ABSTRACT
Background
Elevated oxidant levels and low antioxidant levels in patients with end-stage renal disease (ESRD) play a significant role in the development of endothelial dysfunction, atherogenesis and cardiovascular disease (CVD). A deficiency in vitamin D (Vit.D) is also suggested to be responsible for the generation of oxidative stress (OS) and CVD. Among dialysis patients, conflicting data exist concerning the relationship between hepatitis C virus (HCV) infection and OS. We studied the relationship between 25Vit.D level, HCV infection, and plasma 8 iso-prostaglandin F2 α (8-ISO-PGF2α) as an OS marker in an Egyptian hemodialysis (HD) cohort.
Methods
One hundred and twenty ESRD patients on HD were initially recruited to the study but only 88 patients have met the inclusion and none of the exclusion criteria. Midweek predialysis session blood samples were collected for the measurement of 25(OH) Vit.D, plasma 8-ISO-PGF2α, high sensitivity C – reactive protein (hs-CRP), and intact parathyroid hormone (intact PTH). Patients were stratified into two groups according to the presence or absence of serum antibodies against HCV and their plasma 8-ISO-PGF2α were compared.
Results
Vit.D deficiency was noted in 93% of the participants; the median 8-ISO-PGF2α level was 382 pg/mL. No significant correlation between Vit.D and 8-ISO-PGF2α levels was found. Thirty-two participants (36%) were HCV+ and their 8-ISO-PGF2α levels were significantly lower relative to in the seronegative group (median 171 vs. 647 pg/mL; P < 0.006).
Conclusion
In this Egyptian HD cohort, Vit D deficiency was highly prevalent, yet failed to show any correlation with F2-isoprostanes. HCV+ HD patients might be shielded from OS.
1. Introduction
Oxidative stress (OS) is described as an imbalance between the presence of oxidative products and the capacity of antioxidant defense mechanisms, with overpowering of the former. [Citation1] OS is very prevalent in correlation with chronic kidney disease (CKD), increasing with greater reductions in renal function, and is further exacerbated by hemodialysis (HD) procedures. High levels of OS and reduced antioxidants levels in CKD and HD patients play a crucial role in the development of endothelial dysfunction, atherogenesis, and cardiovascular diseases [Citation2].
Factors affecting OS in patients on HD can be attributed to: both 1) dialysis-related factors such as type of the dialyzer, type and dosage of the anticoagulant, administered drugs, HD solution, presence of a central venous catheter, and length of HD treatment and 2) nondialysis-related factors such as diet, fluid overload, uremia, and other comorbidities [Citation2].
Vitamin D is an important antioxidant and many studies have suggested its antioxidant effect in the upregulation of some antioxidant enzymes [Citation3]. In some studies, Vitamin D deficiency is prevalent in HD patients with a recorded prevalence reaching 80% [Citation4]. This may be the result of many factors such as decreased exposure to sunlight, reduced skin synthesis and greater dietary restrictions [Citation5].
Vitamin D is an essential hormone that plays a central role in regulating mineral and bone metabolism through its pleiotropic effects, and which acts favorably on the cardiovascular and immune systems [Citation6]. Numerous studies have linked Vitamin D deficiency with increased arterial stiffness, vascular calcifications, stroke, left ventricular hypertrophy and mortality in CKD and HD cohorts [Citation6,Citation7].
Notably, some research also suggests that Vitamin D deficiency is involved in enhancing the OS in diabetic patients [Citation8]. However, no strong evidence to date supports the idea that Vitamin D deficiency plays the same role in non diabetics or HD patients. Despite the abundant observational evidence supporting the use of nutritional Vitamin D in HD cohorts, a clear causal relationship with outcomes has not been established [Citation9].
Further, there exist conflicting data regarding the correlation between hepatitis C virus (HCV) infection and OS in dialysis patients. Some have concluded that HCV infection increases OS in dialysis patients [Citation10,Citation11]. On the contrary, others have suggested it is protective against OS [Citation12]. And some did not identify any role of HCV infection in the generation of OS in dialysis patients [Citation13].
F2-isoprostanes are sensitive and accurate biomarkers in the assessment of lipid peroxidation and, thus, the assessment of OS [Citation14]. Isoprostanes are a collection of prostaglandin-like compounds formed by the peroxidation of arachidonic acid via free-radical catalyzation, independent of prostaglandins derived from cyclooxygenase [Citation15]. One member of this group is the 8-ISO-prostaglandin F2α (8-ISO-PGF2α) which can be easily measured in serum or urine [Citation16].
In the HD population, both the OS and factors affecting it remain under debate and require more exploration. Hence, the current study was designed to explore the relationship between the 25(OH) Vitamin D level, HCV infection and plasma 8-ISO-PGF2α as an OS marker in an Egyptian HD cohort.
2. Methods
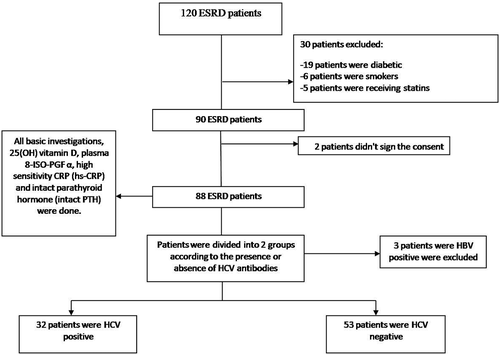
One hundred and twenty ESRD patients on maintenance hemodialysis (MHD) were recruited from Alexandria University Hospital’s HD units, which is a tertiary care hospital. Only 88 of them have met the inclusion and none of the exclusion criteria. Then patients were divided into two groups according to the presence (HCV+) or absence (HCV−) of serum antibodies against HCV ().
Inclusion and exclusion criteria:
Age ≥18 years and MHD (defined as being on regular HD three sessions per week for more than 6 months with each session lasting three to 4 hours) were the inclusion criteria. While, patients on antioxidant therapy (e.g, vitamin E, vitamin C, statins, l-carnitine), smokers, diabetics, and patients who had active inflammatory diseases or active liver disease were excluded from this study.
2.1. Clinical data
All patients were subjected to the following: thorough medical history-taking including the cause of ESRD if known, duration of HD, modality of HD (e.g, low flux, high flux, online hemodiafiltration), vascular access (e.g, arteriovenous fistula, catheter), clinical diagnoses (e.g. diabetes, hypertension), parathyroid surgery (total, subtotal), and drug history (e.g, calcium supplements, natural, or active vitamin D, calcimimetics).
2.2. Laboratory studies
Blood samples were collected for the following laboratory tests at enrollment: serum urea, serum creatinine, complete blood count (Sysmex XE 2100), serum lipid profile, serum albumin, serum calcium, inorganic phosphorus, calcium X phosphorus product.
Serum 25(OH) Vitamin D was measured using enzyme-linked immunosorbent assay (ELISA) test kits (DRG Diagnostics GmbH, Marburg, Germany), serum hs-CRP was measured using nephlometry (Siemens bn prospect), and serum intact parathyroid hormone (PTH) was measured using ELISA kits (Immunotopics, San Clemante, USA) [Citation17].
Blood samples for 8-ISO-PGF2α were drawn with ethylenediaminetetraacetic acid anticoagulant and centrifuged within 10 to 20 minutes of collection for 20 minutes at the speed of 2,000 to 3,000 rpm. Plasma was snap-frozen in liquid nitrogen in plastic vials with tight-fitting caps and stored at −20°C until the measurement of isoprostane content. 8-ISO-PGF2α concentrations in plasma samples were determined using the Sandwich enzyme-linked immunosorbent assay commercial kit for 8-ISO-PGF2α (SinoGeneClon Biotech Co.,Ltd) [Citation18].
2.3. Statistical analysis
Quantitative data are expressed as median and interquartile ranges (IQR), while qualitative data were presented as frequencies and percentages. Comparisons between groups were undertaken with the Mann–Whitney U test or chi-squared test as appropriate. Spearman’s correlation analysis was used to compare the association between Vit.D levels and F2-isoprostanes levels. A P-value of less than 0.05 was considered to be statistically significant in all tests. All analyses were conducted using the Statistical Package for the Social Sciences version 23, (IBM Corporation, Armonk, NY, USA), licensed to Alexandria University.
2.4. Ethics approval
The study was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Alexandria University ethical committee. The ethical approval number is 0201205, IRB NO: 00012098 (Expires 6–10-2022), FWA NO: 00018699 (Expires January 21st, 2026).
3. Results
The median age of the patients was 52 (IQR: 40.5–60) years and 52% were men. The median HD duration was 14 (IQR: 8–16) years and all HD patients were submitted to standard bicarbonate dialysis three times per week with each session lasting 3–4 hours. The type of vascular access was arteriovenous fistula in all patients except for in two patients with permanently tunneled catheters, and the most common cause of ESRD was hypertension. Thirty-two (36%) patients cohort were HCV + and no patients with HCV were receiving antiviral treatment at enrollment.
Intact PTH was increased in all patients except for in five patients who had undergone parathyroidectomy with a median value of 505 pg/mL, the cause for parathyridectomy in the five patients was developing of tertiary hyperparathyroidism that was not responding to the medical therapy. The median value for hs-CRP was slightly elevated in our dialysis patients (3.9 mg/L) with a cutoff of normal value 3 mg/L.
The level of 25(OH) Vit. D was deficient in 93% of patients with a median value of 7 ng/dL. F2- isoprostanes levels were high in all ESRD patients, with a median value of 382 (IQR: 136.4–1174.5) pg/mL .The characteristics of the HD cohort and the laboratory investigations are presented in .
Table 1. Characteristics of the entire HD cohort
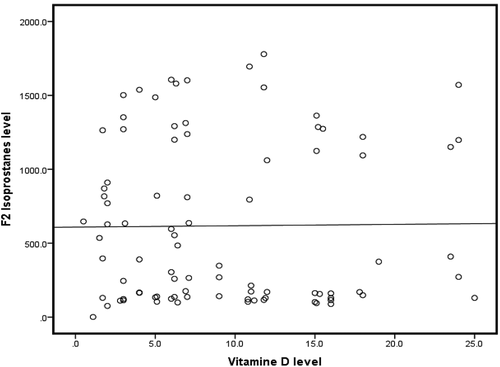
Meanwhile, no significant correlation was found between F2-isoprostane and Vit.D levels with r = −0.051; p = 0.639 ().
In a subgroup analysis patients were divided according to HCV antibodies; in total, 32 patients had HCV antibodies (HCV+) and 53 did not (HCV−). Serum albumin and serum cholesterol findings were significantly lower in the HCV+ group than in the HCV− group (P = 0.010 and P = 0.001).
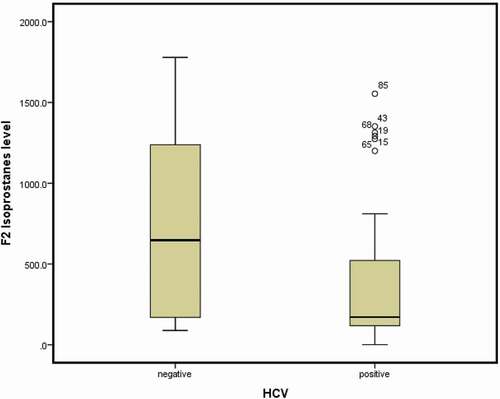
Moreover, F2-isoprostanes were significantly lower in the HCV+ group (median: 171 pg/mL, IQR: 118.4–521 pg/mL) than in the HCV−group (median: 647 pg/mL, IQR: 170–1238 pg/mL) (P = 0.006) (). There were no differences noted between the two groups concerning the other laboratory parameters ().
Table 2. Comparison between HCV positive and negative patients
4. Discussion
Despite the sunny, dry climate of Egypt, the prevalence of Vit D deficiency in the current study population was recorded to be 93%, which exceeds findings reported in previous studies involving HD patients in that some research suggested a prevalence of 80%–90% [Citation4,Citation19]. This may be explained by the high percentage of females (about 48%) in our study cohort, who may be more deprived of sun exposure due to their traditional fully covered way of dressing [Citation20]. Al-Arbagy et al. [Citation21] have found that the prevalence of vitamin D deficiency in a cohort of HD patients from Menoufia governorate in Egypt was 74.3%. The higher prevalence in our study may be due to the difference in the weather between both governorates as Alexandria with its position in the north of Egypt is less sunny than Menoufia.
Conversely, the elevated plasma levels of 8-ISO-PGF2α recorded in our HD patients agree with those of other previous studies [Citation22–24]. This can be explained by two theories: first, renal failure itself causes impaired clearance of the esterified isoprostanes, while, second, HD patients are under continuous OS due to chronic inflammation, uremic toxins, increased inflammatory factors, deficiency of antioxidant such as vitamins and trace elements, and the dialysis procedure itself [Citation25].
Meanwhile, the data presented here suggested no relation between Vit. D deficiency and the increase in F2 -isoprostanes in our HD cohort, in contrast with the study by Javanbakht et al. [Citation8] who reported that serum concentrations of 25(OH) Vit.D were inversely correlated with F2-isoprostane, low-density lipoprotein, and oxidized low-density lipoprotein levels. However, the patients in their study were mainly diabetic non-HD patients, while our patients were nondiabetic patients on HD, suggesting the diversity of factors implicated in cases of high F2-isoprostanes levels.
On the other hand, Makariou et al. [Citation26] in a study assessing patients with metabolic syndrome, determined that the administration of 25(OH)Vit.D in these patients was not associated with more significant reductions in OS markers than the use of dietary intervention alone, which supports our finding that there is no relation between Vit.D deficiency and F2-isoprostanes level.
The large percentage of HCV positivity in our HD population is likely due to the high prevalence of HCV in Egypt, where about 20% of the population suffers from HCV infection. Moreover, up to 50% of HD patients in Egypt are HCV seropositive [Citation27]. Kerollos et al. [Citation28] have found that the repeated blood transfusions and the number of inserted temporary hemodialysis catheters were significant risk factors for seroconversion of HD patients.
The finding that the plasma levels of 8-ISO-PGF2α in our HCV+ group were lower than those in the HCV− group is similar to data reported by Sear et al. [Citation12], who found significantly less OS markers such as superoxide dismutase (SOD), glutathione peroxidase and malondialdehyde existed in the HCV+ HD group (n = 26 patients) in a study involving 73 patients on HD [Citation15].
On the other hand, several studies have indicated that HCV infection per se was incremented as one of the causes of increased OS in patients on HD besides other known factors; these studies suggested that HCV infection is associated with increased OS both in normal and HD populations [Citation10,Citation29,Citation30]. Other research contends that HCV infection has no role in OS in HD subjects [Citation13].
Ultimately, the nature of our data may be explained by several points. First, the other findings in our HCV+ group, such as low serum albumin and total cholesterol may be indicators of a loss of liver biosynthetic capacity even before reaching clinically advanced liver failure. Depending on these findings, other biosynthetic activities of the liver (including proinflammatory cytokine synthesis) may also be impaired, which may lead to the decreased OS activity [Citation12,Citation31].
Several studies have reported that hypercholesterolemia increased the production of plasma F2-isoprostanes [Citation32,Citation33] as F2-isoprostanes are the end-metabolites of lipid peroxidation, which explains our results showing decreased levels of cholesterol in the HCV+ group and, hence, decreased levels of plasma F2-isoprostanes. Second, various genotypes of HCV pair with different OS-induction capabilities; in fact, a sharp decrease in an already-reduced GSH antioxidant level has been observed in patients chronically infected with genotype 1a/b HCV (which found more frequently in Europe where most earlier studies were performed) as compared with other genotypes, indicating that aggressive disease is associated with this genotype, thus increasing the OS [Citation34].
Finally, Da Silva et al. [Citation35] and Fabrizi F et al. [Citation36] have suggested that HCV is less aggressive in dialysis patients, which has been attributed to the protective effect of dialysis treatment on HCV infection via the clearance of HCV RNA by the dialyzate and the entrapment of HCV RNA particles on the membrane surface of the dialyzer.
4.1. Study limitation
The main limitation of this study is the absence of the quantitative PCR for HCV at the time of patients` recruitment. According to our hospital protocol, all patients do PCR for HCV once at the start of dialysis and thereafter they do antibodies every 3 months. At time of recruitment for the study none of our patients was treated from HCV or was receiving HCV treatment so we have measured the PCR to limit the study`s costs. It would be better if we correlated the viral load of HCV infection with the oxidative stress markers.
4.2. Conclusion
Vitamin D deficiency was highly prevalent in this Egyptian HD population, yet failed to show any correlation with F2-isoprostanes. On the contrary, HCV+ HD patients seem to be protected against Oxidative stress. Measuring the HCV load and demonstrating if it correlates with the level of the oxidative stress markers is needed to be performed in the future studies.
“Consent to participate”
All of the included patients signed an informed consent form after receiving full information about the study.
“Data availability”
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Mahmoud S. AbdElHady
Mahmoud S. AbdElHady Assistant lecturer Internal Medicine Nephrology Department Kafrelsheikh university Egypt.
Sara T Ibrahim
Sara T Ibrahim, MDPhD is a Lecturer of Internal Medicine and Nephrology, Faculty of Medicine Alexandria University since April 2020. She is also Assistant of Medical Director of Internal Medicine Hospital (Alexandria Main University Hospitals). She finished her residency program in Alexandria Main University Hospital. Her main research interests are autoimmune renal diseases and chronic kidney diseases. She is member of the international society of nephrology and the Egyptian society of nephrology.She authored 7 peer reviewed publications in high indexed international journals and was cited 10 times till the end of 2021.
Ahmed Adam
Ahmed Adam Professor of Internal Medicine and Nephrology in Faculty of Medicine, Alexandria University, Egypt.Head of the Internal Medicine and Nephrology, Dialysis, Transplantation Department in AUH.Former Senior Registrar in SKI - UKFormer Research Fellow in MN, and KY – USASupervisor of more than 40 MS and Ph D ThesisPublications more than 50 in the field of Kidney, Dialysis, Hypertension and Transplantation.Research interest within the Transplantation field in the area of Infections including BK, CMV, Long term outcome and Rejection.
Abelaziz Elnekidy
Abdelaziz ELnekidy Professor of Radiodiagnosis Faculty of Medcine Alexandria University Egypt. He had many publications in high indexed journals .
Neveen Lewis
Neveen Lewis Assistant professor of Clinical Pathology Faculty of Medcine Alexandria University Egypt. spechial interest in kidney disease research and had many international publications.
Rasha Ibrahim Gawesh
Rasha Ibrahim Gawish Lecturer of internal Medicine Nephrology Department Faculty of Medicine Alexandria University Egypt. she had more than 4 publications in the field of nephrology.
References
- Locatelli F, Canaud B, Eckardt KU, et al. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. NDT. 2003;18(7):1272–1280.
- Liakopoulos V, Roumeliotis S, Gorny X, et al. Oxidative Stress in Hemodialysis Patients: a Review of the Literature. Oxid Med Cell Longev. 2017;2017:e3081856.
- Berridge MJ. Vitamin D deficiency accelerates ageing and age-related diseases: a novel hypothesis. J Physiol. 2017;595(22):6825–6836.
- Drechsler C, Verduijn M, Pilz S, et al. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. NDT. 2011;26(3):1024‐32.
- Melamed ML, Thadhani RI. Vitamin D therapy in chronic kidney disease and end stage renal disease. Clin J Am Soc Nephrol. 2012;7(2):358‐65.
- Christakos S, Dhawan P, Verstuyf A, et al. Vitamin D: metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol Rev. 2016;96(1):365‐408.
- da Silva Canhos MM, de Oliveira RC, Modelli de Andrade LG, et al. Martin Association between vitamin D levels and mortality in hemodialysis patients: a cohort study. Ren Fail. 2020;42(1):225–233.
- Javanbakht MH, Mohammady H, Fooladsaz K, et al. Are Serum Levels of F2-Isoprostane and Oxidized-LDL Related to Vitamin D Status in Type 2 Diabetic Patients? A Case-Control Study. Rep Biochem Mol Biol. 2016;5(1):26‐32.
- Damasiewicz MJ, Toussaint ND. Is nutritional vitamin D supplementation beneficial in dialysis patients? Clin J Am Soc Nephrol. 2015;10(4):544‐6.
- Köken T, Serteser M, Kahraman A, et al. Oxidative stress markers in hepatitis C infected hemodialysis patients. J Nephrol. 2002;15(3):302‐07.
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV co-infection. Hepatology. 2006;43(6):1317‐25. .
- Sezer S, Tutal E, Aldemir D, et al. Hepatitis C infection in hemodialysis patients: protective against oxidative stress? Transplant Proc. 2006;38(2):406‐10. .
- Horoz M, Bolukbas C, Bolukbas FF, et al. Oxidative stress in hepatitis C infected end-stage renal disease subjects. BMC Infect Dis. 2006;6:114.
- Kadiiska MB, Gladen BC, Baird DD, et al. Biomarkers of oxidative stress study II: are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic Biol Med. 2005;38(6):698‐710. .
- Handelman GJ. Evaluation of oxidant stress in dialysis patients. Blood Purif. 2000;18:343–349.
- Ho E, Karimi Galougahi K, Liu CC, et al. Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol. 2013;1(1):483‐91.
- Sunman H, Özkan A, Yorgun H, et al. Vitamin D levels predict the response to cardiac resynchronization therapy in patients with systolic heart failure. Turk Kardiyol Dern Ars. 2016;44:670–676.
- Masiá M, Padilla S, Fernández M, et al. Oxidative Stress Predicts All-Cause Mortality in HIV-Infected Patients. PLoS One. 2016;11(4):e0153456. .
- Bansal B, Bansal S, Mithal A, et al. D deficiency in hemodialysis patients. Indian J Endocrinol Metab. 2012;16(2):270–273.
- El Din US, Fayed A, El Nokeety MM, et al., Vascular Calcification Group. Vitamin-D deficiency is encountered in almost all egyptian stage 3-5 chronic kidney disease patients in spite of the sunny weather. Saudi J Kidney Dis Transpl. 2019;30(6):1389–1397. .
- El-Arbagy AR, El-Zorkany KM, Helwa MA, et al. Assessment of vitamin D in hemodialysis patients. Menoufia Med J. 2020;33:122–126.
- Handelman GJ, Walter MF, Adhikarla R, et al. Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. Kidney Int. 2001;59(5):1960–1966. .
- Kim KM, Jung BH, Paeng KJ, et al. Alteration of plasma total F2-isoprostanes before and after hemodialysis in end-stage renal disease patients. Prostaglandins Leukot Essent Fatty Acids. 2004;70(5):475–478.
- Lim PS, Chang YM, Thien LM, et al. 8-iso-prostaglandin F2alpha as a useful clinical biomarker of oxidative stress in ESRD patients. Blood Purif. 2002;20(6):537–542. .
- Stępniewska J, Gołembiewska E, Dołęgowska B, et al. Oxidative stress and antioxidative enzyme activities in chronic kidney disease and different types of renal replacement therapy. Curr Protein Pept Sci. 2015;16(3):243‐48.
- Makariou SE, Elisaf M, Challa A, et al. No effect of vitamin D supplementation on cardiovascular risk factors in subjects with metabolic syndrome: a pilot randomised study. Arch Med Sci Atheroscler Dis. 2017;2:52–60.
- Elgharably A, Gomaa AI, Crossey MM, et al. Hepatitis C in Egypt - past, present, and future. Int J Gen Med. 2016;10:1–6.
- Kerollos KM, El-Ameen HA, El Wahed LA, et al. Prevalence and seroconversion of hepatitis C among hemodialysis patients in Assiut governorate. Egypt J Intern Med. 2020;32:2.
- Nascimento MM, Suliman ME, Bruchfeld A, et al. The influence of hepatitis C and iron replacement therapy on plasma pentosidine levels in haemodialysis patients. NDT. 2004;19(12):3112‐6.
- Kato A, Odamaki M, Nakamura H, et al. Elevation of blood thioredoxin in hemodialysis patients with hepatitis C virus infection. Kidney Int. 2003;63(3):2262–2268.
- Ríos-Ocampo WA, Daemen T, Buist-Homan M, et al. Hepatitis C virus core or NS3/4A protein expression preconditions hepatocytes against oxidative stress and endoplasmic reticulum stress. Redox Rep. 2019 Dec;24(1):17–26.
- Reilly MP, Praticò D, Delanty N, et al. Increased formation of distinct F2 isoprostanes in hypercholesterolemia. Circulation. 1998;98(25):2822‐8. .
- Davi G, Alessandrini P, Mezzetti A, et al. In vivo formation of 8-Epi-prostaglandin F2 alpha is increased in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1997;17(11):3230‐5. .
- Khadem Ansari MH, Omrani MD, Kheradmand F. Oxidative stress response in patients infected by diverse hepatitis C virus genotypes. Hepat Mon. 2015;15(2):e22069.
- Da SNMO, Kalb AC, Vidales-Braz BM. et al. Oxidative stress: a comparison of different groups of patients with Hepatitis-C in hemodialysis units. Kidney Disord Clin Pract. 2015;12(1):104.
- Fabrizi F, Messa P, Martin P. Impact of hemodialysis therapy on hepatitis C virus infection: a deeper insight. Int J Artif Organs. 2009;32(1):1–11.