ABSTRACT
Introduction: Cell phones are essential for modern life and their usage becomes prevalent. Worries have increased concerning the harmful effects of their radio-frequency electromagnetic radiation. Liver X receptors (LXRs) are ligand-dependent transcription factors that have important roles in lipid metabolism and inflammation, plus several recently emerging roles. Whether it is useful to overcome the hazardous effects of cell phone radiation needs to be clarified. So, this study aimed to investigate the effect of LXRs activation on testicular changes in rats exposed to electromagnetic radiation.
Methods: Thirty adult male Wistar rats were subdivided into control group, experimental group: exposed to cell phone electromagnetic radiation, and treated group: as experimental group and received LXRs agonist T0901317. Serum testosterone (T), Follicle-Stimulating Hormone (FSH), and Luteinizing Hormone (LH), testicular tissue malondialdehyde (MDA) and superoxide dismutase (SOD), and gene expressions of inflammatory and apoptotic markers were assessed. Seminal analysis and testicular histological examination were performed.
Results: LXRs activation in rats exposed to cell phone radiation improved the decreased testicular and gonadotrophic hormones, semen quality parameters, tissue SOD, and anti-apoptotic gene expression as well as the elevated tissue MDA, apoptotic and inflammatory gene expressions, and the testicular histological changes induced by exposure to electromagnetic radiation.
Conclusion: Exposure to cell phone radiation leads to harmful effects on spermatogenesis and LXRs activation could improve these effects via antioxidant, anti-inflammatory properties in addition to its endocrinal action.
1. Introduction
Male infertility is a rising problem around the world; the incidence of male infertility at the reproductive age has been assessed to be up to 7–8% [Citation1]. Idiopathic male infertility is due to decreased sperm quality without organic, genetic, or endocrine etiology [Citation2]. Several environmental factors could be considered as predisposing factors for male infertility like heat or certain chemical agents and a possible link between using cell phones and male sterility was evaluated [Citation3]. The use of mobile phones generates an electromagnetic field (EMF) whose effects on human health have increased the interest to be investigated [Citation4]. The fast evolution of mobile phone usage is accompanied by an equivalent surge in the density of the EMF [Citation5]. Effect of EMF on fertilizing potential of spermatozoa and on testosterone level is controversial. Usage of cell phones by humans [Citation2,Citation6] or exposure to them in animal studies [Citation1,Citation7] leads to defective testicular function and reduction of the sperm count, motility, and viability. However, other researches proved that exposure to EMF did not induce any adverse effects on sperm quantity, quality, or morphology [Citation8].
It was proposed that mobile phone EMF produces free radicals that finally result in oxidative stress and is associated with higher production of pro-inflammatory cytokines and reactive oxygen species (ROS) [Citation3]. Oxidative stress results from imbalance between ROS formation and cell antioxidant defense capacity that is associated with increased germ cell apoptosis, DNA damage, and lipid peroxidation [Citation9]. It was suggested that EMF could induce excessive inflammatory responses via macrophage activation [Citation10]. However, effects of EMF on cytokine expression is a complex process associated with physical and biological aspects of EMF [Citation11].
Liver X receptors (LXRs) are nuclear receptor superfamily and ligand-activated transcription factors that exist in two isoforms: LXRα and LXRβ [Citation12]. LXRs are involved in several physiological processes such as lipid metabolism and cholesterol homeostasis. In addition, they have been reported to play important roles in the modulation of inflammation [Citation13]. LXRα and LXRβ are expressed in the mouse and human testes. In the mouse, LXRα is mainly expressed in Leydig cells, and LXRβ in Sertoli cells, while both are expressed in germ cells. LXR αβ-/- mice become prematurely infertile with increased apoptosis of germ cells, reduced germ cell proliferation, and low testosterone levels [Citation14]. In humans, both LXRα and LXRβ are expressed in testicular biopsy specimens and are expected to have a similar role as in the mouse, so they may be related to premature infertility [Citation15]. Moreover, LXRs exert anti-inflammatory effects by inhibiting production of pro-inflammatory cytokines either directly or indirectly [Citation16].
We hypothesized that prolonged exposure to EMF will produce alteration in testicular functions and LXRs activation could modulate these effects. So, this study aimed to investigate the effect of LXRs activation on testicular changes in rats exposed to electromagnetic radiation.
2. Materials and methods
2.1. Experimental animals
The present research included 30 adult male Wistar rats aged 12–15 weeks weighing 200 ± 20 g that were obtained from animal house of faculty of Veterinary Medicine, Zagazig University, Egypt. The animals were housed in plastic cages (18 inches in length and 12 inches in width) five rats/cage. They were maintained under controlled room temperature (22 ± 2°C) and humidity (50–60%) with 12 h light and 12 h dark cycle and were fed on a standard diet with free access to water. All experimental procedures were following the guide for the care and use of laboratory animals (8th edition, National Academies Press) and have been reviewed and approved by Zagazig University institutional animal care unit committee (ZU-IACUC; Sharkia; Egypt) with approval number: ZU-IACUC/3/F/117/2019).
2.2. Experimental design
Following acclimatization for two weeks, animals were randomly allotted to: control (sham) group (n = 10): in which animals were exposed to cell phones in switch off mode, experimental (EM) group (n = 10): in which animals were exposed to electromagnetic radiation generated by cell phone and treated (EM+T0) group (n = 10): in which animals were exposed to electromagnetic radiation as experimental group and received LXR agonist T0901317. The exposure to mobile phone radiation was done in the morning between 7:00 a.m. and 10:00 a.m.
2.3. EMF exposure system
Cell phones of the same brand and model were used, their properties were as follows: the peak specific absorption rate (SAR) was 0.96 watt/kg (W/kg) and each of them had an 890–915 MHz carrier frequency band. The electromagnetic field radiation generated by cell phones was nearly equal the average of downlink frequency of Orange network in Egypt (935–945) MHz. A call was made from one set of GSMs (Global System for Mobile Communications) mobile phones to another set of mobile phones after ensuring that each of them was powered-on and the answering mode switched on. Animals were exposed to EM radiations from a mobile phone as calling for 60 min daily for 6 weeks. The cell phones were placed inside plastic experimental cages that did not have any metallic fitting and were placed in the center of the cage to provide approximately equal distribution of radiation to the whole body of the rat. The sets of cell phones were put in special sectors in the middle of the cages to avoid any damage that could be produced by rats. Animals could move freely in the cage during the exposure period. Sham animals were exposed to cell phones in switched off mode in a similarly sized cage for the same period in a separate room like that used for the experimental groups [Citation17].
2.4. LXR agonist administration
LXR agonist T0901317 (Sigma-Aldrich, Saint Louis, MO, USA) was dissolved in 100% dimethylsulfoxide (DMSO) at 1 g/ml and was stored at −20°C till time of administration to rats, the DMSO-containing T0901317 was diluted with phosphate-buffered saline (PBS) to a final concentration of 2% [Citation18]. The rats in the EM+T0 group received 10 mg/kg per day LXR agonist T0901317 orally by gavage for six weeks just after 60 min of EMF exposure. Animals in control and EM groups received the same volume of vehicle (2% DMSO in PBS) orally by gavage at the same time for similar period as EM+T0 group.
2.5. Sample collection
At the end of experimental period, rats’ final body weights were assessed then blood samples were obtained from tail veins and allowed to clot for 2 hours at room temperature before centrifugation for 20 minutes. The sera were frozen at −20°C until hormonal assay. The rats were sacrificed under anesthesia (chloral hydrate) inhalation and both testes were excised out, cleared of adhesive tissue, and weighed precisely.
Left testes were used to prepare testicular tissue homogenates by homogenization with 5 to 10 mL of cold buffer containing: 50 mmol/L potassium phosphate and 1 mmol/L EDTA with pH 7.4 followed by centrifugation for 15 minutes at 4°C. The testicular tissue homogenate was frozen at−80°C until further analytical assay.
The right testes were collected and fixed in 10% formalin solution for histopathological examination.
2.6. Hormonal assay
Serum levels of testosterone (T), Follicle-Stimulating Hormone (FSH), and Luteinizing Hormone (LH) were measured colorimetrically by enzyme‐linked immunosorbent assay (ELISA) kits (MyBioSource, Inc. CA, USA) as manufacturers’ instruction.
2.7. Semen analysis
Caudal epididymis was excised and placed in a sperm collecting bottle containing 5 mL pre-warmed Hank’s Balanced Salt Solution buffer (HBSS) at 37°C. It was cut three times to release sperms. The dilution was obtained by adding 0.05 sperm fluid to 0.95 of HBSS. A hemocytometer was used to determine non‐motile sperm count in diluted sperm fluid then multiplied in the dilution factor. Sperm viability was evaluated from smears on a glass microscope slide stained by eosin-nigrosin dye (1.67% eosin, 10% nigrosin, and 0.1 M sodium citrate) by light microscopy in randomly 10 selected fields microscope that evaluated for the percentage of vital (unstained) and dead (stained) spermatozoa [Citation19]. The container of the diluted sperm fluid was submerged in hot water to kill motile sperms then left to reach room temperature and the total number of sperm was counted using hemocytometers then multiplied in the dilution factor. When gross observation showed a small sperm count in sperm fluid (low turbidity), an undiluted aliquot of the fluid was used.
The following equation was used to calculate the percentage of motile sperm: Motile sperm rate (%) = (Number of sperm ـــ number of non‐motile sperm) X100/Number of sperm.
2.8. Oxidative stress assay in testicular homogenate
Oxidative stress induction was indicated by the amount of lipid peroxidation (LPO) by analysis of malondialdehyde (MDA). It was assessed in testicular homogenates spectrophotometrically by using commercially available ELISA kits (Bio diagnostic, Cairo, Egypt).
Antioxidant assessment was indicated by superoxide dismutase (SOD) activity in testicular tissue. It was measured in testicular homogenates spectrophotometrically by using commercially available ELISA kits (Bio diagnostic, Cairo, Egypt).
2.9. Real-time reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from testicular homogenate specimens using the RiboZol™ RNA extraction reagent (Amresco, Solon, Cleveland, Ohio, USA) as the manufacturer’s instructions. RNA was then reverse transcribed using complementary DNAs (cDNAs) by the SensiFAST TM cDNA synthesis kit (Bioline, London, UK). Real-time PCR was performed using 10 μl of SYBER Green PCR Mix (SensiFAST TM SYBER Lo-ROX Kit, Bioline London, UK). The SYBER green data were analyzed with a relative quantification to β‐actin as an internal control. The sets of primers used were listed in . The relative gene expression ratio is calculated from the real-time PCR using the 2-ΔΔCt method [Citation20].
Table 1. Primer sequences
2.10. Histopathological examination
Testes were fixed in 10% buffered formalin solution overnight, dehydrated, and embedded in paraffin. The paraffin-embedded samples were sectioned at 3 μm thickness with a microtome and the slices were subjected to routine hematoxylin and eosin (H&E) staining. The slides were evaluated under a light microscope by a single pathologist who was blinded to the experimental details. Histopathological assessment of spermatogenesis was evaluated in 30 randomly selected seminiferous tubules from each group using Johnsen’s mean testicular biopsy score (MTBS) [Citation1]. MTBS was calculated by dividing sum of all scores by the total number of seminiferous tubules examined ().
Table 2. Histological criteria and Johnsen’s score for spermatogenesis assessment
2.11. Statistical analysis
The results are presented as descriptive statistics (mean ± standard deviation). Statistical analysis was performed using the Statistical Package for Social Science (SPSS) version 25 (SPSS, Inc., IBM Company, Chicago, IL, USA). The normal distribution of data from each group was confirmed using the Kolmogorov-Smirnov normality test. Since the test indicated that variables followed normal distribution, comparisons among the experimental groups were analyzed by one-way analysis of variance (ANOVA) followed by least significance differences (LSD) test to evaluate statistical difference between two groups. P-value <0.05 was considered significant.
3. Results
3.1. Effect of cell phone radiation and LXRs activation on body and testicular weights
Exposure to mobile radiation as well as LXRs activation induced insignificant change in final body weight, weight gain, and testicular weights (p > 0.05). ()
Table 3. Effect of LXRs activation in EMF-exposed rats on body, right and left testicular weights
3.2. Effect of cell phone radiation and LXRs activation on hormonal assay
Exposure to radiation emitted from cell phones led to significant decrease in serum levels of FSH (p = 0.007), LH (p = 0.005) and testosterone (p = 0.003) in comparison to control rats. LXRs activation induced significant increase in serum levels of FSH, LH and testosterone (p < 0.001) in comparison to EM group ().
Table 4. Effect of LXRs activation in EMF exposed rats on hormonal assay, sperm quality, and testicular oxidative stress markers
3.3. Effect of cell phone radiation and LXRs activation on sperm quality
Rats exposed to cell phone radiation exhibited significant decrease in sperm count, motility, and viability (p < 0.001) in comparison to control rats. LXRs activation induced significant increase in sperm quality parameters (p < 0.001) in comparison to EM group (&).
3.4. Effect of cell phone radiation and LXRs activation on oxidative stress markers
Testicular tissue MDA level was significantly higher (p < 0.001) while SOD activity was significantly lower (p < 0.001) in rats exposed to cell phone radiation when compared with the control group. LXRs activation led to significant decrease in MDA level (p < 0.001) and significant increase in SOD activity (p < 0.001) in EM+T0 group when compared with EM group ().
3.5. Effect of cell phone radiation and LXRs activation on mRNA gene expressions
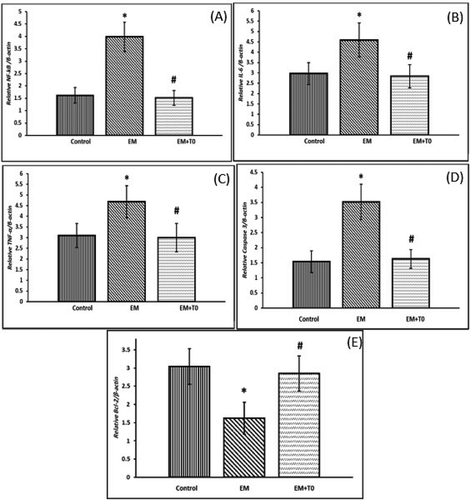
Assessment of inflammation: relative gene expressions of NF‐κB, IL-6, and TNF-α were increased in rats exposed to cell phone radiation (p < 0.001) in comparison to control group. LXRs activation led to significant decrease in NF‐κB, IL-6, and TNF-α expressions (p < 0.001) in EM+T0 group when compared with EM group ().
Assessment of mitochondria-dependent apoptosis: relative gene expression of Caspase 3 was significantly higher (p < 0.001) while relative gene expression of Bcl-2 was significantly lower (p < 0.001) in rats exposed to cell phone radiation when compared with the control group. LXRs activation led to significant decrease in Caspase 3 expression (p < 0.001) and significant increase in Bcl-2 expression (p < 0.001) in EM+T0 group when compared with EM group ().
Figure 2. Effect of cell phone radiation and LXRs activation on gene expressions of NF‐κB (A), IL-6 (B), TNF-α (C), Caspase 3 (D) and Bcl-2 (E). Data are represented as mean ± standard deviation. Significance (P < 0.05): (*) significant when compared with control group, (#) significant when compared with EM group
3.6. Effect of cell phone radiation and LXRs activation on testicular histological assessment
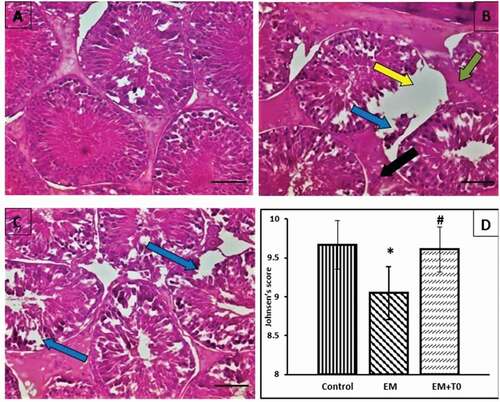
Rats in control group showed normal structure of the testes, rats exposed to mobile radiation showed rupture of the basement membrane, dropped off spermatogenesis cells, intertubular fibrosis, and Leydig cells hyperplasia. Rats exposed to mobile and LXRs activation showed seminiferous tubules lined by nearly normal layers of spermatogenesis cells with mature sperms, the basement membrane of nearly normal thickness, no Leydig cells hyperplasia, and slight focal drop of spermatogenesis cells. Rats exposed to cell phone radiation exhibited significant decrease in Johnsen’s score (p < 0.001) in comparison to control rats. LXRs activation induced significant increase in Johnsen’s score (p < 0.001) in comparison to EM group ().
Figure 3. Photomicrograph of of H & E stain x400 magnification of rat right testis in (A) control group showing normal structure of the testes, (B) mobile radiation exposed group showing: rupture of the basement membrane (yellow arrow), dropped off spermatogenic cells (blue arrow), inter tubular fibrosis (green arrow) and leydig cells hyperplasia (black arrow), (C) mobile radiation exposed and LXRs activated group showing seminiferous tubules lined by nearly normal layers of spermatogenic cells with mature sperms, the basement membrane of nearly normal thickness, no leydig cells hyperplasia and slight focal drop of spermatogenic cells (blue arrow), (D) bar chart showing comparison of Johnsen’s mean testicular biopsy score: data are represented as mean ± standard deviation. Significance (P < 0.05): (*) significant when compared with control group, (#) significant when compared with EM group
4. Discussion
Here in the present study, we hypothesized that exposure to cell phone radiation has hazardous effects on male fertility and LXRs activation could be protective against these effects. We classified these protective effects into four categories including testicular endocrine function, anti-inflammatory and antioxidant status, anti-apoptosis effect besides sperm quality parameters assessment.
Our histological assessment showed that rats exposed to mobile radiation exhibited significant decrease in spermatogenesis with increased disorganization and separation of germ cells in the seminiferous tubules that resulted in spermatogenesis arrest. These changes are similar to that reported by other studies [Citation19,Citation21,Citation22] while Tumkaya et al. reported no histological abnormalities in testicles of rats exposed to mobile phones [Citation23]. However, LXRs activation diminished the EMF-induced damages. These findings could be explained by the cross-links between testicular endocrine status and spermatogenesis as serum levels of testosterone could reveal the normal and/or disturbed spermatogenesis [Citation24]. Moreover, spermatogenesis is hormonally regulated by the hypothalamic-pituitary-gonadal (HPG) axis, with pulsatile secretion of gonadotropins (LH and FSH) leading to androgen synthesis by Leydig cells. In view of these facts, we assessed the testicular endocrine status by evaluating the serum levels of FSH, LH and testosterone [Citation25].
Similar to previous reports about chronic exposure of male Wistar rats to cell phone electromagnetic radiation [Citation26], the exposure to cell phone EMF resulted in diminished serum level of gonadotropic hormonal profile (FSH, LH and testosterone). Moreover, several studies reported the dropped testosterone level in electromagnetism-exposed animals [Citation8,Citation27–29].
However, the effect of exposure to cell phone EMF on gonadotropic hormonal level is controversial. It was reported that 30 days’ exposure to mobile phone radiation for 6 h/day leads to insignificant decrease in serum testosterone levels [Citation19]. Another study on rats showed that EMF significantly lowered serum testosterone with no noticeable changes in FSH, LH levels [Citation22]. Moreover, it was found that mice [Citation30] and rats [Citation31] exposed to EMF exhibited an increase in testosterone levels.
Meanwhile, LXRs agonist up-regulated the lowered serum FSH, LH and testosterone levels in rats exposed to EMF reflecting the ameliorative effect of LXRs activation on endocrine status, which in turn notably improved the histological changes. In legalization with our findings, Volle et al. have reported that LXRs are important for androgen synthesis as the LXR agonist T0901317 induces a 13.3-fold increase in intratesticular testosterone in wild-type mice and low FSH and testosterone levels were observed in LXR αβ-/- mice [Citation14].
In the current study, remarkable elevation of testicular MDA (oxidative stress marker) and significant reduction in testicular SOD activity (endogenous free radical scavenger) were revealed in electromagnetism-exposed rats suggesting that the defective spermatogenesis function is associated with augmented oxidative stress and deterioration in antioxidant activity, that leads to a surge in oxidative stress. However, the animals in T0901317-treated group signified decreased MDA content and improved SOD activity.
Many researchers have reported elevated levels of ROS as well as a reduction in the antioxidant capacity after EMF exposure at different frequencies and power levels [Citation3,Citation20,Citation26,Citation32]. Similarly, in a human study; Agarwal et al. revealed an increase in ROS levels together with a reduction in ROS-TAC score after exposure to EMF from cellular phones while total antioxidant capacity (TAC) of semen revealed no significant changes from the unexposed group [Citation33]. However, other studies failed to find any significant increase in ROS levels in sperm post cell phone radiation exposure in rats [Citation34] or human [Citation35].
LXRs activation is typically known to have valuable effects in several chronic diseases via regulating cholesterol homeostasis and anti-inflammatory action. In this study, we established that LXRs have an antioxidant potential in testicular tissue of rats exposed to cell phone radiation. LXRs antioxidant action was proved in other tissues as peripheral nerve [Citation36], myocardial ischemia reperfusion injury [Citation37], and diabetic nephropathy [Citation38] in studies performed on LXRs knockout mice. The antioxidant effect of LXRs agonists was also evaluated in liver fibrosis [Citation39] and hypertensive rats [Citation40].
On the basis of that EMF could modulate gene expression and cytokine production and oxidative stress is also associated with high levels of pro-inflammatory cytokines that are related to unfavorable semen parameters [Citation11], relative gene expressions of NF‐κB, IL-6, and TNF-α were assessed. Higher NF‐κB, IL-6, and TNF-α expression in testicular homogenates of rats exposed to mobile phone EMF were noticed in the current study while administration of LXRs agonist showed anti-inflammatory action and improved these changes.
The effect of EMF radiation on inflammatory cytokines was investigated in vivo and in vitro and the conclusions are controversial [Citation11]. It was reported that exposure to EMF radiation results in downstream effects such as increased production of more free radicals and cytokines [Citation41]. Moreover, Kim et al. showed in their study on mice that exposure to EMFs can increase the production and expression of pro-inflammatory cytokines (IL-1b, IL-6, and TNF-α) [Citation10]. In human study, it was reported that long-term exposure to EMFs probably affects immune responses, by stimulating the production of pro-inflammatory cytokines [Citation42].
On the contrary, lower plasma NF‐κB and interleukin IL-6 were observed in occupationally exposed workers to EMFs for twenty years [Citation43]. Moreover, a study performed on male workers with high occupational EMF exposure reported that chronic exposure to EMF could affect reproductive functions by decreasing male plasma testosterone but no significant associations of EMF exposure with inflammatory pathway biomarkers were observed [Citation44].
LXRs exert anti-inflammatory effects through inhibiting nuclear factor-κB -dependent induction of pro-inflammatory genes [Citation16]. Studies on male reproductive system from LXR αβ-/- mice revealed another possible role for LXRs in testicular macrophages as LXRs may reduce NF‐κB signaling and reduce levels of the pro-inflammatory cytokines IL-6 and TNF-α [Citation45].
In inflammatory conditions, the mitochondria are exposed to free radicals, leading to mitochondria-dependent apoptosis. There was a relationship between free electrons released from mitochondria and the excessive production of ROS as 1–3% of all electrons in the electron transport chain of mitochondria generate superoxide radicals [Citation46]. As a compensatory mechanism, some proteins such as Bcl-2 are systematized on mitochondrial membrane as antiapoptotic factor to maintain its integrity [Citation47]. Moreover, the caspase 3 activates the cytoplasmic endonucleases leading to damage to the nuclear materials and proteins as well as DNA fragmentation [Citation48].
Minding these facts, here in the current study we investigated relative gene expression of caspase 3 and Bcl-2 in rat testicular tissue and our observations revealed a significant reduction in Bcl-2 with significant increase in caspase 3 expressions after exposure to electromagnetic radiation and these changes were improved by LXRs agonist administration. The apoptotic effect of cell phone radiation was previously reported [Citation3,Citation49]. However, Falzone et al. found no evidence of any outcome of EMF exposure on apoptosis activity such as caspase activation, DNA fragmentation, or ROS generation in human spermatozoa [Citation50].
The anti-apoptotic effect of LXRs could be attributed to its antioxidant and anti-inflammatory action, however, it was reported that LXRs may regulate the balance between the proliferation and apoptosis of germ cells. In LXRs knockout mice, lack of LXR-β reduced proliferation and lack of LXR-α amplified apoptosis and these changes cannot be compensated leading to severe impairment of spermatogenesis [Citation14].
Lastly, the sperm quality that is affected by all the previous changes has been evaluated by assessing sperm count, motility, and viability. The sperm quality evaluation showed that sperm count, motility, and viability were decreased in rats exposed to cell phone radiation while LXRs agonist administration improved sperm quality parameters.
Reliable with our findings, it was reported that cell phone radiation decreased sperm parameters in humans [Citation3,Citation5,Citation33] and rats [Citation20,Citation21]. In contrast to our results, exposure to EMF did not induce any negative effects on sperm quantity, quality, and morphology [Citation8,Citation51,Citation52]. LXRs improving action on sperm quality could be attributed to its antioxidant, ant-inflammatory properties besides its endocrinal action.
5. Conclusion
In conclusion, the usage of cell phones that becomes essential for modern life is associated with obligatory exposure to EMF leads to harmful effects on spermatogenesis and LXRs activation could improve these effects via antioxidant, anti-inflammatory properties as well as its endocrinal action. Further studies to elucidate the effect of natural modulators of liver X receptors on cell phone radiation-induced alternations in male reproductive quality are recommended.
Compliance with ethical standards, ethical approval
All experimental procedures were following the guide for the care and use of laboratory animals (8th edition, National Academies Press) and have been reviewed and approved by Zagazig University institutional animal care unit committee (ZU-IACUC; Sharkia; Egypt) with approval number: ZU-IACUC/3/F/117/2019).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Randa Salah Gomaa
Conception and design: R.S.G., N.M.M. acquisition, analysis, and interpretation of data: N.M.M., A.E.S, R.S.G. Drafting the work or revising: R.S.G., A.E.S, N.M.M. Final approval of the manuscript: N.M.M, R.S.G., A.E.M.
References
- Oh JJ, Byun SS, Lee SE, et al. Effect of electromagnetic waves from mobile phones on spermatogenesis in the era of 4G-LTE. Biomed Res Int. 2018;2018:1801798. PMID: 29789776
- Agarwal A, Deepinder F, Sharma RK, et al. Effect of cell phone usage on semen analysis in men attending infertility clinic: an observational study. Fertil Steril. 2008;89(1):124–128. PMID: 17482179
- Kesari KK, Behari J. Evidence for mobile phone radiation exposure effects on reproductive pattern of male rats: role of ROS. Electromagn Biol Med. 2012;31(3):213–222. PMID: 22897402
- Bhatt CR, Redmayne M, Abramson MJ, et al. Instruments to assess and measure personal and environmental radiofrequency-electromagnetic field exposures. Australas Phys Eng Sci Med. 2016;39(1):29–42. PMID: 26684750
- Liu K, Li Y, Zhang G, et al. Association between mobile phone use and semen quality: a systemic review and meta-analysis. Andrology. 2014;2(4):491–501. PMID: 24700791
- Wdowiak A, Wdowiak L, Wiktor H. Evaluation of the effect of using mobile phones on male fertility. Ann Agric Environ Med. 2007;14(1): 169–172. PMID: 17655195.
- Çetkin M, Kızılkan N, Demirel C, et al. Quantitative changes in testicular structure and function in rat exposed to mobile phone radiation. Andrologia. 2017;49(10):e12761. PMID: 28124386
- Amara S, Abdelmelek H, Garrel C, et al. Effects of subchronic exposure to static magnetic field on testicular function in rats. Arch Med Res. 2006;37(8):947–952. PMID: 17045109
- Pandey N, Giri S. Melatonin attenuates radiofrequency radiation (900 MHz)-induced oxidative stress, DNA damage and cell cycle arrest in germ cells of male Swiss albino mice. Toxicol Ind Health. 2018;34(5):315–327. PMID: 29562845
- Kim SJ, Jang YW, Hyung KE, et al. Extremely low-frequency electromagnetic field exposure enhances inflammatory response and inhibits effect of antioxidant in RAW 264.7 cells. Bioelectromagnetics. 2017;38(5):374–385. PMID: 28370033
- Mahaki H, Tanzadehpanah H, Jabarivasal N, et al. A review on the effects of extremely low frequency electromagnetic field (ELF-EMF) on cytokines of innate and adaptive immunity. Electromagn Biol Med. 2019;38(1):84–95. PMID: 30518268
- Komati R, Spadoni D, Zheng S, et al. Ligands of therapeutic utility for the liver X receptors. version 2. Molecules. 2017;2(1):88–111. PMID: 28067791 .
- Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129(4): 357–367. PMID: 19535829.
- Volle DH, Mouzat K, Duggavathi R, et al. Multiple roles of the nuclear receptors for oxysterols liver X receptor to maintain male fertility. Mol Endocrinol. 2007;21(5):1014–1027. PMID: 17341595
- Rondanino C, Ouchchane L, Chauffour C, et al. Levels of liver X receptors in testicular biopsies of patients with azoospermia. Fertil Steril. 2014;102(2):361–71.e5. PMID: 24842676
- Fessler MB. The challenges and promise of targeting the liver X receptors for treatment of inflammatory disease. Pharmacol Ther. 2018;181:1–12. PMID: 28720427.
- El-Maleky NF, Ebrahim RH. Effects of exposure to electromagnetic field from mobile phone on serum hepcidin and iron status in male albino rats. Electromagn Biol Med. 2019;38(1):66–73. PMID: 30388901 .
- Tachibana H, Ogawa D, Matsushita Y, et al. Activation of liver X receptor inhibits osteopontin and ameliorates diabetic nephropathy. J Am Soc Nephrol. 2012;23(11):1835–1846. PMID: 23085633 .
- Bahaodini A, Owjfard M, Tamadon A, et al. Low frequency electromagnetic fields long-term exposure effects on testicular histology, sperm quality and testosterone levels of male rats. Asian Pac J Reprod. 2015;4(3):195–200.
- Mailankot M, Kunnath AP, Jayalekshmi H, et al. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics (Sao Paulo). 2009;64(6):561–565. PMID: 19578660
- Gautam R, Singh KV, Nirala J, et al. Oxidative stress-mediated alterations on sperm parameters in male wistar rats exposed to 3G mobile phone radiation. Andrologia. 2019;51(3):e13201. PMID: 30461041
- Okechukwu CE. Effects of mobile phone radiation and exercise on testicular function in male wistar rats. Niger J Exp Clin Biosci. 2018;6(2):51–58.
- Tumkaya L, Kalkan Y, Bas O, et al. Mobile phone radiation during pubertal development has no effect on testicular histology in rats. Toxicol Ind Health. 2016;32(2):328–336. PMID: 24097363
- Toocheck C, Clister T, Shupe J, et al. Mouse spermatogenesis requires classical and nonclassical testosterone signaling. Biol Reprod. 2016;94(1):11–24. PMID: 26607719
- Jarvis S, Williamson C, Bevan CL. Liver X Receptors and Male (In) fertility. Int J Mol Sci. 2019;20(21):5379–5392. PMID: 31671745 .
- Oyewopo AO, Olaniyi SK, Oyewopo CI, et al. Radiofrequency electromagnetic radiation from cell phone causes defective testicular function in male wistar rats. Andrologia. 2017;49(10):e12772. PMID: 28261838
- Meo SA, Al-Drees AM, Husain S, et al. Effects of mobile phone radiation on serum testosterone in wistar albino rats. Saudi Med J. 2010;31(8): 869–873. PMID: 20714683.
- Sepehrimanesh M, Saeb M, Nazifi S, et al. Impact of 900 MHz electromagnetic field exposure on main male reproductive hormone levels: a rattus norvegicus model. Int J Biometeorol. 2014;58(7):1657–1663. PMID: 24357488
- Ozguner M, Koyu A, Cesur G, et al. Biological and morphological effects on the reproductive organ of rats after exposure to electromagnetic field. Saudi Med J. 2005;26(3): 405–410. PMID: 15806208.
- Forgács Z, Somosy Z, Kubinyi G, et al. Effect of whole-body 1800MHz GSM-like microwave exposure on testicular steroidogenesis and histology in mice. Reprod Toxicol. 2006;22(1):111–117. PMID: 16434166
- Ozlem Nisbet H, Nisbet C, Akar A, et al. Effects of exposure to electromagnetic field (1.8/0.9 GHz) on testicular function and structure in growing rats. Res Vet Sci. 2012;93(2):1001–1005. PMID: 22130559
- Ribeiro EP, Rhoden EL, Horn MM, et al. Effects of subchronic exposure to radio frequency from a conventional cellular telephone on testicular function in adult rats. J Urol. 2007;177(1):395–399. PMID: 17162098
- Agarwal A, Desai NR, Makker K, et al. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertil Steril. 2009;92(4):1318–1325. PMID: 18804757.
- Dasdag S, Zulkuf Akdag M, Aksen F, et al. Whole body exposure of rats to microwaves emitted from a cell phone does not affect the testes. Bioelectromagnetics. 2003;24(3):182–188. PMID: 12669301
- Falzone N, Huyser C, Franken DR, et al. Mobile phone radiation does not induce pro-apoptosis effects in human spermatozoa. Radiat Res. 2010 Aug;174(2):169–176. PMID: 20681783.
- Hichor M, Sundaram VK, Eid SA, et al. Liver X receptor exerts a protective effect against the oxidative stress in the peripheral nerve. Sci Rep. 2018;8(1):2524–2536. PMID: 29410501
- He Q, Pu J, Yuan A, et al. Activation of liver-X-receptor α but not liver-X-receptor β protects against myocardial ischemia/reperfusion injury. Circ Heart Fail. 2014;7(6):1032–1041. PMID: 25277999
- Patel M, Wang XX, Magomedova L, et al. Liver X receptors preserve renal glomerular integrity under normoglycaemia and in diabetes in mice. Diabetologia. 2014;57(2):435–446. PMID: 24201575
- Heeba GH, Mahmoud ME. Therapeutic potential of morin against liver fibrosis in rats: modulation of oxidative stress, cytokine production and nuclear factor kappa B. Environ Toxicol Pharmacol. 2014;37(2):662–671. PMID: 24583409
- Prahalathan P, Kumar S, Raja B. Morin attenuates blood pressure and oxidative stress in deoxycorticosterone acetate-salt hypertensive rats: a biochemical and histopathological evaluation. Metabolism. 2012;61(8):1087–1099. PMID: 22386933
- Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17(8):958–965. PMID: 23802593
- Hosseinabadi MB, Khanjani N, Samaei SE, et al. Effect of long-term occupational exposure to extremely low- frequency electromagnetic fields on pro-inflammatory cytokine and hematological parameters. Int J Radiat Biol. 2019;95(11):1573–1580. PMID: 31329007
- Zhang D, Zhang Y, Zhu B, et al. Resveratrol may reverse the effects of long-term occupational exposure to electromagnetic fields on workers of a power plant. Oncotarget. 2017;8(29):47497–47506. PMID: 28537898
- Wang Z, Fei Y, Liu H, et al. Effects of electromagnetic fields exposure on plasma hormonal and inflammatory pathway biomarkers in male workers of a power plant. Int Arch Occup Environ Health. 2016;89(1):33–42. PMID: 25808749
- Bhushan S, Tchatalbachev S, Lu Y, et al. Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J Immunol. 2015;194(11):5455–5464. PMID: 25917085
- Ott M, Gogvadze V, Orrenius S, et al. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12(5):913–922. PMID: 17453160
- Sinha K, Das J, Pal PB, et al. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87(7):1157–1180. PMID: 23543009
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013;5(4):a008656. PMID: 23545416
- Liu Q, Si T, Xu X, et al. Electromagnetic radiation at 900 MHz induces sperm apoptosis through bcl-2, bax and caspase-3 signaling pathways in rats. Reprod Health. 2015;12:65–73. PMID: 26239320.
- Falzone N, Huyser C, Franken DR, et al. Mobile phone radiation does not induce pro-apoptosis effects in human spermatozoa. Radiat Res. 2010;174(2):169–176. PMID: 20681783
- Tas M, Dasdag S, Akdag MZ, et al. Long-term effects of 900 MHz radiofrequency radiation emitted from mobile phone on testicular tissue and epididymal semen quality. Electromagn Biol Med. 2014;33(3):216–222. PMID: 23781998
- Trošić I, Mataušić-Pišl M, Pavičić I, et al. Histological and cytological examination of rat reproductive tissue after short-time intermittent radiofrequency exposure. Arh Hig Rada Toksikol. 2013;64(4):513–519. PMID: 24384757