support the idea that adequate dialysis may be critically important in reducing uremic pruritus in hemodialysis patients as uremic pruritus has been associated with the accumulation of middle molecular weight uremic toxins such as β2-microglobulin and dialysis clearance of β2-microglobulin with hemodialysis treatment using a high-flux membrane has been reported to be much higher with adequate dialysis patients and consequently with reduced uremic pruritus [Citation4]. Similarly, early study of chronic kidney disease associated pruritus reported high prevalence rates suggesting the negative correlation between dialysis adequacy and uremic pruritus [Citation16]. In addition, another study used visual analog scale as a material for pruritus assessment, high levels of blood urea nitrogen and β2-microglobulin showed increased scores for blood urea nitrogen and β2-microglobulin [Citation17].
Our study did not find any association between Parathyroid hormone levels and pruritus. This finding is in agreement with that of another study, which had shown that parathyroid hormone levels were not correlated to pruritus in the patients on hemodialysis [Citation18]. Conversely, another study found that, uremic pruritus severity is associated with high parathyroid hormone level due to its major effect on serum phosphorous and calcium levels [Citation19].
In our study, statistically significant association was found between high serum phosphorus level and pruritus (p value = 0.011) but with serum calcium, level it was not significant.
Our study was conducted on 100 patients 56 males and 42 females; however, there was no association between the uremic pruritus and the population-based characteristics of the patient’s sex. Another study had been found that Male gender associated with chronic kidney disease associated pruritus as in the study of the Dialysis Outcomes and Practice Patterns Study database, male gender was associated with a 1.1 greater adjusted odd of having moderate to severe pruritus [Citation20]. In addition, in Japanese cohort study, male gender was associated with ~1.5 greater adjusted odds of moderate or severe pruritus [Citation21].
However, in smaller and unadjusted analyzes, gender was not being consistently correlated with more severe pruritus similar to our study [Citation22].
As regard different causes of renal failure were found in our studied cases included diabetes mellitus, polycystic kidney disease, hypertensive nephrosclerosis, analgesic nephropathy, Lupus nephritis, amyloidosis, preeclampsia, cardiorenal syndrome, and on basis of our study data we found that there was no association between the cause of renal failure and pruritus. Like our results, another study found that uremic pruritus is not associated with the cause of renal failure, but it may be due to a combination of coexisting medical problems, particularly diabetes mellitus, lung disease, cardiovascular disease, neurological disease, liver disease, smoking, and higher body mass index [Citation22].
In addition, there was a statistically significant increase in serum bilirubin (p value = 0.019) in pruritic group rather than in non-pruritic group.
The cause of the elevated serum bilirubin levels remains unclear, a possible explanation was addressed in a previous publication in which it is noted that serum bilirubin levels are linked to a TA‐repeat UGT1A1*28 polymorphism in the promoter region of the hepatic bilirubin uridine diphosphate‐glucuronosyltransferase (UGT1A1) gene, which is the main gene responsible for bilirubin degradation and predicts long‐term cardiovascular events and mortality in chronic hemodialysis patients [Citation23].
6. Conclusion
Inadequate hemodialysis and increased hsCRP serum level play an important role in aggravating intensity and severity of uremic pruritus.
Disclosure statement
The authors report no conflict of interest.
Additional information
Notes on contributors
Iman I. Sarhan
Professor Dr. Iman Ibrahim Sarhan, Professor of Internal Medicine and Nephrology, Ain Shams University , Faculty of Medicine, member of Egyptian Society of Nephrology and transplantation.
Mona Abdel-Halim Ibrahim
Dr Mona Abdelhalim Ibrahim, Lecturer of Dermatology, Venerology and andrology Ain Shams University, member of International Society for cutaneous lymphoma.
Nahla M. Teama
Dr Nahla Mohamed Teama, Lecturer of Internal Medicine and Nephrology Ain Shams University, Faculty of Medicine, member of Egyptian Society of Nephrology and Transplantation.
References
- Tayyebi A, Samanehshasti TD, Eynollahi B, et al. The relationship between blood pressure and dialysis adequacy in dialysis patients. Iran J Crit Care Nurs. 2012;5(1):49–52.
- Rocco MV, Lockridge RS Jr, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the frequent hemodialysis network nocturnal trial. Kidney Int. 2011;80(10):1080–1091.
- Amini M, Aghighi M, Masoudkabir F, et al. Hemodialysis adequacy and treatment in Iranian patients: a national multicenter study. Iran J Kidney Dis. 2011;5(2):103–109.
- Ko MJ, Wu HY, Chen HY, et al. Uremic pruritus, dialysis adequacy, and metabolic profiles in hemodialysis patients: a prospective 5-year cohort study. PLoS One. 2013;8(10):e71404.
- Narita I, Alchi B, Omori K, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69(9):1626–1632.
- Rayner H, Baharani J, Smith S, et al. Uraemic pruritus: relief of itching by gabapentin and pregabalin. Nephron Clin Pract. 2013;122(3–4):75–79.
- Manenti L. Tansinda P and Vaglio A. Uraemic pruritus: clinical characteristics, pathophysiology and treatment. Drugs. 2009;69(3):251–263.
- Bazzino O, Ferreiros ER, Pizarro R, et al. C-reactive protein and the stress tests for the risk stratification of patients recovering from unstable angina pectoris. Am J Cardiol. 2001;87(11):1235–1239.
- Weinstein R, Kershaw G, Bailey J, et al. Safety and efficacy of autologous hemopoietic progenitor cell collection in tandem with hemodialysis in multiple myeloma with myeloma cast nephropathy. J Clin Apher. 2014;29(2):83–89.
- Ashmore SD, Jones CH, CG N, et al. Ondansetron therapy for uremic pruritus in maintenance hemodialysis patients. Am J Kidney Dis. 2000;35(5):827–831.
- Pauli-Magnus C, Mikus G, Alscher DM, et al. Naltrexone does not relieve uremic pruritus: results of a randomized, placebo-controlled crossoverstudy. J Am Soc Nephrol. 2000;11(3):514–519.
- Snit M, Gawlik R, Lacka-Gazdzik B, et al. Substance P and intensity of pruritus in hemodialysis and peritoneal dialysis patients. Med Sci Monit. 2013;19:723–732.
- Fallahzadeh MK, Roozbeh J, Geramizadeh B, et al. Interleukin-2 serum levels are elevated in patients with uremic pruritus: A novel finding with practical implications. Nephrol Dial Transplant. 2011;26(10):3338–3344.
- Razeghi E. Tavakolizadeh S and Ahmadi F. Inflammation and pruritus in hemodialysis patients. Saudi J Kidney Dis Transpl. 2008;19:62–66.
- Mettang T, Krumme B, Bohler J, et al. Pentoxifylline as treatment for uraemic pruritus-an addition to the weak armentarium for a common clinical symptom? Nephrol Dial Transplant. 2007;22:2727–2728.
- Aoki R, Kawamura T, Goshima F, et al. Mast cells play a key role in host defense against herpes simplex virus infection through TNF-α and IL-6 production. J Invest Dermatol. 2013;133(9):2170–2179.
- Mettang T, Pauli-Magnus C, Alscher DM. Uraemic pruritus - new perspectives and insights from recent trials. Nephrol Dial Transplant. 2002;17(9):1558–1563.
- Welter Ede Q, Frainer RH, Maldotti A, et al. Evaluating the association between alterations in mineral metabolism and pruritus in hemodialysis patients. A Bras Dermatol. 2011;86(1):31–36.
- Narita I, Iguchi S, Omori K, et al. Uremic pruritus in chronic hemodialysis patients. J Nephrol. 2008;21(2):161–165.
- Pisoni RL, Wikstrom B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495–3505.
- Kimata N, Fuller DS, Saito A, et al. Pruritis in hemodialysis patients: results from the Japanese Dialysis Outcomes and Practice Patterns StudyO (JDOPPS). Hemodial Int. 2014 Jul;18(3):657–667.
- Ramakrishnan K, Bond TC, Claxton A, et al. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int J Nephrol Renovasc Dis. 2013;7:1–12.
- Chen YH, Hung SC, Tarng DC. Serum bilirubin links UGT1A1*28 polymorphism and predicts long‐term cardiovascular events and mortality in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(3):567–574.