ABSTRACT
Background: Traumatic brain injury is a major public health concern, resulting in substantial mortality and long-term disability. So, it was important to search for novel therapeutic modalities that play an important role in reducing cerebral edema and had neuro- protective effect. A number of studies showed the feasibility, safety, and effectiveness of early parenteral administration of progesterone in acute severe traumatic brain injury patients compared with standard care.
Objective: The study aimed to assess the effect of the progesterone on the neurologic outcome in non-operable patients with acute severe traumatic brain injury.
Patients and Methods: This study was conducted on 50 patients presented with severe traumatic brain injury with GCS less than or equal to 8 after resuscitation and stabilization including both sexes. They were categorized into two groups according to applying the progesterone protocol. Patients were subjected to informed consent from next of kin, history taking, clinical neurological examination, routine laboratory and radiological investigations as needed and progesterone administration to patients in group 2which was given intramuscularly in dose of 1 mg/kg every12h for 7 consecutive days.
Results: Early parenteral administration of progesterone was associated with significant decrease in the mortality rate at day 28, significant improvement in Glasgow outcome scale of the patients by the end of day 28, significant improvement in level of consciousness of the patients (GCS), SOFA score, and CT brain Marshall scoring.
Conclusion: Progesterone administration in acute severe traumatic brain injury patients was associated with significant decrease in 28th day mortality, period of ICU stay, and mechanical ventilation days. Also, it was feasible and safe without noted complications.
1. Introduction
Traumatic brain injury(TBI) is a major public health concern, resulting in substantial mortality and long-term disability; it continues to be a major health epidemic, not just in terms of prevalence but also in terms of the deaths and injuries that occur in the population that they most commonly affect [Citation1]. It is estimated that TBI accounts for 45–50% of all traumatic deaths and is the leading cause of death among trauma victims [Citation2].Head injury is divided into blunt and penetrating types; road traffic injuries are the most common cause of closed-head injury worldwide. These include injuries to drivers of cars, pedestrians, motorcyclists, and bicyclists [Citation3]. Falls are the second most common cause of injury, while gunshot injuries are the main cause of penetrating head injury [Citation3].
Brain trauma is an acute biomechanical event characterized by multiple processes of pathophysiology which evolve in a continuum over time which includes white matter degradation, protein misfolding, persistent inflammatory response, neurorestorative processes.
Brain damage results in extensive brain structure, perfusion, metabolism and functional abnormalities. With closed head trauma, the initial effect leads to a mechanical destruction of cellular integrity and supporting tissue membranes [Citation4]. Numerous secondary effects eventually arise when blood and fluid retention in the brain causes the patient’s condition to deteriorate [Citation5].
Head injuries are classified into primary head injuries which occur at impact time and secondary head injuries which include breakdown of blood–brain membranes, release of inflammatory factors, free radical overload, excessive release of neurotransmitter glutamate (excitotoxicity), calcium and sodium ions into the neurons, and mitochondrial dysfunction.
Due to long-term morbidity of traumatic brain injury, it was very important to search for novel therapeutic modalities that play an important role in reducing effect of traumatic brain injury [Citation6].And from that point was the role of sex hormones. Early parenteral administration of sex hormones has anti-apoptotic, anti-inflammatory and anti-oxidant properties through various models, and may promote reparative processes that avoid long-term TBI sequelae. Progesterone belongs to an essential category of endogenous steroids called neurosteroids, such as pregnenolone and dehydroepiandrosterone (DHEA). It is metabolizable in all areas of the central nervous system. Neurosteroids are neuromodulators that control neurotransmission and myelination and are neuroprotective and neurogenic.
Potential long-term reparative action of progesterone produces a significant protein that regulates and guides differentiation of neural stem cells, thereby affecting brain repair by producing new neurons whenever possible. Progesterone-induced acceleration of protein production can represent a potential pathway for neuroregeneration and neuroprotection [Citation7].
1.1. Aim of the study
The study aimed to assess the effect of the progesterone on the neurologic outcome in non-operable patients with acute severe traumatic brain injury.
2. Patients and methods
This study was conducted on 50 patients presented with severe traumatic brain injury with Glasgow coma scale(GCS) less than or equal 8 after resuscitation and stabilization including both sexes. Informed consent was taken from patients’ next of kin as well as approval from local ethical committee.
Type of study was comparative prospective randomized single-blinded study. Patients were randomly allocated to one of two groups according to simple randomization method where cases in odd number (case 1, 3, 5 …) were allocated in group 1 and cases in even number (2, 4, 6 …) were allocated in group 2.
Group 1:Included 25 patients to whom main lines of management of traumatic brain injury were applied without giving progesterone (control group)
Group2:Included 25 patients who received progesterone in addition to main lines of management of traumatic brain injury (progesterone group or cases).
Selected patients were subjected to the following:
History taking which included personal data; name, age, and gender, past history of chronic diseases as diabetes mellitus, hypertension, cardiac, pulmonary, neurological, renal, hepatic problems and thyroid diseases, and drug history.
Clinical examinationwhich included vital signs (blood pressure, heart rate, temperature, and respiratory rate), complete neurological examination on admission including GCS [Appendix A], acute physiology, and chronic health evaluation (APACHE II) score [Appendix B] calculated on admission, ECG, CXR, CT brain done for all patients on admission, and MRI when needed.
Laboratory investigations including CBC, urea, creatinine, SGOT, SGPT, total protein, serum albumin, electrolytes as Na, K and PT, PTT, total and direct bilirubin.
Measurements and outcome parametersincludingassessment of GCS daily, SOFA score [Appendix C] every 48 hours, and CT brain Marshall scoring at day1, and 7 according to the following criteria as described in . Outcome data as Glasgow outcome scale (GOS) [Appendix D] at day 7 and day 28,7th and 28th day mortality, ICU stay and mechanical ventilation days were recorded.
Table 1. Marshall scoring of TBI
2.1. Progesterone administration
Patients in group II received progesterone (prontogest amp 100 mg/2 ml by MARCRYL company) within 8 hours after the documented time of injury at a dose of 1 mg/kg via intramuscular injection in the buttocks and then once per 12 hours for 7 consecutive days.
2.2. Statistical analysis of the data
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). The significance of the obtained results was judged at the 5% level.
3. Results
The mean age of patients in the progesterone group was 33.36 ± 11.56 years, while the mean age of patients in the control group was 35.28 ± 13.61 years. The male to female ratio was almost 2:1 in the two groups, without statistically significant difference between the two groups regarding age and sex. ()
Table 2. Comparison between the two studied groups according to demographic data
The mean APACHE II score on admission for the progesterone group was 18.68 ± 2.94 while for the control group was 17.88 ± 3.18 with no significant difference.()
Table 3. Comparison between the two studied groups according to APACHEII score and SOFA score
The mean SOFA score at day 1 for the progesterone group was 11.32 ± 2.27 while the mean for the control group was 11.48 ± 2.83.The SOFA score was significantly lower in progesterone group compared to control group at day 3, 5 and 7. (p = 0.007,<0.001,<0.001, respectively). Also, the mean SOFA score at day 7 was significantly lower than that at day 1 in both groups. ()
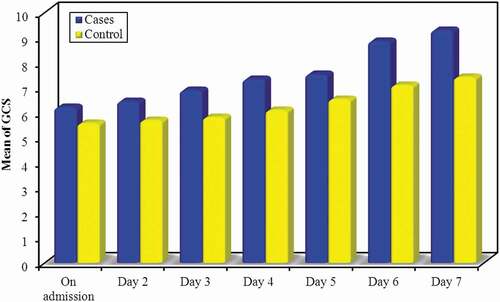
According to GCS, there was significant improvement of GCS at day4, 6 and day 7 in comparison to day 1 in progesterone group, respectively (p = 0.048, p < 0.001, p < 0.001).Alsothe GCS was significantly higher in the progesterone group in comparison to control group starting from day 2 till day 7(p = 0.045, 0.012, 0.007,0.004, 0.002, 0.003, respectively, )
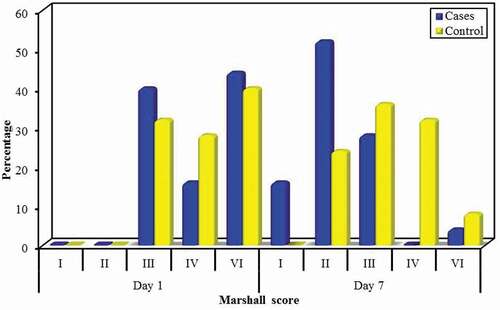
Regarding CT brain (Marshall scoring), there was statistically significant difference between the progesterone and control group at day 7 (MCp = 0.002) with improvement toward the progesterone group. Also there was significant improvement regarding Marshall scoring in day 7 in comparison to day 1 in progesterone and control groups (p < 0.001, p = 0.001, respectively), as we notice that a lot of cases with grade III, IV, and VI changed to grade I and II. ()
3.1. Outcome parameters
According to Glasgow Outcome Scale (GOS) at day 28, there was statistically significant difference between the two studied groups (p = 0.012) that was better toward the progesterone group. Also there was significant worsening of GOS at day 28 in comparison to day 7 in control group (p < 0.001, ).
Table 4. Comparison between the two studied groups according to GOS, mechanical ventilation days, ICU duration and mortality rate
According to ICU duration, the mean for the progesterone group was 7.20 ± 1.71 days while the mean for the control group was 10.48 ± 2.76 days; there was significant lower ICU duration for the progesterone group compared to the control group (p < 0.001, ).
According to mechanical ventilation days, the mean for the progesterone group was 4.24 ± 1.33 days while the mean for the control group was 8.52 ± 2.16 days; there was significant lower duration of mechanical ventilation days consumed by the progesterone group in comparison to the control group (p < 0.001, ).
Regarding 7th day mortality, progesterone group showed lower mortality than control group but without significance (6 patients versus 12 patients, respectively, p = 0.077).However, the 28th day mortality showed that the progesterone group had statistically significant lower mortality than the control group (8 patients versus 17 patients, respectively, p = 0.024, ).
4. Discussion
Brain injuries can be life-threatening and have major impact on the mortality rate all over the world so it was important to search for novel therapeutic modalities that play an important role in reducing cerebral edema and had neuro- protective effect [Citation8].
In our study, we assessed the role of sex hormones (progesterone) that has anti-apoptotic, anti-inflammatory, and anti-oxidant properties in patients with severe TBI. It was conducted on 50 patients presented with severe traumatic brain injury with GCS less than or equal 8 after resuscitation and stabilization including both sexes; they were categorized into 2 groups.
Patients in group 2 received progesterone within 8 h after the documented time of injury at a dose of 1 mg/kg via intramuscular injection and then once per 12 hours for 7 consecutive days
In Apr 2008, Xiao et al. [Citation9], clinical trial, Patients for the treatment group were given progesterone at 1.0 mg/kg via intramuscular injection and then once per 12 hours for 5 consecutive days
On December 25, 2014, Wright et al., [Citation10] clinical trial, patients for the treatment group were given progesterone within 4 h after injury and consisted of a 1 h loading dose, 71 h of maintenance infusion, and a 24-h infusion taper. The study drug was infused continuously through a dedicated intravenous catheter at a dose of 14.3 ml per hour for 1 h and then at 10 ml per hour for 71 h; the dose was then tapered by 2.5 ml per hour every 8 h, for total treatment duration of 96 h.
In our study, we noted that there was significant improvement of GCS in progesterone group compared to control group from day 2 up to day 7.
Also we noted that there was significant improvement of GCS at day 6 and day 7 in comparison to day 1 in both groups.
In agreement with our study, in Xiao et al., [Citation9] clinical trial, daily evaluations of neurologic status done via the GCS score for all patients involved in the study, with the number of patients with GCS from 3 to 5 was 22 for the progesterone group and 20 for the control group and the mean GCS scores increased progressively in the two groups during the 14-day acute phase of the study, but in contrast to our study, there was no apparent differences among the treatment groups.
In Wright et al., [Citation10] clinical trial, seventy-two patients had an index GCS score of 4 to 8; the remainder (28 patients) had an index GCS score of 9 to 12 with significant improvement of GCS as severe traumatic brain injury patients (index GCS scores 4 to 8) randomized to placebo remained in coma longer than those who received progesterone (10.1 days versus 3.9 days).
In our study, the mean SOFA score at day 7 was significantly lower than that at day 1 in both groups denoting improvement in all patients after receiving management.
Also in our study, we compared the duration of mechanical ventilation between the two studied groups which was less in progesterone group with mean 4.24 ± 1.33 (days) in comparison to the control group with mean 8.52 ± 2.16 (days) and that was statistically significant (p < 0.001).
Also, the period of ICU stay was less in progesterone group with mean 7.20 ± 1.71 (days) in comparison to the control group with mean 10.48 ± 2.76 (days) and that was statistically significant (p < 0.001).
Also, in our study, we compared the mortality rate at day 7 between the two studied groups which was lower in the progesterone group in comparison to the control group but was not statistically significant (p = 0.077). However, the mortality rate at day 28 in progesterone group was significantly lower than the control group (p = 0.024).
Death of the patients was mainly from other causes than trauma as septic shock due to hospital-acquired infections as respiratory tract infections, urinary tract infections, bed sores, Acute Respiratory Distress Syndrome (ARDS) and multi-organ failure (MOF) with no complications noted after progesterone administration
A meta-analysis of randomized clinical trials for effect of progesterone administration on the prognosis of patients with severe traumatic brain injury done on January 11, 2019 with the aim of this study was to assess the neuroprotective effect of progesterone administration on severe traumatic brain injury (TBI) for different follow-up periods and administration route by completing a meta-analysis of randomized clinical trials [Citation11].
Patients treated with progesterone had better neurologic outcomes within 3 months post-injury than those given placebo (RR = 1.51, 95% CI [1.12–2.02], P = 0.007). High heterogeneity was observed among trials concerning GOS scores at 6 months post-injury (P = 0.06, I 2 = 60%), and similar neurologic outcomes were observed for both the progesterone and placebo groups (RR = 1.09, 95% CI [0.93–1.27]). Also, progesterone administration improved the clinical outcomes of severe TBI patients within 3 months post-injury but may not have significant long-term benefits at 6 months post-injury [Citation11].
4.1. Study limitation
Proposed dose of progesterone in our study was via intramuscular injection and then once per 12 hour for 7 consecutive days which can be prolonged in other studies for better assessment of progesterone effect, the lack of advanced medical advices such as intracranial pressure monitoring catheters, the small sample size enrolled in the study. Also, the study was applied on restricted types of patients.
4.2. Conclusions
Early parenteral administration of progesterone in acute severe traumatic brain injury patients was associated with significant decrease in the morality rate at day 28,and significant decrease in the period of ICU stay and mechanical ventilation days. It was also associated with significant improvement in Glasgow outcome scale of the patients by the end of day 28.Progesterone administration was associated with significant improvement in level of consciousness of the patients (GCS), SOFA score and CT brain Marshall scoring. Progesterone administration was feasible and safe without noted complications.
Supplemental Material
Download JPEG Image (3.1 MB)Disclosure statement
We have no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Notes on contributors
Amr Hassan Dahroug
Amr Hassan Dahroug, MD critical care medicine, Faculty of Medicine, Alexandria University
Tayseer Mohamed Zaytoun
Tayseer Mohamed Zaytoun, MD critical care medicine, Faculty of Medicine, Alexandria University
Mahmoud Salah Anwar Hussien
Mahmoud Salah Anwar Hussien, critical care medicine, Faculty of Medicine, Alexandria University
References
- James EB. Critical care of patient with traumatic brain injury. In: Irwin RS, Rippe JM, editors. Irwin and Rippe’s Intensive Care Medicine. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2003. p. 1789–1798.
- Combes P, Fauvage B, Colonna M, et al. Severe head injuries: an outcome prediction and survival analysis. Intensive Care Med. 1996;22:1391–1395.
- Moulton R. Head injury. In: Young GB, Ropper AH, Bolton CF, editors. Coma and impaired consciousness. Ch 6. New York: McGraw-Hill, Health Professions Division; 1998. p. 149–181.
- Prins M, Greco T, Alexander D, et al. The pathophysiology of traumatic brain injury at a glance. Dis Model Mech. 2013;6(6):1307–1315.
- Gennarelli TA. Mechanisms of brain injury. J Emerg Med 1993;11:5-11.
- Parikh S, Koch M, Narayan RK. Traumatic brain injury. InterAnesthesiolClin. 2007;45:119–135.
- Pepe PE, Wigginton JG, Gatson JW, et al. Single dose estrogen infusion can amplify brain levels of sonic hedgehog (SHH), a signal protein for neuro stem cells and repair following the indirect brain injury resulting after severe torso burns. Crit Care. 2013;17:287.
- Hardman S, Rominiyi O, King D, et al. Is cranial computed tomography unnecessary in children with a head injury and isolated vomiting? BMJ. 2019;365:l1875.
- Xiao G, Wei J, Yan W, et al. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61.
- Wright DW, Kellermann AL, Hertzberg VS, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402.
- Pan ZY, Zhao YH, Huang WH, et al. Effect of progesterone administration on the prognosis of patients with severe traumatic brain injury: a meta-analysis of randomized clinical trials. Drug Des Devel Ther. 2019;13:265–273.
Appendix A.
Glasgow Coma Scale
Appendix B.
APACHE II score
Appendix C.
The sequential organ failure assessment (SOFA) score
Appendix D.
The Glasgow Outcome Scale (GOS)
Is a global scale for functional outcome that rates patient status into one of five categories: Dead, Vegetative State, Severe Disability, Moderate Disability or Good Recovery.