ABSTRACT
Background
All drugs profoundly modify our biological processes and may manifest as adverse drug reactions (ADRs), which are unpredictable and inevitable consequences. Antibiotics are a common cause of ADR, necessitating stopping or change of antibiotics. The incidence of ADRs increases with the number of drugs prescribed in a prescription, and antibiotics are rarely prescribed as monotherapy.
Aim
The study aimed to assess frequency, class of antibiotics, symptoms, causality, the severity of antimicrobial-associated ADRs, and see the demographic distribution.
Methods
ADRs were collected and filled in suspected ADR forms and sent via vigiflow to the National Coordination Centre-Pharmacovigilance Programme of India (NCC-PvPI). These ADR reports, termed individual case safety reports (ICSRs), were analyzed from Jan 2016 to Dec 2019.
Results
A total of 414 (54.33%) ICSRs of 762 were identified as antimicrobial-associated. Adults in the age group 19–65 years accounted for 345 (83.09%) of ADRs. A total of 192 (46.38%) were males, and 222 (53.14%) were females. Skin and subcutaneous tissue System organ class was involved in 54% of cases. In the causality assessment, 268 (64.49%) were “probable,” 123 (29.71%) were “possible,” and 23 (5.56%) were “certain.” On severity assessment, 256 ADRs (61.83%) were mild, 133 (32.12%) were moderate, and 25 (6.03%) were severe. A total of 54 antimicrobial agents, excluding anti-tubercular drugs, were identified, and antibacterial accounted for 268 (64.73%) ADRs, followed by antiviral 90 (21.73%), antiprotozoal agents 33 (7.97%) antimalarials anti-scabicidal, antifungal accounting for the remaining.
Conclusion
Antimicrobials play a crucial role in treating infections, and utmost vigilance during antimicrobials prescription reduces the frequency and severity of the ADRs, thereby reducing the morbidity and mortality and the pharmacoeconomic burden to the health care system. Pharmacovigilance must be boosted to ensure the safe and effective use of antibiotics and reduce the occurrence of ADRs.
1. Introduction
Antibiotics are the most prescribed medication worldwide, and their use is constantly increasing. The World Health Organization (WHO) report on surveillance of antibiotic consumption between 2016 and 2018 shows an overall consumption ranging from 4.4 to 64.4 Defined Daily Doses (DDD) per 1000 inhabitants per day [Citation1]. India is among the leading consumer of antibiotics, and its consumption increased from 3.2 billion DDDs in the year 2000 to 6.5 billion DDDs in 2015, amounting to a 103% rise [Citation2]. Globally, consumption of antibiotics increased from 8.2 to 13.6 DDD per 1000 inhabitants per day from the year 2000 to 2015, amounting to a 65% rise [Citation3]. This surge in antibiotic usage in India is attributed to increased incidence of infectious diseases, mass manufacturing of generic antibiotics that are cheaper, increased income, and availability of government health insurance schemes. Availability of antibiotics without prescription is another major issue in India; therefore, red strip labeling of packages has been made mandatory to reduce dispensing without prescription. Increased use of antibiotics is associated with an increase in antimicrobial resistance and an increased incidence of adverse drug reactions (ADRs) [Citation4]. Adverse drug reaction (ADR), as defined by the World Health Organization (WHO), is “a response to a drug which is noxious and unintended, and which occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease, or for the modifications of physiological function” [Citation5]. ADRs can occur with any class of drugs, and over half of the hospitalized patients receive at least one antibiotic during their hospital stay, of which 55.5% ADRs are definitely preventable and accounts for 20–50% of the drug expenditure in the hospitals [Citation6]. According to a study conducted in Johns Hopkins Hospital, Maryland, between 2013 and 14, 20% of the hospitalized patients experienced at least one antibiotic-associated ADR and its frequency increase as the number of antibiotics increase [Citation7]. The overall incidence of ADRs is 0.15% to 30% [Citation8]. A systematic review in India reports that the median incidence of ADRs leading to hospitalization is 2.85% and those developed during hospitalization as 6.34% [Citation9]. Despite drug safety studies done during clinical trials and manufacturing, diagnosing, and quantifying prescription-related ADRs remains a challenge. This is because clinical trials are limited to a few hundred to thousand patients chosen based on inclusion and exclusion criteria, unlike patients in clinical settings with various comorbidities and lifestyles. Antibiotic-associated ADRs have resulted in 43% of antibiotics withdrawal, mostly cephalosporins, and fluoroquinolones approved between 1980 and 2009 within 15 years of approval [Citation10]. Development of ADR with an antibiotic compels the clinician to prescribe an antibiotic either of the altered spectrum or efficacy and toxicity, posing a threat to patient safety. The incidence of ADRs associated with certain antibiotics is predicted while for some it is not. For example, penicillin’s most serious hypersensitivity reaction is anaphylaxis and is fatal in about 0.001% of patients; and skin rash of all types due to ampicillin occur in 9% patients [Citation11]. However, frequency of certain ADRs is not known, and such ADRs mandate drug regulatory authorities to update drug usage information.
In line with this inkling, the WHO established the Pharmacovigilance program in 1968 for pooling data in ADRs from multiple countries. This program is coordinated at its collaborating center in Uppsala Monitoring Centre (UMC), Sweden, with more than 160 countries participating, including India [Citation11]. Pharmacovigilance Programme of India (PvPI), maintained by the Indian Pharmacopeia Commission (IPC), functions as the National Coordination Centre (NCC) started in 2010 and became a WHO collaborating center in 2017 and is responsible for ensuring the safety of medicines used by the Indian population. India collects about 50,000 domestic ADRs yearly and shares them with the World Health Organization (WHO) Programme for International Drug Monitoring in VigiBase [WHO global database of individual case safety reports (ICSR)] through vigiflow. More than 250 ADR monitoring centers (AMC) are functioning for reporting ADRs in the Indian database. PvPI aims to enhance patient care and safety concerning the use of medicines and provide reliable information by regularly sending drug safety and therapeutic device alerts [Citation12,Citation13]. The AMC at our tertiary hospital contributes ICSRs to the NCC, and in addition, regularly analyzes the ADRs, deals with under-reporting issues, and sensitizes the health care workers to report any suspected ADR to reduce drug-related morbidity and mortality [Citation14].
India is the fourth largest pharmaceutical producer globally with more than 60,000 formulations and is emerging as a clinical trial hub exposing a large population to newer drug treatments and related ADRs [Citation15]. Therefore, it is imperative to identify antibiotic-associated ADRs as early as possible to ensure their management, formulate guidelines for adequate and appropriate consumption, and frame ADR reporting and prevention policy. However, specific antibiotic-associated ADR data from India is not available, more so from central India, necessitating more studies from regional and state AMCs. A previous study from our AMC analyzed ADRs occurring due to all medicines and reported the highest ADRs due to antimicrobial, so a need for longer duration and specific antimicrobial-associated ADR profiling was felt [Citation14]. With this context in mind, this study was conducted to analyze the frequency, antibiotics involved, System Organ Class (SOC) affected, causality, and severity of antimicrobial-associated ADRs in a tertiary hospital of central India. Antimicrobial-associated ADRs reported from both outpatient and inpatient settings, by all routes and for all ages were included, enabling us to produce a comprehensive picture of the overall incidence and profile of ADRs.
2. Materials and methods
2.1. Data source
The study was conducted at the Department of Pharmacology, PT JNM Medical College, and associated B. R. Ambedkar hospital, Raipur, Chhattisgarh, India. It is a 1100 bedded tertiary care teaching hospital. Suspected ADR form was used to collect ADR information as per the NCC-PvPI standard operating procedure. The ADRs were sent to IPC-PvPI (Indian database) via vigiflow as Individual case safety reports (ICSRs). Data was extracted from the Indian Database between January 2016- December-2019 (4 years). Collection and assessment follow the procedure described by Singh et al. [Citation14].
2.2. Study design
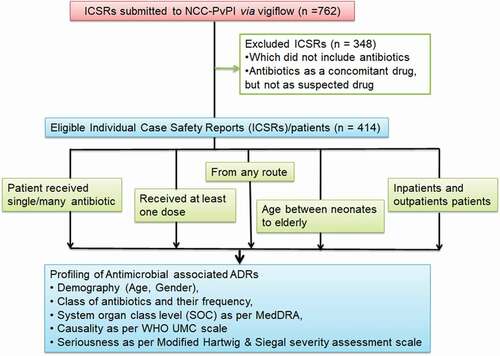
The database search comprised all the ICSRs reported from Pt JNM Medical College Raipur associated BR Ambedkar Hospital, Raipur Chhattisgarh, India. Only those suspected ADRs involving at least one antimicrobial agent with at least one dose in all age groups, by any route of administration, and both outpatient and inpatient patients, were included in the study (). ADRs related to anti-tubercular drugs were excluded from the study as separate hospital functions to implement the National Tuberculosis Elimination Program and report the ADRs directly to the IPC-PvPI. The identification of the patients and reporters was kept confidential.
The ADR profiling was done explicitly under the following heads: demography, antimicrobial agent implicated, SOC involved [Medical Dictionary for Regulatory Activities (MedDRA Version 22.1)], as MedDRA terminology helps to standardize ADR terminology and enhance profiling [Citation16]. The causality assessment was done using the WHO UMC causality scale (causality assessed by the AMC causality assessment committee to avoid incongruity between assessors) [Citation17]. Severity assessment was done by Modified Hartwig and Siegel severity assessment scale and classified as mild, moderate, or severe [Citation18,Citation19].
2.3. Statistical analysis
The data was incorporated in the MS-Excel sheet, and the categorical data in numbers were converted to percentages to achieve readily comparable information and quantify the difference between them.
3. Results
3.1. Demography
During the study period, a total of 414 (54.33%) antimicrobial-associated ICSRs were analyzed of 762 ICSRs. Adults in the age group 19–65 years accounted for 345 (83.33%) of ADRs, and the remaining age groups comprised only 69 (16.67%) ADRs. In addition, 222 (53.62%) ICSRs were of females, and 192 (46.38%) of males, as illustrated in .
Table 1. Analysis of demographic, and causality of adverse drug reactions (ADRs)
3.2. Drug frequency
A total of 54 antimicrobial agents were involved () comprising: antibacterial [268 (64.73%)], antiviral [90 (21.73%)], antiprotozoal [33 (7.97%)], antileprotic and antifungal [9 each (2.17% each)], antimalarial [3 (0.72%)] and antiscabicidal [2 (0.48%)]. Anti-tubercular drugs were excluded. The cephalosporins [104 (25.12%)], antiretroviral agents [90 (21.7%)], penicillin, and its semisynthetic derivatives [78 (18.84%)] quinolones and antiprotozoal [33 (7.97%) each] were the top five offenders. Among individual antimicrobial drugs, the top five agents causing ADR were ceftriaxone [75 (18.11%)], fixed drug combination (FDC) of tenofovir/lamivudine/efavirenz (TLE) [68 (16.42%)], piperacillin tazobactam FDC and Ciprofloxacin [24 (5.79%) each], and cefixime [20 (4.83%)].
Table 2. Individual drug frequency reported in individual case safety reports [(ICSRs) n = 414].a
3.3. System organ class (SOC) involvement
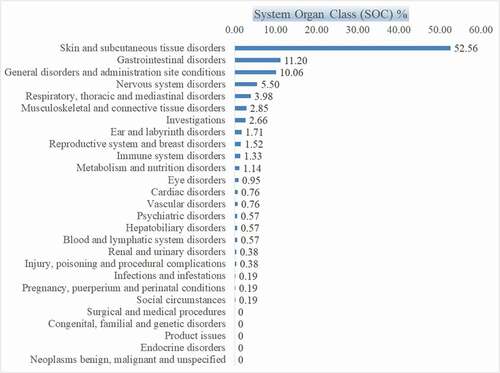
Some ICSRs showed involvement of multiple systems in the body, so of 414 ICSRs reported, 527 SOC involvement was noted as per Medical Dictionary for Regulatory Activities (MedDRA) terminology using version 22.1 (). “Skin and subcutaneous tissue” was involved in 277 (52.56%) reports, followed by “gastrointestinal disorders” [59 (11.19%) reports] and “general disorders and administrative site” [53 (10.05%) reports]. On individual SOC analysis, drugs most implicated in the SOC of skin and subcutaneous tissue () were antiviral FDC of TLE (17.32%), ceftriaxone (12.19%), and amoxicillin-clavulanic acid (10.46%); and manifested as a maculopapular itchy rash, urticaria, and in some cases as skin exfoliation. In the SOC Gastrointestinal disorders (), the drugs most implicated were FDC of TLE (23.72%) and amoxicillin-clavulanic acid (22.03%); and manifested as vomiting, abdominal pain, oral mucositis, and diarrhea. also shows the involvement of other SOCs and drug frequencies. Immune system disorders were reported in only seven ICSRs manifesting as anaphylaxis, and the drugs implicated were ceftriaxone, amoxicillin-clavulanic acid, and dapsone in 4, 2, and 1 case, respectively. No drugs could be assigned to SOC of endocrine disorders, neoplasm, product issues, surgical and medical conditions, and congenital disorders.
Table 3. Antimicrobial-associated adverse drug reactions (ADRs) involved in skin and subcutaneous tissue (SOC).a.
Table 4. Antimicrobial-associated adverse drug reactions (ADRs) involved in different system organ class (SOC).a.
3.4. Causality
As per the “WHO UMC scale,” the causality was “certain” in 23 (5.5%) ICSRs, “possible” in 123 (29.71%) ICSRSs, and “probable” in 268 (64.73%) ICSRs. No cases were assigned to “unlikely,” “unclassified,” and “unclassifiable” ().
3.5. Severity assessment
On severity assessment (), 61.83% ADRs were “mild” (level 1 and 2), 32.12% were “moderate” (level 3 and 4), and 6.03% were “serious” (level 4,5, and 6). Serious ADRs were most frequent with ceftriaxone, amoxicillin-clavulanic acid, and FDC of TLE. Fatal outcomes reported in four cases were suspected to be due to ceftriaxone (two cases) and FDC of TLE and dapsone (one case each).
Table 5. Severity of adverse drug reactions (ADRs) as per modified Hartwig and Siegel severity assessment scale
4. Discussion
Suspected ADRs are common in both inpatient or outpatient settings and are a common cause of morbidity and mortality. The global or national frequency of antimicrobial-associated ADRs is unknown and varies across different countries and within our country. A higher occurrence of antimicrobial-associated ADR was found in this study (54.4%) compared to studies reporting a lower occurrence (17% to 20%). This could be due to variation in the type of studies, their inclusion and exclusion criteria, and settings [Citation7,Citation20,Citation21]. However, one study reports a still higher frequency (62.8%) of ADR [Citation8,Citation22]. The higher frequency of antimicrobial-associated ADRs in this study could be due to reasons cited in various studies. India is a low-income country with an increased incidence of bacterial and non-bacterial infections, poor quality of air and associated respiratory tract infections and overcrowding, contributing to the easy spread of infections [Citation3]. Availability of antibiotics without a prescription, non-essential prescription, easy availability of cheap/affordable generic antibiotics, and accessibility of health insurance, both public and private, adds to the problem [Citation23]. Most antibiotic prescriptions contain concomitant medication for symptomatic relief, and these medicines might interact, leading to increase in adverse events. The increase in ADR frequency is exponential rather than linear and is 3.6% in patients receiving up to three drugs and increase to 11.1% when four or more drugs are used [Citation24]. Hurwitz N also reports an increase in ADR frequency from 3.3% to 19.8% when the number of drugs increased from 5 or less to 6 or more [Citation25]. Antibiotics-associated ADRs frequently affect the skin and subcutaneous tissue, the largest organ and the most visible, leading to early reporting [Citation8]. The female preponderance in this study is consistent with other antibiotic-associated ADR studies [Citation7,Citation8,Citation20]. Females are reported to be at 1.5 to 1.7 fold higher risk than males, and this could be due to pharmacokinetic and pharmacodynamic differences in gender and drug use habit, as women seek medical attention more than males for trivial problems leading to receiving more drugs [Citation26–28].
The frequently implicated classes of antimicrobials were cephalosporins (25.12%), antiretrovirals (21.73%) followed by penicillins (18.84%). Among individual drugs, ceftriaxone (18.11%), fixed-dose combination of tenofovir/lamivudine/efavirenz [(TLE) (16.42%)], and amoxicillin-clavulanic acid (11.83%) were the top offenders. It is challenging to compare this study with other studies because of different inclusion and exclusion criteria and varied methodology. Shehab et al., in their study, excluded topical antibiotics, report 19% of the visit to the emergency department due to antibiotic-associated ADRs, and allergic manifestations (78.7%) was most common; Penicillin and cephalosporin were implicated in 36.9% and 12.2% ADRs [Citation29]. Similarly, R Kiguba et al. conducted their study among hospitalized patients only; the most frequent ADR was gastrointestinal symptoms (50%) followed by neurological symptoms (24%), and ceftriaxone was the most common antibiotic (43%) [Citation20]. Hagiya H et al. conducted the study among hospitalized patients who received only systemic antibiotics and reported gastrointestinal, hepatobiliary, and dermatological manifestations in decreasing frequency, and piperacillin-tazobactam (20.7%) as the most frequently implicated drug [Citation21]. Jong et al. report the most frequent involvement of skin and subcutaneous tissue conditions followed by gastrointestinal disorders and penicillin and quinolones (16%) followed by third generation cephalosporins (14.9%) as the most frequently implicated antibiotics [Citation8]. Richa et al. conducted a similar study in India between 2010 and 2013 and reported that only 15.15% of ADRs were due to antibiotics; dermatological symptoms seen in 47.44% and gastrointestinal disorders in 39.28% reports; and ceftriaxone injections followed by azithromycin oral tablets implicated in 35.71%, and 7.39% ADRs [Citation30]. All the above study done in the last 15 years are different; none includes all classes of antimicrobials: antibacterials, antivirals, antifungals, and antiprotozoals. However, penicillins and cephalosporins both belong to β-lactam group of antibiotics is common in all the above studies. Penicillin and its semisynthetic derivatives and cephalosporins are frequently involved in immediate hypersensitivity reactions mostly mediated by immunoglobulin E [Citation31]. β-lactams act as haptens, cross reactivity between penicillin and cephalosporin and unknown prior exposure to penicillin in any form and frequent prescription to these drugs could be the reason for this increased ADRs [Citation31–33].
According to the MedDRA SOC classification, ADRs involving “Skin and subcutaneous tissue” were most frequent (54.02%), followed by “gastrointestinal disorders” and “general disorders and administrative site conditions” (10.15% each). Some drugs involved more than one organ system, so we report 527 SOCs involvement for 414 ICSRs. Few system organ class involvement was not observed in this study, as shown in , like endocrine disorders, neoplasms, congenital familial and genetic disorders, surgical and medical procedures, and product issues. The ADRs involving these SOCs often go unreported or are misallocated to other SOCs because of the difficulty in implicating a drug as its cause. Treating clinicians must possess a high suspicion index to label these effects as drug-induced. For example, glucose metabolism disorders and diabetic complications are multiaxial and can be linked to both SOC- “metabolism” and “nutrition disorders.” So, if one had to report gatifloxacin (now withdrawn) associated dysglycemia, it can be reported in any one of the two SOC [Citation34]. Metronidazole, sulfamethoxazole, dapsone, and isoniazid-induced pancreatitis can be attributed to either endocrine or gastrointestinal disorders SOC [Citation35]. It is often reported as gastrointestinal SOC as the patient presents with abdominal pain, vomiting, and diarrhea. Laboratory findings of raised pancreatic enzyme and radiological investigations for confirmatory diagnosis are seldom done in the initial phase to diagnose it as drug-induced pancreatitis to label it as an endocrinal effect. Antimicrobials causing congenital disorders or teratogenicity are well documented and include sulfonamides, aminoglycosides, chloramphenicol, tetracycline, erythromycin, and vancomycin, and are usually not prescribed during pregnancy [Citation36]. Therefore, the occurrence of ADRs in SOC congenital familial and genetic disorders is rare. The SOC product issue is focused on issues related to products rather than clinical or patient-related concepts and mostly goes unreported. Identifying neoplasms, both benign and malignant, as an adverse outcome of antibiotics or any medication requires in-depth knowledge, eliciting a detailed history, long-term follow-up, and maintaining the patient’s electronic medical records, which may be possible after a few decades in India.
The WHO UMC scale was used for the causality assessment of the individual case report. “Probable” ADRs, followed by “possible” and “certain,” follow the same frequency pattern reported in other studies [Citation8,Citation30,Citation37]. This higher frequency of probable is ascribed to the fact that it is convenient to establish a causal time relationship of the adverse reaction with the suspected drug and exclude its occurrence due to disease or other drug and improvement on withdrawal. Reporting an ADR as “certain” is problematic because diagnostic tests specific for the adverse drug effect are usually absent, and a re-challenge is ethically unjustified [Citation17].
Severity assessment using modified Hartwig and Siegel severity assessment scale allocates a majority of the ADRs as mild, followed by moderate and severe, which corroborates with Indian studies [Citation6,Citation37]; however, one study shows a preponderance of moderate severity ADRs [Citation38]. The most offending agent in severe ADR was Ceftriaxone, and this could be due to higher ceftriaxone prescriptions in our setting. In its report on antibiotic consumption, “WHO” mentions high consumption of cephalosporins and quinolones in some countries and very high consumption of third-generation cephalosporins in all states in India [Citation1]. Other risk factors for ceftriaxone-related ADRs include rapid intravenous injection and not eliciting the previous history of allergic reaction. It must be mentioned here that routine intradermal testing is done in most hospitals, preventing many ADRs. We report four fatalities; two with injectable ceftriaxone and one each with oral TLE and Dapsone. Ceftriaxone has been implicated for the highest number of deaths in the Iranian database also [Citation39]. A study on ADRs due to antimicrobial agents reports TLE-based regimens accounting for 66.9% of the total ADRs but do not mention any fatal reaction [Citation40]. We must keep in mind that antiretroviral therapy is relatively safe, and, 5–40.8% of death in patients with human immunodeficiency virus (HIV) occur within the first six months of initiating treatment and could be due to advanced clinical stage of the disease, low baseline CD4 count predisposing to infections, and poor treatment adherence [Citation41]. Fatality reported with dapsone was diagnosed as Dapsone Hypersensitivity Syndrome (DHS) and is well known as a rare potentially fatal reaction if not recognized and managed timely [Citation42]. The incidence of DHS is 0.5–3.6% with a mortality of 9.9%; acute clinical courses, mucosal involvement, hepatitis, older age, and low socioeconomic status of the patient account for higher risk of fatal outcome [Citation43].
The strength of our study is its broad inclusion of all antimicrobials, including antibacterials, antivirals, antifungals, antiprotozoals, and long study duration. However, there are several limitations to our study. First, this study was conducted at a single ADR monitoring center and lacked reports from other state centers. Second, ADRs are usually reported voluntarily, and mild reactions might have gone unreported. Third, few reports contained more than one antibiotic, the clinical manifestation of suspected adverse effect was ascribed to the antibiotic well known for the documented adverse effect with a possibility of missing out on “new signals.” Fourth, our findings cannot be generalized to other hospitals in our state or country, which have a different antibiotic utilization pattern, and there is a difference in the clinician’s knowledge, experience, and observational skills for reporting ADRs.
5. Conclusion
Ceftriaxone was responsible for the highest risk for antibiotic-associated ADRs, followed by a tenofovir-based TLE regimen. The most frequent clinical symptoms were of skin and subcutaneous tissue SOC, followed by gastrointestinal disorders. The causality of a majority of ADRs was probable, and the severity was mild. The national coordinating center must publish a similar study and compare it with other developed countries to find if our population is more prone to specific antibiotic-associated ADRs. Periodic analysis of antibiotic safety data will help assess the accurate burden of ADRs in terms of patient morbidity and mortality, human resources, and financial resources; and help formulate guidelines and policies to prevent or reduce the frequency and severity of ADRs, and contribute to antibiotic stewardship.
Disclosure of potential conflicts of interest
Manju Agrawal, Preeti Singh, and Usha Joshi declare that they have no competing interests.
Acknowledgments
The authors thank the National Coordination Centre-Pharmacovigilance Programme of India, under the Indian Pharmacopoeia Commission, and Dr. Bhim Rao Ambedkar Memorial Hospital, & Pt. Jawaharlal Nehru Memorial Medical College, Raipur, Chhattisgarh, India, for support during collection and analysis of data.
Additional information
Notes on contributors
Manju Agrawal
Manju Agarwal, MBBSMD assistant professor pharmacology Pandit Jawaharlal Nehru medical College Raipur Chhattisgarh India. Dr. Manju Agarwal is a competent dedicated faculty in the institute with more than 10 years teaching experience in pharmacology and 15 years of experience in clinical field. She is a trained biomedical researcher with more than 25 publications in various journals of national and international repute.
Preeti Singh
Preeti Singh, is a pharmacologist, currently working as pharmacovigilance associate at ADR Monitoring Center, Deptt. of Pharmacology, Pt. Jawaharlal Nehru Memorial Medical College Raipur Chhattisgarh India 492001 under Pharmacovigilance Programme of India (PvPI). She has 3 years of teaching experience in pharmacy & pharmacology and nearly 06 years of experience in Pharmacovigilance. She has published more than 14 articles in various journals of national and international repute. Her current h-index is 5 and i10-index is 3. She has also authored a comprehensive book on Pharmacovigilance, which is currently in press. Her area of expertise is toxicity study and pharmacovigilance.
Usha Joshi
Usha Joshi, Professor & HOD at Pandit Jawaharlal Nehru medical College Raipur Chhattisgarh India. She has more than 11 years of experience in teaching. She is a trained biomedical researcher with more than 15 publications in various journals of national and international repute. Under her supervision ADR Monitoring centre is running by the PvPI.
References
- World Health Organization. WHO report on surveillance of antibiotic consumption: 2016-2018 early implementation. Geneva, Switzerland: World Health Organization;. 2018.
- Nadeem M, Maqdoom M. A prospective study of antimicrobial utilization in post-operative care unit of a teaching hospital in South India. Int J Basic Clin Pharmacol. 2020 Jan;24(9):310–314.
- Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci. 2018;115(15):E3463–70.
- Jemal A, Ward E, Hao Y, et al. Trends in the leading causes of death in the United States, 1970-2002. Jama. 2005;294(10):1255–1259.
- World Health Organization. The importance of pharmacovigilance- Safety Monitoring of Medicinal Products.Geneva, Switzerland: World Health Organization; 2002.
- Shamna M, Dilip C, Ajmal M, et al. A prospective study on adverse drug reactions of antibiotics in a tertiary care hospital. Saudi Pharm J. 2014;22(4):303–308.
- Tamma PD, Avdic E, Li DX, et al. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308–1315.
- Jung IY, Kim JJ, Lee SJ, et al. Antibiotic-related adverse drug reactions at a Tertiary Care Hospital in South Korea. Biomed Res Int. 2017;2017:2017.
- K Patel T, B Patel P. Incidence of adverse drug reactions in Indian hospitals: a systematic review of prospective studies. Curr Drug Saf. 2016;11(2):128–136.
- Outterson K, Powers JH, Seoane‐Vazquez E, et al. Approval and withdrawal of new antibiotics and other antiinfectives in the US, 1980–2009. J Law Med Ethics. 2013;41(3):688–696.
- World Health Organization. Pharmacovigilance: ensuring the safe use of medicines. Geneva, Switzerland: World Health Organization; 2004.
- Kalaiselvan V, Srivastava S, Singh A, et al. Pharmacovigilance in India: present scenario and future challenges. Drug Saf. 2019;42(3):339–346.
- Kalaiselvan V, Thota P, Singh GN. Pharmacovigilance programme of India: recent developments and future perspectives. Indian J Pharmacol. 2016;48(6):624–628.
- Singh P, Agrawal M, Hishikar R, et al. Adverse drug reactions at adverse drug reaction monitoring center in Raipur: analysis of spontaneous reports during 1 year. Indian J Pharmacol. 2017;49(6):432–437.
- Kumar A. Past, present and future of pharmacovigilance in India. Syst Rev Pharm. 2011;2(1):55–58.
- MedDRA M Introductory guide for Standardised MedDRA Queries (SMQs) Version 21.0. 2018.
- Behera SK, Das S, Xavier AS, et al. Comparison of different methods for causality assessment of adverse drug reactions. Int J Clin Pharm. 2018;40(4):903–910.
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–2232.
- Srinivasan R, Ramya G. Adverse drug reaction-causality assessment. Int J Res Pharm Chem. 2011;1(3):606–612.
- Kiguba R, Karamagi C, Bird SM. Antibiotic‐associated suspected adverse drug reactions among hospitalized patients in Uganda: a prospective cohort study. Pharmacol Res Perspect. 2017;5(2):e00298.
- Hagiya H, Kokado R, Ueda A, et al. Association of adverse drug events with broad-spectrum antibiotic use in hospitalized patients: a single-center study. Intern Med. 2019;58(18):2621–2625.
- Selva P. An analysis of adverse drug reactions reported in a Tertiary Care Hospital. Eur J Mol Clin Med. 2021;7(8):3601–3610.
- Shet A, Sundaresan S, Forsberg BC. Pharmacy-based dispensing of antimicrobial agents without prescription in India: appropriateness and cost burden in the private sector. Antimicrob Resist Infect Control. 2015;4(1):1–7.
- Koh Y, Kutty FBM, Li SC. Drug-related problems in hospitalized patients on polypharmacy: the influence of age and gender. Ther Clin Risk Manag. 2005;1(1):39–48.
- Hurwitz N. Predisposing factors in adverse reactions to drugs. Br Med J. 1969;1(5643):536–539.
- Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol. 2001;2(6):349–351.
- Watson S, Caster O, Rochon PA, et al. Reported adverse drug reactions in women and men: aggregated evidence from globally collected individual case reports during half a century. EClinicalMedicine. 2019;17:100188.
- Domecq C, Naranjo CA, Ruiz I, et al. Sex-related variations in the frequency and characteristics of adverse drug reactions. Int J Clin Pharmacol Ther Toxicol. 1980;18(8):362–366.
- Shehab N, Patel PR, Srinivasan A, et al. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735–743.
- Tandon VR, Sharma S, Khajuria V, et al. Adverse drug reactions profile of antimicrobials: a 3-year experience, from a tertiary care teaching hospital of India. Indian J Med Microbiol. 2015;33(3):393.
- Sogn DD. Penicillin allergy. J Allergy Clin Immunol. 1984;74(4):589–593.
- Atanasković‐Marković M, Veličković TĆ, Gavrović‐Jankulović M, et al. Immediate allergic reactions to cephalosporins and penicillins and their cross‐reactivity in children. Pediatr Allergy Immunol. 2005;16(4):341–347.
- Bogas G, Mayorga C, Martín-Serrano Á, et al. Penicillin and cephalosporin cross-reactivity: role of side chain and synthetic cefadroxil epitopes. Clin Transl Allergy. 2020;10(1):57.
- Rehman A, Setter SM, Vue MH. Drug-induced glucose alterations part 2: drug-induced hyperglycemia. Diabetes Spectr. 2011;24(4):234–238.
- Badalov N, Baradarian R, Iswara K, et al. Drug-induced acute pancreatitis: an evidence-based review. Clin Gastroenterol Hepatol. 2007;5(6):648–661.
- Arulappen AL, Danial M, Sulaiman SAS. Evaluation of reported adverse drug reactions in antibiotic usage: a retrospective study from a tertiary care hospital, Malaysia. Front Pharmacol. 2018;9:809.
- Moore N, Berdaï D, Blin P, et al. Pharmacovigilance–the next chapter. Therapies. 2019;74(6):557–567.
- Sundaran S, Udayan A, Hareendranath K, et al. Study on the classification, causality, preventability and severity of adverse drug reaction using spontaneous reporting system in hospitalized patients. Pharmacy. 2018;6(4):108.
- Shalviri G, Yousefian S, Gholami K. Adverse events induced by ceftriaxone: a 10‐year review of reported cases to Iranian Pharmacovigilance Centre. J Clin Pharm Ther. 2012;37(4):448–451.
- Bandyopadhyay A, Chaurasia RC, Yadav RK, et al. A prospective study of adverse drug reactions to antiretroviral therapy in a tertiary care hospital at Allahabad, Uttar Pradesh.
- Biset Ayalew M. Mortality and Its predictors among HIV infected patients taking antiretroviral treatment in Ethiopia: a systematic review. AIDS Res Treat. 2017;2017:2017.
- Vinod KV, Arun K, Dutta TK. Dapsone hypersensitivity syndrome: a rare life threatening complication of dapsone therapy. J Pharmacol Pharmacother. 2013;4(2):158.
- Lorenz M, Wozel G, Schmitt J. Hypersensitivity reactions to dapsone: a systematic review. Acta Derm Venereol. 2012;92(2):194–199III.