ABSTRACT
Background
Portal hypertensive gastropathy (PHG) is an overlooked complication of liver cirrhosis, as it is a source of acute upper gastrointestinal bleeding and cause of chronic blood loss.
Objective
To assess the role of narrow band endoscopy in the diagnosis of PHG in cirrhotic patients.
Methods
Fifty patients with liver cirrhosis were examined by both conventional White Light Endoscopy (WLE) and Narrow Band Technology Variable Intelligent Staining Technology (VIST) using Sonoscape endoscope HD500. Biopsies were taken from the body of gastric mucosa during endoscopy.
Results
The prevalence of PHG among patients with liver cirrhosis is around 94% by WLE, 92% by VIST, and 55.3% by pathology. There is no statistical significance between VIST and WLE in case of PHG p = 0,750. The risk of developing oesophageal varices grade 3 in severe PHG is higher than in no or mild PHG (OR = 6.8571, 95% CI 1.6270 to 28.9001, p = 0.0087).
Conclusion
VIST is comparable and complementary to WLE in diagnosis of PHG. There is poor correlation between pathology and WLE in diagnosis of PHG.
1. Introduction
Portal hypertensive gastropathy (PHG) is an overlooked complication of liver cirrhosis. Although the exact mechanism for PHG is unclear, portal hypertension is assumed to be the driving force for its development [Citation1]. Changes in splanchnic hemodynamics together with imbalance between vasodilators and vasoconstrictors (angiotensin II, endothelin, thromboxane A2, and norepinephrine) make the gastric mucosa more vulnerable to injury [Citation2].
PHG can be either asymptomatic or present with acute upper gastrointestinal bleeding. Chronic blood loss associated with PHG is also reported. It is diagnosed when there is 2 g/dl drop in hemoglobin level in the previous 6 months with no documented history of non-steroidal anti-inflammatory drugs (NSAIDs) [Citation3]. The severity of PHG is associated with higher risk of mortality in patients [Citation4].
There is a wide variation in the prevalence of PHG among patients with liver cirrhosis ranging from 20% to 80% [Citation5]. This was explained by variation in endoscopic findings as well as presence of different classifications and diagnostic criteria [Citation6]. Endoscopic examination of gastric mucosa from the body, fundus, and less commonly antrum shows mosaic pattern of polygonal erythematous areas surrounded by pale borders giving a snakeskin appearance [Citation3]. On histological examination, PHG appears as mucosal and submucosal capillary and venular dilatation and congestion associated with derangement of the microcirculation but without evident inflammation or microthrombi [Citation7].
PHG is diagnosed mainly by endoscopic tool rather than histopathology examination due to the fact of poor correlation between endoscopic and histological findings [Citation8]. Over years, different classifications were proposed to define the degrees of PHG and to decrease the interobserver disagreement like McCormak classification, New Italian Endoscopy Club (NIEC) classification, and Baveno classification [Citation6]. McCormak was the first to describe PHG and he proposed a classification that divided the forms of PHG into mild and severe. But the problem was in the intermediate forms and their descriptions. After that in 1994, NIEC proposed another classification to overcome the defects in the previous one and deals more with the intermediate stage. But this classification was too complex with several grades in the intermediate stage, as it divided the mosaic pattern into pink, red center, or red. In 1996, Baveno score system was proposed depending on a score system where it divided red markings in either isolate or confluent and the mosaic pattern in mild (pink mosaic pattern) and severe (red mosaic pattern). In addition, the presence of gastric antral vascular ectasia was added [Citation9]. The aim of any classification is to be simple, accurate and to increase interobserver reliability. But unfortunately, till now there is no ideal classification.
The objective of this study is to estimate the prevalence of PHG among our patients with liver cirrhosis using WLE, VIST and histopathology and correlate the grade of PHG to the severity of liver disease and to the grade of oesophageal varices.
2. Methods
2.1. Ethics statement
The protocol was approved by the Ethics Committee of Alexandria University, Faculty of Medicine. The nature of the study, potential hazards, and anticipated benefits were explained to the patients. All patients provided a written informed consent before inclusion in the study, in accordance with the principles of the Declaration of Helsinki (revision of Edinburgh, 2000).
2.2. Patients
Patients with liver cirrhosis, regardless of etiology, listed for upper GIT endoscopy in Alexandria Main University Hospital, between November 2019 to July 2020, were included. Any patient on proton pump inhibitor, beta-blocker or history of NSAIDs use in the preceding month was excluded from the study. The severity of liver disease was stratified based on Child-Pugh class and model of end stage liver disease (MELD) score.
HCV RNA PCR, HBV DNA PCR, autoimmune markers and metabolic panel were done to identify cause of liver cirrhosis. Laboratory investigations included complete blood picture and complete liver profile including liver enzymes (ALT and AST), INR, total and direct bilirubin and serum albumin.
Abdominal ultrasound was done to all patients to assess liver echogenicity, spleen size, portal vein diameter and presence of ascites.
2.3. Endoscopic procedure
All patients were examined by both conventional White Light Endoscopy (WLE) and Narrow Band Technology Variable Intelligent Staining Technology (VIST) using Sonoscape endoscope HD500. WLE was performed assessing the presence and severity of PHG and oesophageal varices. The severity of PHG was graded as either mild or severe according to the Baveno classification. “Mild PHG” is characterized by pink mosaic pattern and “Severe PHG” is characterized by red mosaic pattern [Citation3]. Oesophageal varices (O.V) were graded according to Modified Paquet’s classification. While VIST view depended on Narrow Band Imaging criteria of PHG where red spots represent red mucosa plus intramucosal hemorrhage around capillaries and Mosaic like pattern represents swelling of gastric pits, and dilatation and convolution of capillaries surrounding the gastric pits and clarified gastric areas [Citation10]. All findings were confirmed by consensus of two endoscopists to minimize inter-observer variability.
2.4. Biopsy procedure
Gastric mucosal biopsies were taken from the body for histopathological examination. Fundus was spared to minimize the risk of bleeding. Biopsies were stained with hematoxylin and eosin. Expert pathology panel unaware of the endoscopic results examined the slides. PHG is diagnosed if there is ectasia and congestion of mucosal and submucosal venules and capillaries without inflammation or fibrin thrombi [Citation3].
2.5. Statistical methods
For qualitative variables, frequencies were calculated. For quantitative variables, the median and range values were estimated.
Tests used for comparing groups: Kruskal Wallis, Spearman’s Rank correlation coefficient. Sensitivity and specificity of the diagnostic tools were calculated, and for the degree of agreement, we used McNemar test. All results with p value <0.05 were considered significant.
3. Results
3.1. Patient characteristics
Fifty (50) patients with liver cirrhosis were enrolled in the study. Clinical and laboratory characteristics of these patients are summarized in .
Table 1. Baseline clinical and laboratory characteristics of patients
During endoscopy session, 47 patients were diagnosed by PHG by WLE, 24/47(48%) had mild PHG and 23/47(46%) had severe PHG. Oesophageal varices (O.V) presence was higher among patients with severe PHG than no and mild PHG, but with no statistical significance, p = 0.119. The risk of developing OV grade 3 in severe PHG is higher than in no or mild PHG (OR = 6.8571, 95% CI 1.6270 to 28.9001, p = 0.0087). The correlation between the grade of varices and severity of PHG was very weak (rs = 0.26045, p (2-tailed) = 0.06774) with no statistical significance.
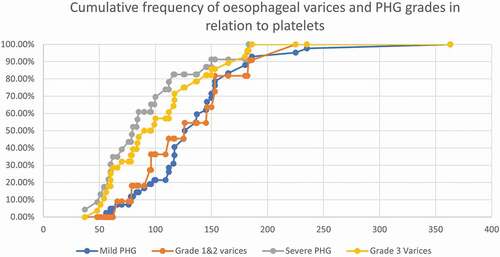
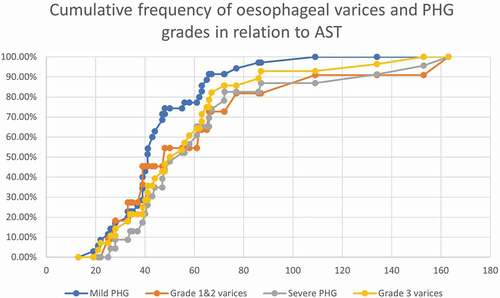
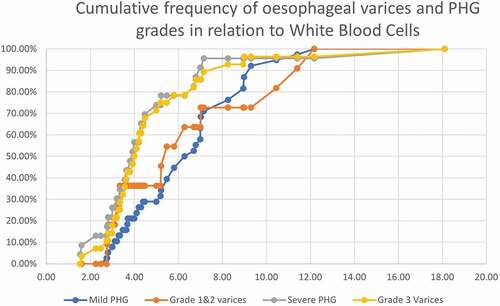
There was no statistical significance as regards the age, sex, and other parameters between the three groups (). The main results that were found significant: Platelet count was lower in severe PHG (80x103/cc) than mild PHG (121x103/cc) and no PHG (150x103/cc), with statistical power, p = 0.016. And AST was higher in severe PHG than mild and no PHG, (p = 0.024). show the cumulative frequency of PHG and oesophageal varices grades in comparison to platelets, WBC, and AST. Severe PHG and OV grade 3 cumulative frequency exceeded 50% at lower platelets and WBC count than patients with milder grade (). Severe PHG and OV grade 3 had closely related cumulative frequency curves to mild PHG and OV grade 1&2 in relation to AST level ().
Table 2. Comparison of different clinical characteristics between different categories of PHG
Figure 2. The cumulative frequency of oesophageal varices and PHG grades in relation to White blood cells.
There are two main patterns of gastric mucosa detected by VIST, the ridge/villous pattern and circular pattern. The antrum shows small light circular pattern surrounding dark areas, while in the body dark circles surround the light mucosa. VIST also differentiated two patterns of blood vessels in the gastric mucosa. Subepithelial capillary network (SECN) is observed regularly arranged in both the antrum and body [Citation11,Citation12] ().
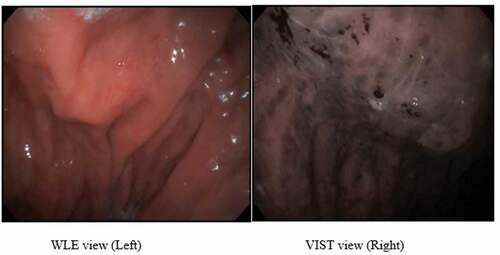
Figure 4. WLE view of patient with severe PHG during endoscopy session showing diffuse hemorrhagic red spots (Left) and VIST view with dilated capillaries surrounding gastric pits with areas of hemorrhage (Right).
The degree of concordance between results of VIST when compared to WLE showed a strong agreement between the two modalities and non-inferiority of VIST when compared to WLE in the diagnosis of PHG, Kappa measure of agreement 0.645 and p < 0.001. It diagnosed 46 out of the 47 cases of PHG, VIST has 66.7% and 97.9% specificity and sensitivity respectively.
Histopathology of gastric mucosa diagnosed 26/47 cases, it has 66.7% specificity and 55.3% sensitivity compared to WLE findings, kappa measure of agreement was 0.053 and p < 0.459. There was no agreement between WLE and histopathology of gastric mucosa for diagnosis of PHG .
Table 3. Comparison and agreement of PHG detection between WLE versus pathology and VIST
Finally, the prevalence of PHG among the patients was calculated around 94% by WLE, 92% by VIST and 55.3% by histopathological examination.
4. Discussion
PHG is an underestimated complication of liver cirrhosis that is assumed to result from hemodynamic changes associated with portal hypertension. In the absence of gold standard tool for diagnosis of PHG, our study elucidated that the prevalence of PHG among patients with liver cirrhosis varies according to the diagnostic tool used. PHG prevalence reached 94% when diagnosed by WLE which was comparable to VIST (92%), while histopathology showed less prevalence (55.3%). This discrepancy between WLE and histopathology findings could be explained by the possibility of non-target mucosal biopsy, which yields lower prevalence of PHG by pathology. This limitation was overcome in our study by taking multiple biopsies from gastric body. Furthermore, fundal and thick gastric mucosa biopsies are required to examine deeply located submucosal blood vessels which is usually not feasible during surveillance endoscopy. This practice is carried by some endoscopists to avoid or minimize the risk of bleeding from PHG [Citation2,Citation8,Citation13].
Even though, our results were not in line with a number of studies which showed good correlation between PHG and Child Pugh score [Citation14]. There was good correlation between PHG and platelet count and WBC, p = 0.016 and 0.044 respectively. Thrombocytopenia and leukopenia in patients with PHG are attributed to hypersplenism associated with portal hypertension which is one of the key players in the etiology of thrombocytopenia in cirrhotic patients [Citation15]. This implies that patients with severe PHG had advanced degree of portal hypertension than mild cases. AST was higher in the group with severe PHG; this could be related to the advanced liver condition with the grade of PHG.
Severe PHG group had more cases with grade 3 esophageal varices (16/23), than grade 1 and 2. There was no correlation between grade of PHG and oesophageal varices, p = 0.119. Tiwari et al. [Citation16] reported similar results, they found no correlation between PHG degree and size of varices. It was found the risk of OV grade 3 in severe PHG 6.8 times than in no and mild PHG, that highlights the effect of severity of PHG on the grade of varices.
El Shazly et al. [Citation17] and Achim et al. [Citation18] showed good efficacy of Narrow Band Technology in the diagnosis of PHG, giving additional details to what was observed by WLE, which matches our results.
There was no agreement between WLE and pathology in diagnosis of PHG, kappa measure of agreement was 0.053. Our results are comparable to several studies, but Ma et al study showed lower prevalence of PHG by WLE than ours. Ma et al. excluded mild PHG without snake-skin appearance which could explain the difference between our results [Citation2,Citation8]. This highlights the importance of WLE as gold standard tool for diagnosis of PHG to overcome the limitations of pathology in the diagnosis.
The limitation of our study is the limited sample size because the study was suspended because of the COVID-19 pandemic. Inclusion of a larger sample of patients is recommended to confirm the trends observed in the current sample with a higher degree of confidence. Further integration of endoscopic technology with artificial intelligence is trendy, with the promise of reducing human diagnostic errors and improving the diagnostic yield of the procedure [Citation19].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Randa Salah Eldin Abdelmoneim Ibrahim
Randa Salah Eldin Abdelmoneim Ibrahim, assistant lecturer of Internal Medicine, Alexandria University Faculty of Medicine, Alexandria, Egypt
Amr Aly Abdelmoety
Amr Aly Abdelmoety, Professor of Internal Medicine, Alexandria University Faculty of Medicine, Alexandria, Egypt
Nahed Baddour
Nahed Baddour, Professor of Pathology, Alexandria University Faculty of Medicine, Alexandria, Egypt
Perihan Salem
Perihan Salem, Professor of Internal Medicine, Alexandria University Faculty of Medicine, Alexandria, Egypt
Marwa Metawea
Marwa Metawea, Lecturer of Internal Medicine, Alexandria University Faculty of Medicine, Alexandria, Egypt
References
- El Shahawy MS, Shady ZM, Gaafar A. The efficacy of argon plasma coagulation versus carvedilol for treatment of portal hypertensive gastropathy. Digestion. 2019;101(6):651–658.
- Ma C, Chen C-H, Liu T-C. The spectrum of gastric pathology in portal hypertension—An endoscopic and pathologic study of 550 cases. Pathol Res Pract. 2016;212(8):704–709.
- Rockey DC. An update: portal hypertensive gastropathy and colopathy. Clin Liver Dis. 2019;23(4):643–658.
- Bang CS, Kim HS, Suk KT, et al. Portal hypertensive gastropathy as a prognostic index in patients with liver cirrhosis. BMC Gastroenterol. 2016;16(1):93.
- Gjeorgjievski M, Cappell MS. Portal hypertensive gastropathy: a systematic review of the pathophysiology, clinical presentation, natural history and therapy. World J Hepatol. 2016;8(4):231–262.
- Casas M, Vergara M, Brullet E, et al. Inter and intra-observer concordance for the diagnosis of portal hypertension gastropathy. Rev Esp Enferm Dig. 2018;110(3):166–172.
- Chandanwale SS, Gupta N, Sheth J, et al. Histopathological study of portal hypertensive gastropathy using gastric biopsy. Med J DY Patil Univ. 2017;10(6):562.
- Bella MR, Casas M, Vergara M, et al. Utility of histology for the diagnosis of portal hypertensive gastroenteropathy. Concordance between the endoscopic image and gastrointestinal biopsies. Role of the CD34 marker. Gastroenterol Hepatol (English Edition). 2019;42(3):150–156.
- de Macedo GFS, Ferreira FG, Ribeiro MA, et al. Reliability in endoscopic diagnosis of portal hypertensive gastropathy. World J Gastrointest Endosc. 2013;5(7):323–331.
- Paghadhar S, Jain M, Mahadevan B, et al. Interpretation of benign gastric mucosal lesions using narrow-band imaging. J Digest Endosc. 2020;11(2):106–111.
- Abdelmoaty AS, Sakr MA, Aboelmaaty ME, et al. Evaluation of upper endoscopic findings before and after transjugular intrahepatic portosystemic shunt in Egyptian patients with budd-chiari syndrome. Afro-Egypt J Infect Endem Dis. 2021;11(1):18–26.
- Al-Taee AM, Cubillan MP, Hinton A, et al. Accuracy of virtual chromoendoscopy in differentiating gastric antral vascular ectasia from portal hypertensive gastropathy: a proof of concept study. World J Hepatol. 2021;13(12):2168.
- Bansal A, Ulusarac O, Mathur S, et al. Correlation between narrow band imaging and nonneoplastic gastric pathology: a pilot feasibility trial. Gastrointest Endosc. 2008;67(2):210–216.
- Mandhwani R, Hanif FM, Ul Haque MM, et al. Noninvasive clinical predictors of portal hypertensive gastropathy in patients with liver cirrhosis. J Transl Int Med. 2017;5(3):169–173.
- Peck‐Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37(6):778–793.
- Tiwari PS, Sudhamshu K, Sharma D, et al. Prevalence of portal hypertensive gastropathy in chronic liver disease and correlation with the severity of liver disease. Cureus. 2019;11(8):e5454.
- El Shazly YM, Said H, Rafik MM, et al. Narrow band imaging: a new tool for diagnosis of portal hypertensive gastropathy. Nat Sci. 2014;12:40–47.
- Achim AC, Vesa SC, Dumitru E. The efficacy of virtual chromoendoscopy in the diagnosis of portal hypertensive gastropathy. J Gastrointestin Liver Dis. 2016;25(3):289–293.
- Hajjar AE, Rey J-F. Artificial intelligence in gastrointestinal endoscopy: general overview. Chin Med J (Engl). 2020;133(3):326–334.