ABSTRACT
Introduction: Malnutrition is common in patients with End-Stage Renal Disease (ESRD) on hemodialysis (HD) which significantly affects their quality of life. ESRD is associated with thyroid dysfunction which may affect morbidity and mortality. Changes in thyroid function in this population could be a marker of malnutrition. Our aim was the assessment of the nutritional status of patients with ESRD on HD and its association with thyroid function.
Methods: A cross-sectional study was conducted on 84 patients with ESRD on HD. Nutritional status was assessed by anthropometric measurements and Subjective Global Assessment (SGA) Score. Serum FT3, FT4, and TSH concentrations were determined. CBC, kidney function tests, serum albumin, serum iron, total iron-binding capacity, serum cholesterol, and CRP were measured. Patients’ comorbidity status was determined using the Charlson Comorbidity Index (CCI).
Results: The mean SGA score for studied patients was 13.73 ± 4.4, mean values of thyroid functions were: TSH 2.99 ± 2.93uIU/ml; FT4 1.08 ± 0.21 ng/dl and FT3 2.55 ± 0.52 pg/ml. According to SGA score, 26.2% of patients had normal nutritional status, 69% had mild to moderate malnutrition and 4.8% had severe malnutrition. SGA had significant negative correlation with FT3, while there was no significant correlation between it and FT4 or TSH. Serum FT3 concentration inversely correlated with age (r = −0.25, P= 0.02), CCI (r = −0.48, P= 0.0001), CRP (r = −0.46, P= 0.0001), and SGA (r = −0.49, P= 0.0001), and positively correlated with serum albumin (r = 0.47, P= 0.0001). In multivariate regression analysis, SGA was independently associated with FT3 (β, −1.36; 95% confidence interval, −2.5 to −0.2, P= 0.02)
Conclusions: Malnutrition is prevalent among patients with ESRD on HD. FT3 is a marker of malnutrition and could be used as an accessible and reproducible periodical method to detect such states.
1. Introduction
End-stage renal disease (ESRD) leads to a lot of hormonal and metabolic disorders [Citation1]. There is a strong cross relationship between the thyroid function and kidney diseases such that the kidney plays a role in the regulation, metabolism, and elimination of thyroid hormones. The thyroid hormones, on the other hand, are involved in the kidney growth, development, and maintenance of water and electrolyte homeostasis [Citation2,Citation3]. Dialysis is also associated with changes in the levels of circulating thyroid hormones which, in turn, may have prognostic implications. Moreover, drugs used to treat thyroid dysfunction may affect kidney function and drugs treating kidney diseases may affect thyroid function [Citation4]. It has been reported that patients with ESRD have reduced levels of tri-iodothyronine (T3) and thyroxine (T4) with elevated thyroid-stimulating hormone (TSH) in absence of overt hypothyroidism. Subclinical hypothyroidism was reported in 21.82% of patients with ESRD on HD [Citation5]. ESRD affects thyroid functions in several ways through low blood hormone level, reducing the binding to transport proteins, disturbing the peripheral hormone metabolism and inducing the uptake of iodine by the thyroid gland decreasing the tissue hormone quantity [Citation6].
Poor nutritional status and protein energy wasting (PEW) are common among ESRD patients on HD and are considered strong predictors of poor outcome in this population [Citation7]. Many factors are implicated in protein malnutrition and wasting in these patients, including an inadequate nutrient intake, metabolic acidosis, hormonal dysregulation, changes in bowel flora and sustained inflammation. All these factors can result in increased rates of morbidity and mortality [Citation8].
Although thyroid dysfunction is a well-known complication of ESRD, yet, its association with the nutritional status of ESRD patients on HD is not well understood.
Our aim was to assess the nutritional status of ESRD patients on HD and its association with thyroid function.
2. Subjects and methods
From May 2018 till October 2018, a cross-sectional study was conducted on 84 patients with ESRD undergoing HD aged from 30 years to 80 years. They were enrolled from Mit-Khakan Dialysis Unit, Egypt.
2.1. Selection criteria for the patients
The subjects included in this study were selected according to inclusion and exclusion criteria.
2.1.1. Inclusion criteria
Adult patients (Age ≥18 years) with ESRD on maintenance HD for more than 3 months.
2.1.2. Exclusion criteria
Patients with previously identified thyroid illness, patients on medications that might interfere with the thyroid function, patients with acute infection, patient exposed to hospitalization, major surgeries or recent trauma less than 6 months and patients known to have malignancy.
2.2. All cases were subjected to
Detailed medical history, full clinical examination, and laboratory investigations that included: complete blood count (CBC); kidney function tests (serum urea, serum creatinine); serum albumin, serum iron, total iron-binding capacity, serum cholesterol, C-reactive protein (CRP) and serum thyroid hormones (TSH, Free T3 (FT3), and Free T4 (FT4)). The samples were drawn immediately before dialysis session and divided into two tubes. One tube had been centrifuged and plasma separated and kept at −20°C until time of hormonal assay. The other one was used immediately for detecting the biochemical measurements. Thyroid hormones (TSH, FT3, and FT4) were measured by chemiluminescence with Immulite (1000) using kit (Diagnostic Products Corporation; Los Angeles, CA, USA).
Anthropometric measures included: weight, height, body mass index (calculated by dividing weight (kg) to height squared (m2)), midarm circumference (measured using a flexible tape in the halfway point between the acromion of the scapula and the olecranon of the ulna).
Comorbidity was estimated using modified Charlson comorbidity index (mCCI) (excluding age-factor), which is a composite score of multiple comorbid conditions. It is a popular tool of assessing comorbidity and is a strong predictor of outcome in patients with ESRD [Citation9]. Testing the adequacy of dialysis kt/v was done using single pool equation.
Nutritional status was assessed by subjective global assessment score (SGA score): The SGA is an integrated tool that utilizes the clinical judgment of a practitioner to identify patients at risk of or with malnutrition. It consists of seven items, two components related to physical examination (indicator of fat and muscle loss and nutritional status-associated with changes in fluid balance) and five components of medical history (weight change, diet, gastrointestinal symptoms, functional capacity, disease, and nutrition relationship needs). Each component has a score between one (normal) to five (very severe). Thus, malnutrition score has a total score between 7 and 35. Patients having score between 7 and 10 are considered as well-nourished patients. Score between 11 and 22 are considered as having mild to moderate malnutrition. Likewise score between 23 and 35 are considered as severely malnourished [Citation10].
2.2.1. Statistical analysis
Results were tabulated and statistically analyzed by using a personal computer using MICROSOFT EXCEL 2016 and SPSS v. 21 (SPSS Inc., Chicago, IL, USA). Statistical analysis was done using: Descriptive: e.g. percentage (%), mean and standard deviation. Analytical: that includes: Chi-Squared (χ2), t-test, and Pearson’s correlation coefficient (r). A value of P less than 0.05 was considered statistically significant.
3. Results
The study included 84 hemodialysis patients, 45 (53.6%) males and 39 (46.4%) females with the mean age of 57.42 ± 11.09 years (range, 30 to 80 years). The duration of hemodialysis ranged from 4 months to 11 years with a mean duration of 38.23 ± 24.37 months. 41.7% of patients had hypertension, 8.3% had diabetes mellitus, 19% had both hypertension and diabetes while 31% of the studied group hadn`t neither hypertension nor diabetes.
Anthropometric and biochemical parameters are shown in . The mean weight for studied patients was 75.3 kg, the mean height was 170.19 cm, the mean BMI was 25.94 kg/m2, the mean MAC was 28.43 cm, the mean KT/V for studied patients was 1.2 and the mean CCI for studied patients was 5.54. The mean hemoglobin for studied patients was 9.7 g/dl, the mean creatinine was 8.4 mg/dl, the mean serum iron was 0.7 mg/l, the mean TIBC was 2.35 mg/l, the mean albumin was 3.55 g/dl, the mean urea was 172.42 mg/dl, the mean cholesterol was 178.29 mg/dl and the mean CRP was 10.14 mg/l.
Table 1. Anthropometric and biochemical characteristics of the patients (N = 84)
The mean hormonal values of thyroid function were: TSH 2.99 ± 2.93 µIU/ml (range: 0.01–17.1); FT4 1.08 ± 0.21 ng/dl (range: 0.65–1.62) and FT3 2.55 ± 0.52 pg/ml (range: 1.18–3.64).
The mean SGA score for studied patients was 13.73 ± 4.4 (range: 7–25). According to SGA score, 26.2% of patients had normal nutritional status while 69% of them had mild to moderate malnutrition and 4.8% had severe malnutrition.
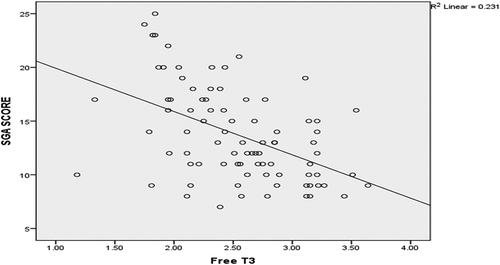
SGA had significant negative correlation with FT3 (), while there was no significant correlation between it and FT4 or TSH. There was also a significant negative correlation between SGA and weight, BMI, MAC, serum albumin, hemoglobin, creatinine, cholesterol, and significant positive correlation between it and duration of dialysis, CRP, and CCI (P-value <0.05), while there was no statistically significant correlation between SGA and age, TIBC, serum iron, urea, or KT/V (P-value >0.05) ().
Table 2. Correlation between SGA and other variables (N = 84)
The multivariate regression analysis, including variables with P values <0.05 in the univariate analysis, demonstrated that FT3, BMI, albumin, cholesterol, CRP, and CCI were significant independent determinants of SGA among hemodialysis patients ().
Table 3. Univariate regression analysis for SGA (N = 84)
Table 4. Multivariate regression analysis for SGA (N = 84)
Serum FT3 concentration inversely correlated with age (r = −0.25, P= 0.02), CCI (r = −0.48, P= 0.0001), CRP (r = −0.46, P= 0.0001), and SGA (r = −0.49, P= 0.0001), and positively correlated with serum albumin (r = 0.47, P= 0.0001) and KT/V (r = 0.25, P= 0.02) ().
Table 5. Correlation between free T3 and other variables (N = 84)
4. Discussion
One of the most common complications that occur in ESRD patients, with a significant negative impact on life, is malnutrition. Often overlooked or underestimated, malnutrition is clearly associated with increased mortality risk [Citation11].
Our results show that the age of the studied group was 57.42 ± 11.09 years. These results agree with another study that reported an increase in prevalence of ESRD with aging particularly after 50 years in both genders [Citation12]. Also, these results go in agreement with an epidemiological study carried out at Menoufia governorate, Egypt which showed that the mean age at the start of dialysis is 52 years [Citation13]. This age is slightly lower than the average worldwide age of ESRD which is more than 60 years in 73% of studies done on 6541 patients with ESRD in a meta-analysis [Citation14]. This may reflect the standard of medical care and early diagnosis of patients with chronic kidney disease. As regarding sex, 53.6% were males, and 46.4% were females in the present study. This is in accordance with other studies which reported the increased incidence of ESRD 50% more in men than in women [Citation15,Citation16] although the incidence of chronic kidney disease is more in women [Citation17].
Results of this study demonstrated that HTN is the commonest cause of ESRD (41.7%) in these patients. These results agree with most of the studies where hypertension and diabetes mellitus are the main causes of renal failure [Citation18].
Based on SGA score about (69%) had mild to moderate malnutrition and (4.8%) had severe malnutrition. This result go in agreement with a Jordanian study which showed that out of 180 patients on regular HD, about 61% were malnourished, 8.5% of them had severe malnutrition [Citation19]. This also agreed with a study done in Cairo Egypt which showed that the prevalence of malnutrition among HD patients was 67% (50% were mild to moderate malnourished and 17% were severe malnourished) [Citation20]. These results were lower than the prevalence reported by another Egyptian study done among HD patients in Assiut city which revealed about 85% malnourished patients (81.6% mild to moderate malnutrition and 3.6% severe malnutrition) [Citation21]. This difference can be explained by lower educational level, socioeconomic state, and health-care facilities in South Egypt area compared to the capital city. In Saudi Arabia, malnutrition was detected in 79% of ESRD patients on HD according to SGA [Citation22]. These differences in prevalence may be due to environmental diversity and different diet regimens in various regions of the Middle East area.
Our study showed also that SGA score correlated negatively with anthropometric measures (body weight, body mass index, midarm circumference) and laboratory features of malnutrition (serum albumin, hemoglobin level and cholesterol). This goes in agreement with the EQUAL study which reported significant correlations between SGA and nutritional markers such as plasma albumin, creatinine, BMI, and body weight [Citation23]. This also agreed with another study that showed SGA score have a negative correlation with BMI and positive correlation with CRP, and linked it with increased inflammation and mortality [Citation24].
SGA correlated positively with the duration of dialysis. This is in agreement with other studies which stated that the duration of dialysis can be associated with malnutrition [Citation25]. As increased duration of dialysis may lead to loss of nutrients, inflammation and hypercatabolism so, long duration on dialysis may be regarded as one of the main causes of malnutrition.
We also found that SGA had significant positive correlation with CRP as inflammatory marker and associated comorbidities represented by CCI. These findings agreed with a cross-sectional analysis performed in a monocentric study involving 126 patients on maintenance HD. They reported a negative association between the degree of malnutrition evaluated by SGA and serum albumin, and a significant positive correlation with CRP [Citation26]. Chronic inflammation in malnourished patients could be the link through which malnutrition increase the mortality in patients with ESRD [Citation24,Citation27].
Regarding the association between thyroid function and nutritional status, our results showed that SGA, as a marker of nutritional status, had a significant negative correlation with FT3; the FT3 was a significant independent determinant of SGA. While there was no significant correlation between SGA and neither free T4 nor TSH. This goes in agreement with Koo et al., who found that the proportion of malnourished patients assessed by SGA were significantly higher in patients with lower T3 levels [Citation28].
This also agreed with the study done by Fernández-Reyes which showed that half of dialysis patients had decreased levels of serum FT3 without alteration on FT4 or TSH. They also confirmed that low FT3 levels correlate with malnutrition and inflammation parameters and could be considered an early marker for catabolism or protein energy wasting [Citation29]. In the present work, FT3 had significant positive correlation with serum albumin and negative correlation with CRP. These results go in agreement with the study done by Fan et al., who concluded that low T3 syndrome is closely associated with malnutrition-inflammation complex syndrome [Citation30]. The main source of extrathyroidal FT3 is the peripheral conversion of T4 to T3 by the 5 – deiodinase system [Citation31]. Both protein energy malnutrition and pro-inflammatory stimuli can interfere with the activity of the 5-deiodinase system, resulting in reduced T3 production.
Our results showed also that FT3 had negative correlation with age and CCI which agreed with Koo et al. [Citation28] Similarly, Yavuz D et al, showed that serum FT3 concentration inversely correlated with age, CCI, CRP, and positively correlated with serum albumin. They concluded that plasma FT3 level is one of the earliest surrogate markers of thyroid dysfunction, and this reduction correlates negatively with the severity of illness and associated malnutrition [Citation32].
In 2016, another study stated that plasma FT3 was in positive correlation with serum albumin. It was also observed that FT3 had been highly correlated with inflammatory markers IL-6 and CRP, so FT3 was considered as a good inflammatory marker and strong predictors of mortality [Citation33].
As a result, our findings confirm that the decrease in FT3 in hemodialysis patients is mainly the body’s adaptive response to reduced kidney function aiming to reduce baseline metabolism and avoiding catabolism. However, this adaptation may be accompanied by malnutrition, inflammation and increased mortality.
4.1. Limitations of the study
The findings of this study have to be seen in light of some limitations. The small number of the study sample as this study was done at one hemodialysis unit. This was a cross-sectional study; thus, it is not possible to infer a causal relationship between the thyroid dysfunction and malnutrition.
4.1.1. Conclusions
Malnutrition is a common problem among hemodialysis patients. It is associated with decreased levels of FT3, which is associated with poor outcome and increased mortality. For clinicians, periodical measurement of FT3 levels could be an accessible and reproducible method to detect such states.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Walid Shehab-Eldin
Walid Shehab-Eldin is a Assistant Professor of Diabetes, Endocrinology, and obesity in Internal Medicine Department, Menoufia Faculty of Medicine.
Mohamed Ahmed Shaaban
Mohamed Ahmed Shaaban is a Professor of Diabetes, Endocrinology, and obesity in Internal Medicine Department, Menoufia Faculty of Medicine.
Mai Abdel Samed Atia
Mai Abdel Samed Atia Nephrology Resident in Nephrology unit, Shebin Elkom Fever Hospital, Menoufia.
Shimaa Kamal Zewain
Shimaa Kamal Zewain Lecturer of Diabetes, Endocrinology, and obesity in Internal Medicine Department, Menoufia Faculty of Medicine.
References
- Kuczera P, Adamczak M, Wiecek A. Endocrine abnormalities in patients with chronic kidney disease. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2015;36(2):109–118.
- Rhee CM. The interaction between thyroid and kidney disease: an overview of the evidence. Curr Opin Endocrinol Diabetes Obes. 2016 Oct;23(5):407–415.
- Iglesias P, Bajo MA, Selgas R, et al. Thyroid dysfunction and kidney disease: an update. Rev Endocr Metab Disord. 2017 Mar;18(1):131–144.
- Xu H, Brusselaers N, Lindholm B, et al. Thyroid function test derangements and mortality in dialysis patients: A systematic review and meta-analysis. Am J Kidney Dis. 2016 Dec;68(6):923–932.
- Da Costa AB, Pellizzari C, Carvalho GA, et al. High prevalence of subclinical hypothyroidism and nodular thyroid disease in patients on hemodialysis. Hemodial Int. 2016 Jan;20(1):31–37.
- Lim VS. Thyroid function in patients with chronic renal failure. Am J Kidney Dis. 2001 Oct;38(4 Suppl 1):S80–4.
- Jadeja YP, Kher V. Protein energy wasting in chronic kidney disease: an update with focus on nutritional interventions to improve outcomes. Indian J Endocrinol Metab. 2012 Mar;16(2):246–251.
- Zha Y, Qian Q. Protein nutrition and malnutrition in CKD and ESRD. Nutrients. 2017 Feb 27;9(3):208.
- Hall WH, Ramachandran R, Narayan S, et al. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004 Dec;20(4):94.
- Keith JN. Bedside nutrition assessment past, present, and future: a review of the Subjective Global Assessment. Nutr Clin Pract. 2008 Aug-Sep;23(4):410–416.
- Abdulan IM, Onofriescu M, Stefaniu R, et al. The predictive value of malnutrition for functional and cognitive status in elderly hemodialysis patients. Int Urol Nephrol. 2019 Jan;51(1):155–162.
- Yu M, Ryu DR, Kim SJ, et al. Clinical implication of metabolic syndrome on chronic kidney disease depends on gender and menopausal status: results from the Korean National Health and Nutrition Examination Survey. Nephrol Dial Transplant. 2010 Feb;25(2):469–477.
- Zahran A. Epidemiology of hemodialysis patients in Menofia governorate, delta region, Egypt. Menoufia Med J. 2010;24:211–220.
- Van Deudekom FJ, Schimberg AS, Kallenberg MH, et al. Functional and cognitive impairment, social environment, frailty and adverse health outcomes in older patients with head and neck cancer, a systematic review. Oral Oncol. 2017 Jan;64:27–36.
- Nagata M, Ninomiya T, Doi Y, et al. Trends in the prevalence of chronic kidney disease and its risk factors in a general Japanese population: the Hisayama Study. Nephrol Dial Transplant. 2010 Aug;25(8):2557–2564.
- Albertus P, Morgenstern H, Robinson B, et al. Risk of ESRD in the United States. Am J Kidney Dis. 2016 Dec;68(6):862–872.
- Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016 Oct 4;165(7):473–481.
- Swarnalatha G, Ram R, Prasad N, et al. End-stage renal disease patients on hemodialysis: a study from a tertiary care center in a developing country. Hemodial Int. 2011 Jul;15(3):312–319.
- Tayyem RF, Mrayyan MT, Heath DD, et al. Assessment of nutritional status among ESRD patients in Jordanian hospitals. J Ren Nutr. 2008 May;18(3):281–287.
- Zaki DS, Mohamed RR, Mohamed NA, et al. Assessment of malnutrition status in hemodialysis patients. Clin Med Diagn. 2019;9(1):8–13.
- Abozead SE, Ahmed AM, Mahmoud MA. Nutritional status and malnutrition prevalence among maintenance hemodialysis patients. IOSR J Nurs Health Sci. 2015;4(1):51–58.
- Ali Bokhari SR, Faizan Ali MA, Khalid SA, et al. The development of malnutrition is not dependent on its traditional contributing factors in patients on maintenance hemodialysis in developing countries. Saudi J Kidney Dis Transpl. 2018 Mar-Apr;29(2):351–360.
- Windahl K, Irving GF, Almquist T, et al. Prevalence and risk of protein-energy wasting assessed by Subjective Global Assessment in older adults with advanced chronic kidney disease: results from the EQUAL Study. J Ren Nutr. 2018 May;28(3):165–174.
- Kizil M, Tengilimoglu-Metin MM, Gumus D, et al. Dietary inflammatory index is associated with serum C-reactive protein and protein energy wasting in hemodialysis patients: A cross-sectional study. Nutr Res Pract. 2016 Aug;10(4):404–410.
- Gencer F, Yildiran H, Erten Y. Association of Malnutrition Inflammation Score with anthropometric parameters, depression, and quality of life in hemodialysis patients. J Am Coll Nutr. 2019 Jul;38(5):457–462.
- Essadik R, Msaad R, Lebrazi H, et al. Assessing the prevalence of protein-energy wasting in haemodialysis patients: A cross-sectional monocentric study. Nephrol Ther. 2017 Dec;13(7):537–543.
- Dai L, Mukai H, Lindholm B, et al. Clinical global assessment of nutritional status as predictor of mortality in chronic kidney disease patients. PLoS One. 2017 Dec 6;12(12):e0186659.
- Koo HM, Kim CH, Doh FM, et al. The impact of low triiodothyronine levels on mortality is mediated by malnutrition and cardiac dysfunction in incident hemodialysis patients. Eur J Endocrinol. 2013 Sep 12;169(4):409–419.
- Fernandez-Reyes MJ, Sanchez R, Heras M, et al. Can FT3 levels facilitate the detection of inflammation or catabolism and malnutrition in dialysis patients? Nefrologia. 2009;29(4):304–310.
- Fan J, Yan P, Wang Y, et al. Prevalence and clinical significance of low T3 syndrome in non-dialysis patients with chronic kidney disease. Med Sci Monit. 2016 Apr 8;22:1171–1179.
- Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006 Oct;116(10):2571–2579.
- Yavuz D, Sezer S, Yavuz R, et al. Free triiodothyronine in hemodialysis patients: link with malnutrition and inflammation. Iran J Kidney Dis. 2014 May;8(3):212–217.
- Abd-Elhafeez HA, El-Sayed EE, Lotfy AM, et al. Serum triiodothyronine (T3) hormone evaluation in patients with chronic kidney disease and its value as an inflammatory marker. Life Sci J. 2016;13(6):40–45.