ABSTRACT
Background: The abundance of proteins in human system has made it a major target for glucose auto-oxidation. Likewise, chromosomal instability, describes an oxidative DNA damage that can be accelerated by chronic hyperglycemia. This work investigates the extent and contribution of diabetes oxidation/stress on protein carbonylation and chromosomal instability among 120 type 2 diabetics (60 with vascular complications and 60 without any vascular complications) and 50 apparently healthy control subjects. Anthropometric data and fasting venous blood specimen was collected from each participant for glyceamic control, antioxidants, protein oxidation, oxidative DNA damage parameters and chromosomal aberration assay using standard methods.
Results: Diabetics without vascular complications shows a significant (p = 0.0000) difference in all measured parameters except 8-OHdG (p = 0.0764) as compared to control subjects. However, diabetics with vascular complications show significant (p = 0.0000) difference of all measured parameters than those without vascular complications.
Conclusion: Our study findings indicate an increased formation of protein carbonyl contents, and chromosomal aberration in diabetics especially among those with vascular complications, likewise, diabetes with vascular complications is associated with increased DM disease activity. Thus, protein oxidative biomarker can serve as a therapeutic tool in the management of diabetes cases while increased chromosomal aberration may indicate an increased risk for cancer among diabetics.
1. Background
Type 2 diabetes mellitus is the commonest metabolic and endocrine disorder in Nigeria and it is characterized by chronic hyperglycemia resulting from defect in insulin metabolism [Citation1]. Chronic hyperglycemia if not properly managed has been fingered as a source of body oxidation and the root cause of long term vascular micro and or macro vascular disease/complications that leads to dysfunction of various organs [Citation2]. Likewise, an increased oxidative stress (which might be due to either increase in oxidant production processes or decrease in antioxidant defense mechanism or both) has been observed and canvased as an accelerated cause of these complications along with chronic hyperglycemia in diabetics [Citation3]. These complications have however, imposed a heavy economic, financial and health burden on the fragile Nigeria health system and the society at large [Citation4].
Due to its abundance and high constant reaction rate, proteins are major target for oxidation from free radicals and two-electron oxidants in biological system [Citation5]. In a nutshell, protein oxidation can be defined as an induced covalent modification of protein amino acid side chains or protein backbone either directly (by reactive oxygen species-ROS/reactive nitrogen species-RNS) or indirectly (by secondary by-products of oxidative stress) resulting in fragmentation of protein/protein-protein cross linkages [Citation6]. In diabetics, hyperglycemia has been reported to promote ROS and RNS accumulation through mechanisms that increase the formation of advanced glycation end-products (AGEs). AGEs formation ultimately leads to bio-molecular oxidation which promotes irreversible tissue damage therefore, protein glycation is basically an end product of glucose auto-oxidation, and it is a process initiated majorly through ROS and RNS build up [Citation7].
Due to increased relative risk of morbidity and mortality among individuals with diabetes as compared with apparently healthy non-diabetes controls, diabetes can be viewed as a premature aging syndrome that affects the overall metabolic shift leading to genotoxic stress and loss of chromosomal integrity [Citation8]. Alterations in genomic system occur when the process of oxidative DNA lesions/damage overwhelms the DNA repair mechanism, leading to accumulation of unrepaired damaged DNA in the system. Thus, chromosomal aberration can be described as the microscopically visible part of a wide spectrum of DNA changes generated by different repair mechanisms of DNA double strand breaks and it represents the biological consequences of human exposure to either ionizing or genotoxic agents or both [Citation9]. Chromosomal aberrations can either be a numerical abnormalities (missing of whole chromosome or addition of extra chromosome to the normal pair) or structural abnormalities (when part of an individual chromosome is missing/extra switched to another chromosome/turned upside down) [Citation10]. Structural chromosomal aberration (sCA) is a product of breakage and incorrect rejoining of chromosomal segments that resulting in varieties of disease conditions. Structural chromosomal aberration can either be balanced (the complete chromosomal set is still present after being rearranged, e.g. inversions, translocations) or unbalanced (after the rearrangement the complete chromosomal set is either missing or having an addition, e.g. deletions, duplications, insertions) [Citation11].
Dicentric chromosome is a product of genomic rearrangement leading to placement of two centromeres on the same chromosomes and substantially occurs naturally in humans [Citation12]. It has been identified as an agent of genomic instability associated with evolution of cancer in humans [Citation13]. Ring chromosomes (RCs) are circular DNA molecules that can be formed in both meiosis and mitosis. It occurs as a result of end joining of two double stranded DNA breaks, telomere-subtelomere junction or inv dup del arrangement. The presence of RCs has been attributed to some developmental (both physical and intellectual) delay [Citation14]. Chromosomal breaks are breaks within a chromosome and occurs as a result of DNA damage (by either radiations or chemicals) or as part of chromosomal recombination mechanism. If the break occurs during the G1 phase of cell cycle it’s known as chromosome breaks while, chromatid breaks occurs at G2 phase of cell cycle. Chromosomal brakes have been documented to disrupt or affect the expression of an important gene causing several syndromes [Citation15]. Acentric fragments chromosome are commonly formed when a cell enters and exits metaphase with an improperly or unrepaired double-stranded DNA break. The unrepaired break however, generates two chromosome fragments (one with a telomere, a centromere, and a broken end, and the other with only a telomere and a broken end). Acentrics can also be generated through translocations between acro-centric chromosomes (robertsonian translocations), or through gene amplification [Citation16]. Chromosome gap is a visible non-staining region (achromatic lesion) at the same locus in both chromatids of a single chromosome and there is minimal misalignment of the chromatid [Citation17,Citation18]. With recent scientific advances, different sCA has been categorized as an unstable karyotypes and a major indicator of cancer evolution [Citation10,Citation19]. With recent scientific advances, sCA has been categorized as an unstable karyotypes and a major indicator of cancer evolution [Citation10,Citation19]. This study therefore investigates the extent and contribution of diabetes oxidation/stress on protein carbonylation and chromosomal instability (structural chromosomal aberration) in type 2 diabetics and possibly explore whether they play a role in the disease progression/development of its associated vascular complications.
2. Materials and methods
2.1. Subjects
One hundred and twenty (120) type 2 diabetes mellitus patients (aged between 30 and 65 years), diagnosed according to the WHO [Citation20] criteria, were recruited from the outpatient department (diabetes clinic) of the State Specialist Hospital, Osogbo. Vascular complication was defined as any documented vascular complications (both macro and micro) that developed at least a year after the diagnosis of diabetes mellitus [Citation21]., fourteen neuropathic subjects diagnosed based on the presence of two or more signs of paresthesia, absent pinprick, light touch sensation, sense of position and absent tendon reflexes or muscular atrophy [Citation22], three coronary heart disease subjects based on the standard 12 lead electrocardiogram with the presence of Q wave or of left bundle [Citation23,Citation24], two nephropathic subjects based on the criteria that a patient repeatedly had either a urinary albumin excretion rate of >200 µg/min or a positive urinalysis for protein using a reagent strip [Citation25,Citation26] and 2 diabetic foot patients diagnosed based on exhibition of any pathological features of the foot resulting directly from foot ulcer which took 2 or more weeks to heal [Citation23]. Exclusion criteria were type 1diabetics or secondary diabetics, smokers, recent surgery and acute or chronic infection or undergoing isotope diagnostic or irradiation therapy in previous 3 months, development of vascular complications prior diabetes diagnosis. The control subjects were fifty apparently healthy subjects recruited among the staff of Osun State University but with similar age and sex distribution to those of the diabetes patients. They were subjected to complete medical examination to exclude the presence of any medical problems. Informed consent was obtained from all the participants before the commencement of the study while, a structured research questionnaire was administered on each subjects to collect relevant demographic diabetes information. In addition, anthropometric data to calculate body mass index (BMI) and blood pressure were collected using standardized methods. The clinical characteristics of the diabetics and control subjects were as indicated in .
Table 1. Socio-demographic/clinical characteristics of the study population
2.2. Blood samples and laboratory analysis
Fasting blood sample was obtained by venipuncture (as all subjects were counseled and encouraged on overnight fasting of 10–12 hours and to take no medication on the morning before sample collection), into heparinzed tube for the estimation of the following biomarkers:
Glycosylated hemoglobin (HbA1c) and fasting plasma glucose (FPG) were estimated using ion-exchange resin method of Spectrum© kit (Egypt) and enzymatic glucose oxidase/peroxidase method of Randox© kit (UK) respectively.
Plasma total antioxidant capacity (pTAC), was also measured on the basis of the ability of plasma antioxidants plasma to reduce Fe3+-TPTZ to a blue Fe2+-TPTZ. by a reductant at low pH. and monitored at 593 nm [Citation27].
Protein carbonyl (PCO) content of plasma samples was quantified, as a marker of protein oxidation, using the method of Mesquita et al. [Citation28]. Briefly, plasma samples (400 µl) was added to equal volume of 10 mM DNPH (prepared in 0.5 M H3PO4) solution to produce dinitrophenylhydrazones (in alkaline medium), which was measured at 450 nm.
Protein thiol (–SH) group estimation: Plasma – SH group was estimated according to modified Ellman’s method [Citation29], based on the ability of the – SH group to reduce 5, 5′-dithiobis, 2-nitrobenzoic acid (DTNB) and form a yellow anionic colored product whose absorbance was measured at 412 nm.
Plasma 8-Oxo-2ʹ-deoxyguanosine (8-OHdG) was estimated as an index of DNA damage according to manufacturers direction using an enzyme linked immunosorbent assay (ELISA) detection kit (Northwest Life Science Specialties, Vancouver, USA).
Chromosomal aberration (CA) assay: 0.5 ml whole heparinized blood sample was added to a culture medium (5 ml) containing RPMI 1640 medium (pH 6.8–7.2), 15% fetal bovine serum (15%) and 10 µg/ml phytohemagglutinin (PHA) for 72 hours at 37°C (for each subject, the cultures were set up in triplicates).CA assay was performed for the entire incubation period of 72 hours. Colchicine (50 µg/ml) was added 4 hours before harvesting of the cell cultures (i.e. at 68 hr). The cells were harvested and processed through treatments of hypotonic solution (0.075 M KCl) and fixatives (3:1 methanol:glacial acetic acid). Blind coded chromosome slides were stained with Giemsa solution (5%, pH 6.8) and scored for structural aberration in first division metaphases through an oil immersion objective of a light microscope (Olympus, Japan) [Citation17,Citation30–32].
2.3. Statistical analysis
Data were expressed as mean ± standard deviation (SD) and assessed by the Student’s t-test. The correlation coefficients were determined by Pearson’s simple linear regression analysis. For CA test, the percentage aberration for each group was calculated and result expressed as mean ± standard error. Statistical significance was accepted at p < 0.05. All statistical analyses were performed with Graphad prism 8.
3. Results
One hundred and twenty (120) type 2 diabetics were divided into two groups of sixty (60) (25 males and 35 females) without vascular complications DM-VC (mean age 53.97 ± 9.71) and sixty (60) (20 males and 40 females) with vascular complications DM+VC (mean age 53.35 ± 8.41) diagnosed with different vascular complications. Age (54.16 ± 9.61) and sex (20 males and 30 females) matched fifty (50) apparently healthy individuals served as control group. None of our subjects smoked tobacco or associated substance within the last 6 months. Other clinical/anthropometric and subject characteristics were as shown in .
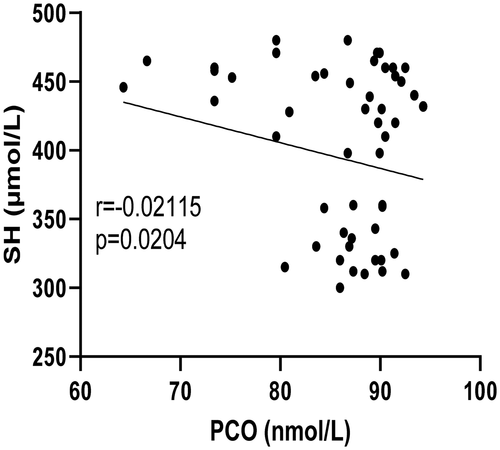
shows the mean levels of FPG, HbA1c level, PCO level and SH level among the study population where there were significant (p < 0.05) differences between the studied parameters in type 2 diabetics and control subjects and also between the diabetics with and without vascular complications. However, there is an inverse relationship between PCO and SH among the test population ().
Table 2. Oxidative indices and DNA damage biomarker of the study population
represents the frequencies of total aberrations seen among the study populations. Type 2 diabetics with vascular complications showed a significantly (p < 0.001) higher value of CA (0.16 ± 0.003) than diabetics without vascular complications (0.09 ± 0.002) and the controls (0.04 ± 0.002) groups. Dicentric type of chromosomal aberration was also the most prevalent aberration seen in our study populations.
Table 3. Chromosomal aberration indices of the study population
shows the relationship between different disease activity variables (HbA1c and diabetes duration) and the measured parameters among the test population. PCO, 8-OHdG and CA were significantly (p < 0.05) increased while SH and pTAC were significantly (p < 0.05) reduced among diabetics having HbA1c values ≥8% than those with HbA1c values <8%. SH was statistically (p < 0.05) lower while, CA was statistically (p < 0.05) higher in diabetics with disease duration ≥5 years than individuals with <5 years disease duration. The difference observed in other parameters were of no statistical significance (p > 0.05) when using disease duration as a yardstick.
Table 4. Oxidative indices and DNA damage biomarker of the test population in different disease activity variables
4. Discussion
In this study, it is clear that there exists a multifactorial involvement of low educational status, increased incidence of obesity and hypertension with development of diabetes in the study population. Although a strong inverse relationship has been established between educational level and incidence of diabetes in North American, Europe and some part of Asia [Citation30,Citation31], there was no available information on any longitudinal study in Nigeria showing an association of educational level with incidence of type-2 diabetes. However, low educational status has been documented to influence an individual’s diet quality, physical inactivity and other unhealthy behavior(s) which are possibly co-clusters promoting diabetes in an individual [Citation32,Citation33].
Various studies have also reported an increased prevalence of obesity and hypertension among type 2 diabetics in Nigeria [Citation34–36]. Increased obesity among Nigeria has been attributed to increased urbanization/westernization with decreased individual physical activity energy expenditure (PAEE) and thus, an independent risk factor for metabolic syndrome [Citation37]. Likewise hypertension, a common feature among diabetics has been shown as an independent risk factor for development of either microvascular or macrovascular complications in diabetes [Citation38]. There is also an increase incidence of diabetes among the young adults (30–49 years) of this study population, a feature also reported by Edo, et al. [Citation34], among his study population and attributed it to increased westernization cum increased sedentary lifestyle among this group of individuals.
Different biochemical factors which are not only involved in the pathogenesis, but also in predicting and preventing the consequences arising out of complications in diabetes mellitus have also been identified. However, this study noted a long standing poor glyceamic control (measured by HbA1c–glycated hemoglobin) as a prominent feature among diabetics especially among those with vascular complications and this goes a long way in accessing the effectiveness of compliance to therapy by patients. Evidently, in-vitro and in-vivo studies have shown that chronic hyperglycemic conditions (as observed in type 2 diabetes) is associated with decreased antioxidants and increase production of reactive oxygen (RO) and reactive nitrogen (RN) species which leads to nitro-oxidative stress affecting biomolecules thereby adversely affecting the vascular (micro and or macro) body system and functioning [Citation6,Citation39,Citation40] and also affecting genomic stability of an organism [Citation41,Citation42].
This study however, investigates the role of free radicals production on protein carbonylation, DNA damage and genomic instability among type 2 diabetes and its associated vascular complications. To assess this we demonstrated the plasma levels of antioxidant/reducing substances (pTAC & SH), level of oxidant (PCO) and genomic instability (8-OHdG & sCA), among type 2 diabetics and to this end, our study revealed a significantly high protein carbonyl contents, DNA damage and frequency of structural chromosomal aberration while antioxidants were significantly lower as compared with non-diabetes subjects.
The increased protein carbonylation however, has been attributed to chronic increase in RO/RN species which has the capacity to react directly (in an irreversible non-enzymatic manner) with proteins to form a highly reactive carbonyl species-PCO (a pre-cursor biomarker in AGEs formation) [Citation43]. Likewise, the increased free radicals have been implicated to induce and increase the varieties of DNA lesions/damage (oxidized bases, abasic sites, DNA strand breaks and formation of cross-links between DNA and proteins) causing various form of chromosomal aberration within the body system [Citation44] as evident in our study
So far there was insufficient literature about the presence of sCA (dicentrics, acentric fragments, breaks, rings and gaps) in type 2 diabetes individuals in our locality. In this present investigation we observe an increased rate of sCA in diabetics with vascular complications as compared to diabetics without vascular complications and normal control individuals. This increased rate can be attributed to increased oxidative stress [Citation41,Citation42] as evidently shown in our study. The significantly high frequency of chromosomal aberrations among type 2 diabetics may therefore indicate that diabetes individuals can be at a higher risk of developing cancers than the control subjects (apparently healthy individuals). Though, some studies have documented an increased risk in cancer development among diabetes individuals [Citation41], but the underlying mechanism is not clearly understood and the development of sCA may therefore be a pointer to increased cancer development among diabetes. Moreover, some anti diabetes medication has been implicated as a potential cytotoxic drug capable of eliciting chromosomal aberration among users [Citation45].
The high protein carbonylation, DNA damage and genomic instability as observed in our study of type 2 diabetes is however, attributed to chronic increase in RO/RN species as reported by earlier studies. To further authenticate this study hypothesis of chronic hyperglycemia advancing the course of diabetes disease and formation of vascular complications following excessive ROS and RNS generation, we measure increased protein oxidation, DNA damage and genomic instability in plasma of type 2 diabetics with and without vascular complications with varying levels of disease activity markers including HbA1c and disease durations. Our study findings indicate that the formation of protein carbonyl contents, DNA damage and chromosomal aberration are higher in diabetics with vascular complications and that diabetes with vascular complications is associated with increased DM disease activity. Likewise, we also found increased oxidation and reduced antioxidants among diabetics with higher glycosylated hemoglobin. The higher levels of protein carbonylation, DNA damage and chromosomal aberration observed in diabetics in the present study, together with HbA1C, disease durations provide strong evidence of the involvement of oxidative stress in the progression of type 2 diabetes disease.
5. Conclusion
This study evaluates protein oxidative biomarkers and chromosomal aberration in diabetics with and without vascular complications. Chronic hyperglycemia in diabetes leads to increased diabetes activities, protein carbonylation and DNA/chromosomal damage with subsequent vascular complications among diabetics. Evaluation of protein oxidative biomarkers can therefore, predict diabetes injury due to oxidation while chromosomal aberration can give an insight to DNA damage and repair system in type 2 diabetics. Large-scale longitudinal study (including invitro study to assess the relationship between drugs and chromosomal aberration) that will validate the result of this study in Nigerian population is hereby advocated.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
O.O. Olaniyan
Olayinka Olaolu Olaniyan holds a doctorate degree in Clinical Chemistry, he currently work in the Department of Chemical Pathology, Osun State University, Osogbo, Nigeria. His area of interest is in the field of diabetes and genetics as it relates to human heath and diseases.
O.O Odewusi
Olufunsho Odeyinka Odewusi holds a Ph.D degree of the university of Benin. He's an Associate Professor in the Department of Medical Laboratory Science, Afe Babalola University, Ado-Ekiti, Nigeria, where he currently teaches Clinical Chemistry and Toxicology as an area of specialization.
H.B Osadolor
Humphrey Benido Osadolor is a Professor of Clinical Biochemistry and Toxicology at the Department of Medical Laboratory Science, University of Benin, Benin City, Nigeria. His area of expertise is in the field of environmental toxicology and human health.
References
- Olaniyan OO, Osadolor HB. Erythrocyte G6PD activity and GSH level as risk factors for vascular complications among type 2 diabetics in Osogbo, Nigeria. Alexandria J Med. 2019;55(1):95–99. DOI: https://doi.org/10.1080/20905068.2019.1688986.
- Olamoyegun M, Ibraheem W, Iwuala S, et al. Burden and pattern of micro vascular complications in type 2 diabetes in a tertiary health institution in Nigeria. Afr Health Sci. 2015;15(4):1136–1141.
- Olaniyan OO, Osadolor HB. Blood lipid peroxidation and DNA damage in a Nigerian population of type 2 diabetics. Sokoto J Med Lab Sci. 2019;4(3):29–36.
- Fasanmade OA, Dagogo-jack S. Diabetes care in Nigeria. Available from. Ann Glob Heal [Internet]. 2015;81(6):821–829.
- Davies MJ. Protein oxidation and peroxidation. Biochem J. 2016;473(7):805–825.
- Zhang W, Xiao S, Ahn DU. Protein oxidation: basic principles and implications for meat quality. Crit Rev Food Sci Nutr. 2013;53(11):1191–201. doi: https://doi.org/10.1080/10408398.2011.577540. PMID: 24007423.
- Nawale RB, Mourya VK, Bhise SB. Non-enzymatic glycation of proteins: a cause for complications in diabetes. Indian J Biochem Biophys. 2006;43(6):337–344.
- Boehm BO, Möller P, Högel J, et al. Lymphocytes of type 2 diabetic women carry a high load of stable chromosomal aberrations; A novel risk factor for disease-related early death. Diabetes [Internet]. 2008 Nov cited 2021 Feb 20];57(11):2950–2957. Available from;():. https://pubmed.ncbi.nlm.nih.gov/18650367/
- Obe G, Pfeiffer P, Savage JRK, et al. Chromosomal aberrations: formation, identification and distribution. Mutat Res - Fundam Mol Mech Mutagen [Internet]. 2002 Jul [cited 2020 Nov 10] ;504(1–2):17–36. Available from: https://pubmed.ncbi.nlm.nih.gov/12106643/
- Vargas-Rondón N, Villegas VE, Rondón-Lagos M The role of chromosomal instability in cancer and therapeutic responses [Internet]. Vol. 10, Cancers. MDPI AG; 2018 [cited 2020 Nov 10]. Available from: https://pubmed.ncbi.nlm.nih.gov/29283387/
- Genetic Alliance; The New York-Mid-Atlantic Consortium for Genetic and Newborn Screening Services. Understanding Genetics: A New York, Mid-Atlantic Guide for Patients and Health Professionals. Washington (DC): Genetic Alliance; 2009 Jul 8. APPENDIX F, CHROMOSOMAL ABNORMALITIES. Available from: https://www.ncbi.nlm.nih.gov/books/NBK115545/https://www.ncbi.nlm.nih.gov/books/NBK115545/
- Stimpson KM, Matheny JE, Sullivan BA. Dicentric chromosomes: unique models to study centromere function and inactivation. Chromosom Res. 2012;20(5):595–605.
- Mackinnon RN, Campbell LJ. The role of dicentric chromosome formation and secondary centromere deletion in the evolution of myeloid malignancy the role of dicentric chromosome formation and secondary centromere deletion in the evolution of myeloid malignancy. Genet Res Int. 2011;2011:1–11.
- Pristyazhnyuk IE, Menzorov AG. Ring chromosomes : from formation to clinical potential. Protoplasma. 2017;255(2018):439–449.
- Eid MM, Temtamy SA. Cytogenetic studies of chromosomal breakage diseases. Middle East Journal of Medical Genetics. 2013;2(1):11–22.
- Warecki B, Sullivan W. Mechanisms driving acentric chromosome transmission. Chromosome Res. 2020 Dec;28(3–4):229–246. doi: https://doi.org/10.1007/s10577-020-09636-z. Epub 2020 Jul 25. PMID: 32712740.
- Husain SA, Balasubramanian S, Bamezai R. Chromosome breaks gaps in breast cancer patient. Cytologia (Tokyo). 1996;61(1):47–51.
- Savage JRK. On the nature of visible chromosomal gaps and breaks. Cytogenet Genome Res. 2004;55(104):46–55.
- Lobo I. Chromosome abnormalities and cancer cytogenetics. Nat Educ. 2008;1(1):68.
- World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia : report of a WHO/IDF consultation [Internet]. World Health organisation; 2006. 50 p. Available from: https://www.who.int/diabetes/publications/Definitionanddiagnosisofdiabetes_new.pdf
- Frank RN. Diabetic Retinopathy. N Engl J Med. 2004;350(1):48–58.
- Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy : a position statement by the American diabetes association. Diabetes Care. 2017;40(1):(January):136–54.
- Arambewela MH, Somasundaram NP, Jayasekara HBPR, et al. Prevalence of chronic complications, their risk factors, and the cardiovascular risk factors among patients with type 2 diabetes attending the diabetic clinic at a tertiary care hospital in Sri Lanka. J Diabetes Res. 2018;2018:4504287.
- Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation [Internet]. 2006 May 30 [cited 2021 Feb 13];113(21):2495–2501. Available from: http://www.circulationaha.org
- Nakhoul FM, Zoabi R, Kanter Y, et al. Haptoglobin phenotype and diabetic nephropathy. Diabetologia [Internet]. 2001 May cited 2019 Jul 15];44(5):602–604. Available from;():. http://www.ncbi.nlm.nih.gov/pubmed/11380078
- Gross JL, Mirela J DA, Silveiro SP, et al. Diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):176–188.
- Kaushik A, Jijta C, Kaushik JJ, et al. FRAP (ferric reducing ability of plasma) assay and effect of diplazium esculentum (retz) sw. (a green vegetable of North India) on central nervous system. Indian J Nat Prod Resour. 2012;3(2):228–231.
- Mesquita CS, Oliveira R, Bento F, et al. Simplified 2, 4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem [Internet]. 2014;458:69–71. Available from.
- Chakrabarty J, Vidyasagar MS, Fernandes D, et al. Effectiveness of pranayama on the levels of serum protein thiols and glutathione in breast cancer patients undergoing radiation therapy: a randomized controlled trial. Indian J Physiol Pharmacol. 2013;57(3):225–232.
- Agardh E, Allebeck P, Hallqvist J, et al. 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol [Internet]. 2011Jun [cited 2020 Nov 10];40(3):804–818. Available from: https://pubmed.ncbi.nlm.nih.gov/21335614/
- Shang X, Li J, Tao Q, et al. Educational level, obesity and incidence of diabetes among chinese adult men and women aged 18 – 59 years old : An 11-year follow-up study. PLoS One. 2013;8(6):e66479–e66479.
- Booth SL, Sallis JF, Ritenbaugh C, et al. Environmental and societal factors affect food choice and physical activity: rationale, influences, and leverage points. Nutr Rev. 2001 Mar;59(3 Pt 2):S21–39. doi: https://doi.org/10.1111/j.1753-4887.2001.tb06983.x. PMID: 11330630.
- Adam Drewnowski, SE Specter, Poverty and obesity: the role of energy density and energy costs, The American Journal of Clinical Nutrition,79(1): 6–16, https://doi.org/https://doi.org/10.1093/ajcn/79.1.6
- Edo A, Edo G, Ohenhen A, et al. Age at diagnosis and duration of type 2 diabetes seen in Benin City, Nigeria. African J Diabetes Med. 2015;23(1):18–19.
- Chinenye S, Ogbera A, Fasanmade O, et al. Profile of Nigerians with diabetes mellitus - Diabcare Nigeria study group (2008): Results of a multicenter study. Indian Journal of Endocrinology and Metabolism. cited 2020 Nov 10. 2012;16(4):558. Available from:. /pmc/articles/PMC3401756/?report=abstract
- Gezawa I, Mijinyawa M, Musa B, et al. Prevalence of hypertension and its relationship with indices of obesity in Maiduguri, Northeastern Nigeria. Niger J Basic Clin Sci [Internet]. cited 2020 Nov 10. 2014;11(2):67. Available from:. http://www.njbcs.net/text.asp?2014/11/2/67/140307
- Okubadejo NU, Ozoh OB, Ojo OO, et al. Prevalence of hypertension and blood pressure profile amongst urban-dwelling adults in Nigeria: a comparative analysis based on recent guideline recommendations. Clin Hypertens [Internet]. 2019 Dec; cited 2020 Nov 10 25(1): Available from: https://pubmed.ncbi.nlm.nih.gov/31016027/
- Unadike B, Essien I, Akpan N, et al. Indications for and outcome of diabetic admissions at the university of uyo teaching hospital, uyo. Ibom Med J. 2013;6(1):16–22.
- Almogbel E, Rasheed N. Protein mediated oxidative stress in patients with diabetes and its associated neuropathy : correlation with protein carbonylation and disease activity markers. J Clin Diagn Res. 2017;11(2):21–25.
- Zang Z, Apse K, Pang J, et al. High glucose inhibits glucose-6-phosphate dehydrogenase via cAMP in aortic endothelial cells. J Biol Chem. 2000;275(51):40042–40047.
- Anand S, Nath B, Diabetes SR. - Increased risk for cancers through chromosomal aberrations? Asian Pac J Cancer Prev. 2014;15(11):4571–4573.
- Limoli CL, Giedzinski E. Induction of chromosomal instability by chronic oxidative stress. Neoplasia. cited 2020 Nov 10 2003;5(4):339–346. Available from https://pubmed.ncbi.nlm.nih.gov/14511405/
- Curtis JM, Hahn WS, Long EK, et al. Protein carbonylation and metabolic control systems. Available from. Trends Endocrinol Metab [Internet]. 2012;23(8):399–406.
- Cinkilic N, Kiyici S, Celikler S, et al. Evaluation of chromosome aberrations, sister chromatid exchange and micronuclei in patients with type-1 diabetes mellitus. Mutat Res/Genet Toxicol Environ Mutagen. 2009;676(2009):1–4.
- Bonnefond A, Skrobek B, Lobbens S, et al. Association between large detectable clonal mosaicism and type 2 diabetes with vascular complications. Nat Genet. 2013;45:1040–1045.