?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Introduction: Exercise-induced bronchospasm (EIB) is a transient narrowing of the airway that usually occurs shortly after exercise. It occurs commonly in people with asthma; however EIB has also been reported in individuals without clinical asthma. The study aimed to determine the prevalence and factors associated with EIB among secondary school students.
Methods: It was a descriptive cross-sectional school- based study involving secondary school students aged between 10 and 17 years without previous history of asthma. Subjects undertook a six-minute running test, spirometry was done and the best of three FEV1 readings were obtained pre-exercise, at 5, 10, 15 and 30 minutes post exercise. The diagnosis of EIB was made when there was a decrease in FEV1 of ≥10% from baseline after exercise.
Results: Of the 265participants studied, 34 (12.8%) had EIB at 5 minutes post exercise. EIB was significantly more in the 10–13 years age group (χ2 = 18.416, p = <0.001), there was no significant gender difference. The presence of allergic (vernal) conjunctivitis and rhinitis were both significantly associated with the development of EIB (χ2 = 13.574, p < 0.001 and 0.011 respectively). There was no significant association with previous history of wheeze and exposure to indoor air pollution such as cooking with biomass fuel and parental cigarette smoking.
Conclusion: EIB exist among non-asthmatic school children, and it is more frequent in the early adolescent age-group. Students with allergic conjunctivitis and rhinitis are more likely to manifest exercise induced bronchospasm.
1. Introduction
Exercise-induced bronchospasm (EIB) is a transient airway narrowing that usually occurs shortly after or rarely during exercise [Citation1]. It is a manifestation of airway hyper-responsiveness. Exercise induced bronchospasm can manifest clinically with cough, wheezing, chest tightness or pain and unusual shortness of breath after a burst of strenuous and continuous aerobic exercise [Citation1,Citation2]. Some individuals with EIB may however have a normal physical examination.
The objective diagnosis of EIB is made when there is a decrease in FEV1 of ≥10% from baseline after exercise, with or without symptoms [Citation2,Citation3]. Exercise challenge test used to detect EIB include running on treadmill, bicycle ergometer or a free range nonstop running exercise. The treadmill or bicycle ergometer are standardized and can easily be controlled, however these equipment are relatively expensive [Citation4]. The free range running method is inexpensive and sophisticated equipment is not required for performing it. The free range nonstop exercise has been used in the community settings to evaluate students [Citation5,Citation6]. It has an advantage that it mimics the normal physical activity the individual undertakes on daily basis and it has increased tendencies to precipitate bronchospasm [Citation1,Citation3].
Exercise-induced bronchospasm has been reported in patients with or without asthma, and in individuals with atopy and allergic rhinitis [Citation2]. In the general population, EIB occur in more than 10% of individuals with no history of allergy or asthma, while it may be up to 40–50% in those with allergic rhinitis and about 90% in asthmatic individuals [Citation7]. Epidemiological surveys have shown that the prevalence of EIB in children from the general population varies widely [Citation7,Citation8]. Ng’ang’a et al [Citation9], reported a prevalence of 22.9% among urban school children in Kenya while Kuti et al [Citation6] recorded a prevalence of 23.8% among school children in south west Nigeria. Both studies used a ≥ 10% decrease in FEV1 after a running exercise as diagnostic criteria. Addo-Yobo et al in a study conducted among Ghanaian school children recorded a prevalence rate of 8.3% [Citation10]. These varying prevalence rates are due to the different cutoff values used for diagnosing and also the methods used to detect the response (FEV1 or PEFR).
Exercise induced bronchospasm is often a neglected diagnosis, however it is an important condition as it may limit exercise performance and quality of life of an individual if undetected and untreated. The prevalence of EIB within the non-asthmatic population remains understudied in Nigerian children; in particular, the prevalence of EIB in non-asthmatic school children in Osogbo is unknown. There is thus a need for more studies to allow the prevalence of EIB in individuals without asthma to be better understood. The study aimed to determine the prevalence and factors associated with EIB among secondary school students.
2. Materials and methods
2.1. Study area
The study was conducted in Osogbo local government area of Osogbo. Osogbo is the capital city of Osun state in south-west Nigeria. It lies at 318 m or 1043 feet above sea level.
2.2. Study design
The study was a descriptive cross-sectional school based study involving 265 secondary school students aged between 10 and 17 years. The study was carried out over a period of nine months (January 2018- September 2018). Subjects were eligible for the study if they were between 10–17 years, gave assent and whose care-givers gave informed consent. The students were excluded from the study when there was suspected heart disease, musculoskeletal symptoms (deformity) or any other illness or conditions that could interfere with exercise tolerance. Also, students with history of asthma, abnormal pre-exercise respiratory and cardiac findings such as wheeze, rhonchi and abnormal pulse rate or heart sounds, those unable to carry out an acceptable lung function test, and students who do not attain the predicted maximum heart rate after the six minutes of exercise were excluded from the study.
2.3. Sample size determination
The minimum number of subjects required for this study was determined using the Leslie-Fishers formula [Citation11]:
Where Z is a constant usually set at 1.96, P is a predetermined value of the prevalence of EIB obtained from previous study (6.0%) [Citation5], Q is the proportion of the population not involved in the study i.e. 1-P and D is the degree of accuracy desired usually set as 0.05.
Minimum sample size was determined to be 241. A total of 265 pupils were studied.
2.4. Subject recruitment
Subjects were recruited into the study by multi-stage sampling technique. From the two local government area (LGA) in Osogbo, one LGA was selected by simple balloting using numbers 1 and 2. Number 1 (Osogbo LGA) was randomly selected. The list of secondary schools in the selected local government area (Osogbo LGA) was obtained from the Local Education Authority. Ten per cent of the total number of secondary schools in the selected (Osogbo) local government area was studied. There were 47 secondary schools in Osogbo LGA (16 public and 31 private secondary schools) Ratio of public: private was 1: 1.81 ≈ 1: 2. Thus ten per cent of schools selected by random sampling were two public and four private schools (1: 2). There was proportional allocation of respondents to the selected schools depending on number of pupils in each school. An arm was selected from all the arms of each class by simple random sampling technique (balloting method). List of students within the age group 10–17 years from the selected classes was obtained. Subjects were selected by picking the pupil whose name appear after every 5 pupils from the list until the proportional sample size was completed.
2.5. Study procedure
Questionnaires were administered to the participants using interviewer method. The demographic data, symptoms of allergies, history of wheeze, exposures to indoor air pollution (biomass fuel and cigarette smoke) were obtained. The socio-economic classification was done as described by Ogunlesi et al [Citation12]. Features of vernal (allergic) conjunctivitis sought for were recurrent eye itching, brownish discoloration and/or watery discharge [Citation13]. Features of allergic rhinitis were excessive sneezing, recurrent sniffing, recurrent runny nose and itchy throat in the absence of cold [Citation14]. Eczema was defined as recurrent itchy, scaly, dry skin. Their anthropometric measurements i.e. weight, height were recorded, using a weighing scale with an inbuilt height meter (Bright future Medical- England, model RGZ-160) after ensuring that they were minimally dressed. The Body mass index (BMI) was calculated using Weight (kg) divide by Height (meter)2. The BMI percentile was interpreted as <5 percentile being underweight, ≥ 5 and <85 percentile as normal weight, ≥ 85 and < 95 percentile as overweight and ≥ 95 percentile as obesity. The clinical findings including features of allergies were documented. The pre- and post-exercise respiratory rate and heart rate were also recorded.
2.6. Spirometry and exercise challenge
The spirometry procedure was explained and demonstrated to all the children. The subjects had a practice session pre-exercise to ensure the technique had been mastered. The spirometer was calibrated daily with a 3-Liter calibration syringe. A new disposable mouthpiece was attached to the spirometer for each participant to ensure strict infection control. The subjects were instructed to breathe in as deeply as possible to full inspiration, hold his or her breath just long enough to seal the lips around the mouth piece, and then blow maximally. Nose clip was worn by the participants to prevent air leakage through the nostrils. The spirometry was performed in the sitting position using the Minispir® light spirometer. The FEV1 measurements (pre-exercise) were obtained; the best FEV1 of three acceptable tests was recorded. The predicted reference values used in this study were based on the data of Knudson et al using their gender, weight and height [Citation15]. A six-minute free running test was done, at the end of each sixth minute; the whistle was blown to stop the exercise session for each subject. (The running test was done at same time every day in order to limit the effect of variation in environmental conditions). Their heart rate was expected to reach at least 80% of predicted maximum heart rate (HRmax) after the six-minute exercise [Citation16]. The predicted maximum heart rate was calculated using the formula; 208–0.7 × age [Citation17].
The percentage change in FEV1 post-exercise was calculated using the following equation [Citation3,Citation16]:
A reduction in FEV1 of ≥10% from the baseline value was considered a positive EIB test. Post-exercise measurements (the best FEV1 of three acceptable tests) were taken at 5, 10, 15 and 30 minutes. The FEV1 measurements recorded were those that met the ATS/ERS acceptability criteria [Citation18].
2.7. Data management and analysis
Data was analyzed using Statistical Package for Social Sciences (SPSS) version 20.0 (SPSS Chicago Inc., IL, U.S.A). The prevalence of EIB among the students was calculated by proportion of student with EIB over the total number of study participants. The relationship between categorical variables was determined using Pearson’s Chi-square test and Fisher’s exact test. The level of significance was set at p-value less than 0.05.
2.8. Ethics
The procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in the year 2000. Ethical approval for the study was obtained from the Ethics and Research Committee of Ladoke Akintola University of Technology (LAUTECH) Teaching Hospital, Osogbo, Osun State (LTH/EC/2016/01/256). Approval for the study was also obtained from the Osun State Ministry of Education. Permission was obtained from the school principals and class teachers of the pupils before proceeding with the study. The study objectives and procedures were explained to participants, parental consent and assent was obtained from the participants.
3. Results
A total of 265 students who met the study criteria were enrolled. Of these, 138 (52.1%) were females and 127 (47.9%) were males giving a male to female ratio of 0.9: 1. One hundred and twenty-two students (46.0%) belong to the lower social class. Twenty-three (8.7%) participants had allergic features. ()
Table 1. Socio-demographic, anthropometric characteristics, allergic symptoms and FEV1 measurements
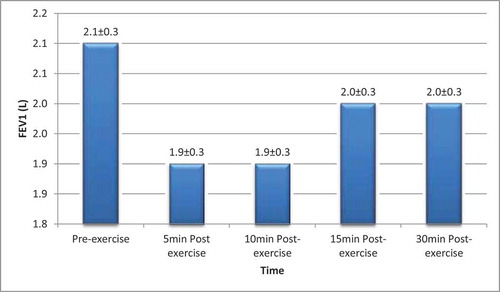
The Mean FEV1 of the participants before and after exercise is as shown in .
3.1. Prevalence of exercise-induced bronchospasm
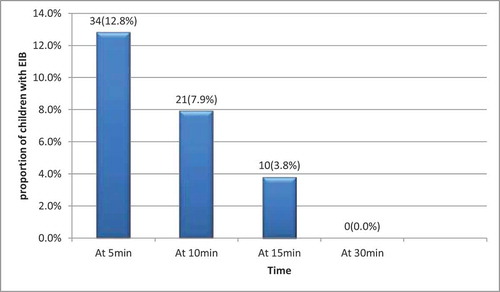
Thirty-four (12.8%) of the 265 participants had EIB at five minutes post exercise, this value reduced to 21(7.9%) at 10 minutes post exercise and 10 (3.8%) at 15 minutes post exercise. No student had EIB at 30 minutes post exercise. ()
3.1.1. Association between socio-demographic profile, anthropometric characteristics and presence of exercise-induced bronchospasm at 5 minute post-exercise
Participants between ages 10–13 years had a higher frequency of EIB. There was a statistically significant association between age and EIB (χ2 = 18.416, p < 0.001). The gender, social class and BMI percentile did not show any significant association with EIB. ()
Table 2. Association between socio-demographic profile, anthropometric characteristics and exercise-induced bronchospasm at 5 minute post-exercise
3.1.2. Association between EIB and allergic features, exposure to smoke from biomass fuel and cigarette smoke
The presence of vernal (allergic) conjunctivitis and allergic rhinitis were significantly associated with EIB among the participants (χ2 = 13.574, p < 0.001 and p = 0.011 respectively). Cooking with biomass fuel and exposure to parental cigarette smoke were not significantly associated with the development of EIB. ()
Table 3. Association between allergic features, exposure to biomass fuel, parental cigarette smoke and exercise induced-bronchospasm at 5 minute post-exercise
4. Discussion
This study shows that EIB exist among apparently healthy secondary school students in Osogbo, with a prevalence of 12.8%. The prevalence observed in this study is comparable to the 13.2% reported by Sudhir et al among urban school children in India [Citation19]. The prevalence of EIB in this study is however lower than the 23.8% reported by Kuti et al in Ilesa, Nigeria [Citation6]. The disparity in the prevalence rates in these studies may be explained by the difference in study participants. Though the study was also conducted among children in an urban setting, the inclusion of children with asthma may explain the higher prevalence. Higher prevalence of EIB has been reported in people previously diagnosed with asthma who are not on anti-inflammatory treatment [Citation20]. These individuals usually experience exacerbation of underlying inflammation and airway hyperactivity during physical activity with resultant bronchospasm. The prevalence of EIB in this study is however higher than the 6.0% reported by Onazi et al among school children in Gusau, Nigeria [Citation5]. The disparity in findings may be due to the different diagnostic criteria. While a decrease in FEV1 of ≥ 10% was used in this study, Onazi et al used a ≥ 15% decrease in PEFR as their diagnostic criteria. The forced expiratory volume in one second (FEV1) is a more accurate measure of airway bronchoconstriction than peak expiratory flow rate [Citation18]. This is because PEFR varies more with each use; it is highly effort dependent and mainly dependent on flow rate in larger airways [Citation21]. Another reason for the difference in the prevalence rate observed in this study and that of Onazi et al may partly be explained by the difference in age categories of the school children evaluated. Onazi et al included younger age school children (5–9 years), while the present study involved school children aged 10–17 years. EIB has been noted to be more in the adolescent than in younger children [Citation5,Citation6].
With regards to the demographic characteristics of the respondents, the early adolescents (age 10–13 years) had a significantly higher frequency of EIB than older children. This is similar to the studies of Onazi et al [Citation5] and Kuti et al [Citation22] who both reported a higher prevalence of EIB in the early adolescent age group; 10–14 and 11–13 years respectively. This finding may be explained by the fact that asthma and related disorders have been observed to be commoner during early puberty, and this is particular so in girls; this is because of the changes in level of female hormones during this period [Citation23]. In addition, this study shows that EIB occurred more commonly in female than males; however this difference was not statistically significant. The gender difference observed in this present study is similar to what was reported by De Baets et al [Citation8] and Joason et al [Citation24]. The higher occurrence in females may be as a result of changes in sex hormone such as estrogen and progesterone during puberty [Citation25]. Evidence have shown that sex hormones play an important role in the development of allergic immune response, estrogen promotes while androgen attenuates allergen-mediated type 2 airway inflammation which is most commonly seen in patients with asthma [Citation26]. Airway inflammation is important in the development of asthma and EIB [Citation27].
In this study, there was no significant association between EIB and BMI. This finding is contrary to previous studies where it was observed that EIB was significantly more frequent in children who are overweight or obese [Citation28]. The absence of EIB in obese children in the present study may be explained by the small number (six) of obese children involved in the study. This finding is, however, similar to some other studies that observed no significant association between EIB and BMI [Citation29]. For instance, Mansournia et al [Citation29] observed that there was no significant association between EIB and BMI, while Lopes et al [Citation30] reports that obesity does not contribute to increased EIB frequency in non-asthmatic adolescents. Findings on EIB and body weight are not universal; hence more longitudinal studies may be needed to ascertain the relationship between BMI and EIB.
In the present study, children in the lower socioeconomic class had the highest frequency of exercise induced bronchospasm, although this difference was not statistically significant. This finding is similar to what was reported by Kuti et al [Citation22] in Nigeria. Other researchers have also reported similar findings [Citation29,Citation31]. Our finding appears to contradict the general belief that EIB and/or asthma are commoner among children of higher socioeconomic class [Citation32]. The reason for this finding is unclear. Other confounding factors that may be related to low social class such as presence of house dust mite, rearing of poultry/pets, were not investigated in this study. However, researchers have postulated that children from low socio-economic class were usually at a higher risk of intestinal parasite and this may contribute to the development of allergy [Citation20].
In our study, allergic (vernal) conjunctivitis and allergic rhinitis were significantly associated with the development of EIB. This finding is in agreement with previous studies [Citation5,Citation6,Citation33]. Atopy (allergy) is a major risk factor for the development of EIB. Epidemiologic data has demonstrated allergic rhinitis in up to 40% of Pediatric population with EIB [Citation2]. Allergic sensitization induces systemic Type-2 T helper-cell immunity which predisposes the individual to airway inflammation thus promoting hyper-reactivity [Citation2]. Eczema and a past history of wheeze were not significantly associated with EIB. Although history of wheeze has been reported in some studies to be associated with EIB [Citation5,Citation6], our finding was quite different. The disparity in findings may be explained by the fact that only one subject had eczema and past history of wheeze. Furthermore, individuals with present history of wheeze and those who had rhonchi on auscultation were excluded from the study.
This study showed no significant association between EIB and exposure to indoor air pollution such as cooking with biofuel mass and parental cigarette smoke. Although this finding is similar to that of some earlier researchers, it is, however, contrary to the general belief that indoor air pollution is significantly associated with asthma and EIB. The reason for this different finding may be because our study participants are school children who stay more outdoor (stay a minimum of eight hours in school) thereby giving them a limited period of exposure to these agents.
In conclusion, this study has shown that EIB occurs in a significant number of non-asthmatic school children, and it is more frequent in the early adolescent age-group. School children with allergic conjunctivitis and rhinitis are more likely to manifest exercise induced bronchospasm.
4.1. Limitation
We did not do skin prick test nor estimated serum Immunoglobulin E (IgE) in the determination of allergic sensitization in our participants.
Acknowledgments
We acknowledge all the students and caregivers who participated in this study. We also thank the principals and the entire staff of the secondary schools who took part in this study for their support and cooperation during the period of data collection.
Disclosure statement
The authors have no conflict of interest to declare.
Additional information
Notes on contributors
A. O. Odeyemi
Dr. Abimbola Odeyemi is a Lecturer and Consultant Paediatrician at the Department of Paediatrics, Bowen University and Bowen University Teaching Hospital, Ogbomoso, Nigeria. Her Research interest is in the area of Lung infection in Children and Pediatric Asthma. She is happily married with Children.
A. O. Odeyemi
Dr. Abiona Odeyemi is a Lecturer and Consultant Physician and Pulmonologist at the Department of Medicine, Bowen University and Bowen University Teaching Hospital, Ogbomoso, Nigeria. His Research interest is in the area of Lung infections, Obstructive Lung Diseases and Sleep Medicine. He is happily married with Children.
O. V. Kayode
Dr. Olamide Kayode is a Consultant Pediatrician at the Paediatrics Unit of Reddington Multi specialty Hospital, Victoria Island, Lagos-state. Nigeria. He is happily married with Children.
S. B. A. Oseni
Dr Saheed Oseni is a Senior Lecturer and Consultant Paediatrician at the Department of Paediatrics and Child Health, Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria. His research interest is in the area of Paediatric Neurology. He is happily married with children.
O. A. Oyedeji
Dr Olusola Oyedeji is an Associate Professor and Consultant Paediatrician at Ladoke Akintola University of Technology (LAUTECH) and LAUTECH Teaching Hospital, Osogbo, Nigeria. His research interest is in the area of Paediatric Pulmonology, allergic and infectious diseases in children. He is happily married with children.
References
- Sinha T, David AK. Recognition and management of exercise-induced bronchospasm. Am Fam Physician. 2003;67(4):769–774.
- Randolph C. Exercise-induced bronchospasm in children. Clin Rev Allerg Immunol. 2008;34(2):205–216.
- Parsons JP, Hallstrand TS, Mastronarde JG, et al. An official American thoracic society clinical practice guideline: exercise -induced bronchoconstriction. Am J Respir Crit Care Med. 2013;187(9):1016–1027.
- Krafczyk MA, Asplund CA. Exercise-induced bronchoconstriction: diagnosis and management. Am Fam Physician. 2011;84:428–434.
- Onazi SO, Orogade AA, Yakubu AM. Exercise -induced bronchospasm among school children in Gusau, Nigeria. West Afr J Med. 2012;3(12):76–80.
- Kuti BP, Omole KO, Kuti DK, et al. Exercise Induced Bronchospasm in Ilesa, Nigeria: A comparative study of rural and urban school children. Am J Respir Crit Care Med. 2017;195:A2223.
- Hough DO, Dec KL. Exercise-induced asthma and anaphylaxis. Sports Med. 1994;18(3):162–172.
- De Baets F, Bodart E, Dramaix-Wilmet M, et al. Exercise-induced respiratory symptoms are poor predictors of bronchoconstriction. Pediatr Pulmonol. 2005;39:301–305.
- Ng’ang’a LW, Odhiambo JA, Mungai MW, et al. Prevalence of exercise induced bronchospasm in Kenyan school children: an urban-rural comparison. Thorax. 1998;53:909–910.
- Addo-Yobo EOD, Woodcock A, Allotey A, et al. Exercise-induced bronchospasm and atopy in Ghana: two surveys ten years apart. PLoSMed. 2007;4(2):e70.
- Araoye MA. Subject selection in research methodology with statistics for health and social sciences. 1st ed. Ilorin (Nigeria): Nathadex Publishers; 2004. p. 115–129.
- Ogunlesi TA, Dedeke IOF, Kuponiyi OT. Socioeconomic classification of children attending specialist paediatric centres in Ogun state, Nigeria. Nig Med Pract. 2008;54(1):21–25.
- Bielory L, Friedlaender MH. Allergic conjunctivitis. Immunol Allergy Clin North Am. 2008;28(1):43–58.
- Druce HM. Allergic and non-allergic rhinitis. In: Middleton EM, Reed CE, Ellis EF, et al., editors. Allergy: principles and practice. 5th ed. Missouri: Mosby St Louis; 1998. p. 1005–1016.
- Knudson RJ, Lebowitz MD, Holberg CJ, et al. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–734.
- Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2000;161:309–329.
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37(1):153–156.
- Miller MR, Crapo R, Hankinson J, et al. Series “ATS/ERS task force Standardisation of lung function testing” General consideration for lung function testing. Eur Respir J. 2005;26:153–161.
- Sudhir P, Prasad CE. Prevalence of exercise-induced bronchospasm in schoolchildren: an urban–rural comparison. J Tropical Pediatr. 2003;49(2):104–108.
- Awopeju OF, Erhabor GE. Exercise and asthma: a review. Afr J Respir Med. 2011; 10–17.
- Adeniyi BO, Erhabor GE. The peak flow meter and its use in clinical practice. Afr J Respir Med. 2011;6:5–8.
- Kuti BP, Kuti DK, Omole KO, et al. Prevalence and factors associated with exercise induced bronchospasm among rural school children in Ilesa, Nigeria. Niger Postgrad Med J. 2017;24:107–113.
- Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. 2017;17(3):19.
- Joason H, Norlander K, Janson C, et al. Nordang L and Emtnar M. the relationship between exercise induced bronchial obstruction and health related quality of life in female and male adolescent from a general population. BMC Pulm Med. 2016;16(1):63.
- Almqvist C, Worm M, Leynaert B. Impact of gender on asthma in childhood and Adolescence: a GA2LEN review. Allergy. 2008;63:47–57.
- Fu I, Freishtat RJ, Gordish-Dressman H, et al. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc. 2014;11(6):939–944.
- Parson JP, Baran CP, Phillips G, et al. Airway inflammation in exercise-induced bronchospasm occurring in athietes without asthma. J Asthma. 2008;45(5):363–367. .
- Del Rio-Navarro B, Cisneros-Rivero M, Berber-Eslava A, et al. Exercise induced bronchospasm in asthmatic and non-asthmatic obese children. Allergol Immuno-pathol. 2000;28:5–11.
- Mansournia MA, Jamali M, Mansournia N. et al. Exercise-induced bronchospasm among students of Tehran University of Medical Sciences. Allergy Asthma Proc. 2007;28:348–352.
- Lopes WA, Radominski RB, Rosario Filho NA, et al. Exercise-induced bronchospasm in obese adolescents. Allergol Immunopathol (Madr). 2009;37(4):175–179.
- Heaman DJ, Estes J. The free running asthma screening test: an approach to screening for exercise induced asthma in rural Alabama. J Sch Health. 1997;67:83–88.
- Liu AH, Covar RA, Spahn JD, et al. Childhood Asthma. In: Kliegman RM, Stanton BF, St. Geme III JW, et al., editors. Nelson textbook of paediatrics. 19th ed. Philadelphia, USA: Saunders; 2011. p. 780–801.
- Mtshali BF, Mokwena KE. The prevalence of exercise-induced asthma among school children. S A Fam Pract. 2009;51(6):489–491.