ABSTRACT
Background
Identifying hig- risk non-small cell lung cancer (NSCLC) patients is the most contentious area in lung pathology for reducing cancer-related morbidity and mortality.
Aim of the work
Was to investigate the immunohistochemical expression of CD8, CTLA4, and PD-L1 among different NSCLC histopathological variants and it’s correlation with different clinicopathological variables.
Material and Methods
Expression of CD8, CTLA4, & PD-L1 was evaluated immunohistochemically in 45 NSCLC cases.
Results
Higher expression of CD8 tumor infiltrating lymphocytes (TILs) was significantly associated with better progression-free survival (PFS). The expression of CTLA4&PD-L1 on tumor cells was significantly associated with lower PFS. However, smoking status of the studied cases showed no statistically significant correlation with expression of any of the studied immunohistochemical markers.
Conclusions
Immunostaining for CD8, CTLA4, and PD-L1 could have a major role in the anticipation of PFS of NSCLC cases regardless of their smoking status.
KEYWORDS:
1. Introduction
Lung cancer is considered one of the most common malignancies worldwide (accounting for ~12% of cancers) and is considered a major leading cause of cancer-related mortality worldwide in both genders. Moreover, there is a tendency toward increasing incidence [Citation1].
Most NSCLC patients undergo lymph node &/or distant metastasis. Identifying high-risk patients is the most important challenge if reducing morbidity and mortality pertinent to NSCLC is addressed [Citation2].
Tobacco smoking is considered a major risk factor for lung cancer [Citation3]. As reported, smokers have risk of 30-fold times of developing lung cancer than nonsmokers [Citation3–5]. The tobacco smoking-induced inflammatory response activates lung cancer through variable mechanisms. Genomic alterations occur through binding of DNA to inflammatory cell–derived reactive nitrogen or oxygen species [Citation6].
Until recently, NSCLC was known to be a non-immunogenic tumor, but now it is highly suggested that inflammatory and immunological responses have a pivotal role in lung carcinogenesis [Citation7].
Immune response is the corner stone in tumor development and progression. The balance between tumor progression, immunosuppression and effective antitumor responses depends on the tumor micro environment (TME). Tumor-infiltrating lymphocytes (TILs) are found in many tumor tissues with higher population of CD3+ and CD8+ T cells. CD8 + T lymphocytes have cytotoxic activity against tumor cells, consequently these T cells may have a major role in antitumor immunity [Citation8].
In general, immune checkpoints are considered to be inhibitory pathways that preserve self-tolerance by balancing the immune responses [Citation8]. Modulating pro- and anti-immune reactions by our body through the immune checkpoint helps to avoid autoimmune reaction [Citation9]. Cancer cells are capable of escaping attack from immune system by stimulating the immunosuppressive mechanism [Citation8]. Among one of the most important checkpoint pathways are, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1) pathways. They are expressed on T cells leading to inhibiting the function of cytotoxic T cells with or without the interaction with its ligands. Blocking these two pathways tip the balance from tumor immune tolerance to immune activation [Citation10].
In view of the pressing need to identify patients at high risk for a guarded/poor prognosis and to determine whether distinct tissue immune microenvironment markers have a preferential effect on clinical outcome in NSCLC, we set forth to study the possible roles of CD8, CTLA4, and PD-L1 as prognostic markers of NSCLC.
2. Material
The material of this study comprises prospective analysis of 45 selected patients obtained from clinical oncology and pathology department at faculty of Medicine, Alexandria University, in the period 2019 through 2020.
2.1. They were grouped into two groups
Group I: 15 smoker patients
Group II: 30 nonsmoker patients
2.2. Inclusion criteria
Patient diagnosed as having radiological lung mass and small biopsies performed for lung mass(core or forceps biopsies)
Patients diagnosed as NSCLC
2.3. Exclusion criteria
Patient diagnosed as having radiological lung mass and resection was performed for lung mass
Patient diagnosed as having SCLC
Patients working in any hazardous environment (other than tobacco smoke) as chemical hazards like asbestos, pesticides and heavy metals.
3. Methods
3.1. In this study the work protocol include the following
We collected full clinical, pathologic and oncological data which include the patients’ age, sex, smoking history,tumor stage, histologic subtype (According to WHO Classification of Lung Tumors 2015) [Citation11] and the treatment received.
3.2. Immunohistochemical study
Four microns thick tissue sections of each paraffin block were cut and mounted onto positively charged slides, then the avidin-biotin method was utilized.
3.3. Immunohistochemical staining Protocol
Deparaffinization in xylene and rehydration in a graded alcohol series (100% to 70%), followed by washing 2 times in the phosphate-buffered saline (PBS), each one for 5 minutes, then incubating in Hydrogen Peroxide Block for 10–15 minutes, in order to block endogenous peroxidase activity for reducing nonspecific background staining was done . After blocking peroxidase activity, washing four times in PBS was done, each one for 5 minutes. Slides to be stained by CD8 were immersed in plastic coplin jars containing sodium citrate buffer (0.01 M Na-citrate monohydrate, pH 6.0) and then incubated in a microwave oven for 10 min twice, after that it is left to cool down to room temperature. Whereas, the slides to be stained by CTLA4 & PD-L1, were immersed in a water bath having EDTA Buffer (1 Mm, pH 8.0) and pre-heated until temperature reached 95–100°C. Slides were then immersed in the staining dish, with a loosely placed lid on the staining dish and incubated for 20 minutes. The staining dish was removed from the water bath to room temperature for allowing the slides to cool for 20 minutes. Subsequently washing by PBS four times each one for 5 min, then incubation with Ultra V Block for 5 min at room temperature for the sake of blocking nonspecific background staining was done. Afterward primary antibodies were applied as follows: CD8 (catalog number: MS-457-S0, Thermo scientific), (diluted 1:100 with PBS), then incubated for 30 minutes in a humidified chamber at room temperature. CTLA4 (catalog number: 004–100, Genome Me) was applied (diluted 1:100 with PBS) and incubated for 30 minutes at room temperature in a humidified chamber. PD-L1 (catalog number: RM0324, Medaysis) was applied (diluted 1:50 with PBS) and incubated for 30 minutes at room temperature in a humidified chamber. After washing 4 times in PBS each one for 5 min, tissue sections were incubated with Biotinylated Goat Antipolyvalent for 10 min, after that washing four times each for 5 min then incubation with streptavidin peroxidase for 10 min and washing four times each for 5 minutes and afterward applying a mixture of one drop DAB chromogen and 2 ml of DAB substrate for 15 minutes, then washing four times in tap water and finally counterstaining with Meyer hematoxylin and covering by a cover slip and examine it by a light microscope.
3.4. Evaluation of immunostaining
CD8 was evaluated as membranous staining of tumor infiltrating lymphocytes for staining frequency(on the basis of the percentage of positively stained lymphocytes) and was scored as 0 (0%),1 (1–25%),2 (26–50%),3 (51–75%),4 (76–100%) whereas the staining intensity was scored as 0 (negative),1 (weak),2 (moderate),3 (strong). Finally, multiplying the score of staining intensity by the labeling frequency score was used to categorize cases into three groups: low (final score ≤3), intermediate (final score >3, ≤ 6), and high (final score>6) [Citation12]. The positive external control tissue (tonsil) was included in every run.
CTLA4 was evaluated as cytoplasmic staining of tumor cells for staining frequency (on the basis of the percentage of positively stained cells) and intensity of staining reaction which was scored as 0, 1+, 2+, 3 +. The final score was as “0” (100% of cells with intensity of 0; expression: negative), score “1a(<50% of cells with intensity of 1+; expression: low-positive), score “1b”(<50% of cells with intensity of 2+ and/or 3+; expression: low-Positive), score “2a” (≥50% of cells with intensity of 1+; expression: positive),score “2b” (≥50% of cells with intensity of 2+ and/or 3+; expression: positive) [Citation13].The external positive control (tonsil) was included in every run.
PD-L1 was evaluated as membranous staining of tumor cells, score 0 = less than 5% of tumor cells staining, score 1 = 5–50% of tumor cells with weak or moderate staining, score 2 = more than 5% of tumor cells with strong staining, or more than 50% with weak to moderate staining and score 5 = un-interpretable tissue due to lack of tumor, core drop-out, or ambiguous staining [Citation14].The external positive control tissue (placenta) was included in every run.
4. Close follow up was attempted for all patients for at least 6 months from the final diagnosis
a. Monitoring local recurrences or distant metastasis.
b. Calculating progression free survival (the period where the patients were free of any sort of disease progression)
5. Statistical analysis of the data
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. Qualitative data were described using number and percent. Quantitative data were described using range (minimum and maximum), mean, standard deviation, and median. Significance of the obtained results was judged at the 5% level (P value = 0.05). ROC curve analysis was done to predict the diagnostic accuracy of each immunohistochemical marker in predicting mortality.
The Used Tests were
1-Chi-square test: For categorical variables, to compare between different groups.
2-Fisher’s Exact or Monte Carlo correction: Correction for chi-square when more than 20% of the cells have expected count less than 5.
6. Results
6.1. Patient characteristics
Clinicopathological characteristics of the enrolled patients with lung NSCLC were illustrated in . The mean age at diagnosis was 58.6 (range 33–82) years with thirty five cases (77.7%) being males. Thirty cases (66.7%) were smokers. The most prevalent histopathology was adenocarcinoma (24/45 cases, 53.3%). The majority of patients (23/45, 51.1%) had stage III disease.
Table 1. Demographic, clinical, and pathological characteristics of the studied cases.
6.2. The correlation between the expression of CD8, and clinicopathological variables of NSCLC () and )
Figure 1. Immunohistochemical staining for immune markers in NSCLC. (a) A case of moderately differentiated squamous cell carcinoma showing tumor infiltrating lymphocytes with strong brown membranous staining for CD8 with total score 12.(X200). (b) A case of acinar adenocarcinoma showing strong brown cytoplasmic staining for CTLA 4 with total score 2B.(X400). (c) A case of poorly differentiated squamous cell carcinoma showing strong brown membranous staining for PD-L1 with total score 2. (X200). (d) A case of acinar adenocarcinoma showing tumor infiltrating lymphocytes with moderate brown membranous staining for CD8 with total score 4.(X200). (e) A case of moderately differentiated squamous cell carcinoma showing moderate brown cytoplasmic staining for CTLA4 with total score 1b. (X400). (d) A case of solid adenocarcinoma showing moderate brown membranous staining for PD-L1 with total score 1. (X400)
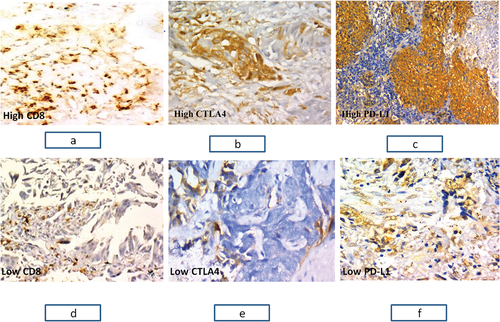
Table 2. Relation between the expression of CD8, and clinicopathological variables of NSCLC(*:significant).
The expression rate of CD8 was 77.1%. The expression of CD8 showed no statistically correlation with the sex, age, and smoking status. There was statistically significant relation between CD8 expression and histopathological variants(P = 0.05), tumor staging(P = 0.047), nodal(P = 0.021) & distant metastasis(P = 0.042), and progression free survival(P = 0.009).
6.3. ROC curve to determine the cut off value of CD8 sensitivity and specificity that can predict mortality within period of less than 6 months ()
Figure 2. ROC curve to determine the cut off value of CD8 sensitivity and specificity that can predict mortality in less than 6 months.
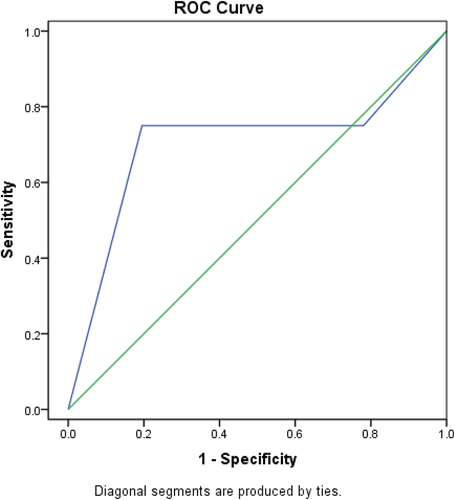
The accuracy of CD8 expression in predicting mortality within period in less than 6 months was further tested by ROC curve analysis in order to detect a cutoff point of which mortality is predicted. A cut off value of 8 was diagnostic of predicting mortality within period of less than 6 months with a sensitivity of 75.0%, specificity of 70.0%, and accuracy 72%.
6.4. The correlation between the expression of CTLA4, and clinicopathological variables of NSCLC () and )
Table 3. Relation between the expression of CTLA4, and clinicopathological variables of NSCLC (*:significant).
The expression rate of CTLA4 was 68.9%. The expression of CTLA4 showed no statistically significant relation with sex, age, smoking status, histopathological variants, tumor staging, nodal & distant metastasis. There was statistically significant relation between CTLA4 expression and progression free survival (P = 0.001).
6.5. ROC curve to determine the cut off value of CTLA4 sensitivity and specificity that can predict mortality within period of less than 6 months ()
Figure 3. ROC curve to determine the cut off value of CTLA4 sensitivity and specificity that can predict mortality in less than 6 months.
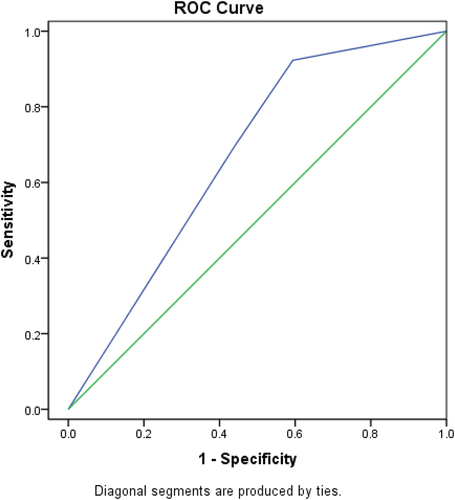
The accuracy of CTLA4 expression in predicting mortality in less than 6 months was further tested by ROC curve analysis in order to detect a cutoff point of which mortality is predicted. A cut off value of 2b was diagnostic of predicting mortality within period time of less than 6 months with a sensitivity of 65%, specificity of 61% and accuracy 63%.
6.6. The correlation between the expression of PD-L1 and clinicopathological variables of NSCLC () and )
Figure 4. ROC curve to determine the cut off value of PD_L1 sensitivity and specificity that can predict mortality in less than 6 months.
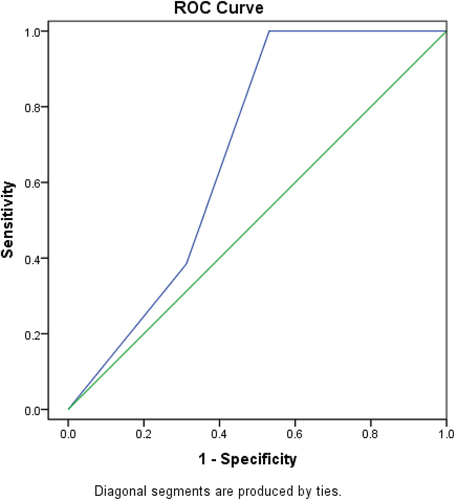
Table 4. Relation between the expression of PD-L1, and clinicopathological variables of NSCLC(*:significant).
The expression rate of PD-L1 was 75.6%. The expression of PD-L1 showed no statistically significant correlation with sex, age, smoking status, histopathological variants, tumor staging and nodal metastasis. There was statistically significant relation between PD-L1 expression with distant metastasis(P = 0.037) and progression free survival(P = 0.002).
6.7. ROC curve to determine the cut off value of PD-L1 sensitivity and specificity that can predict mortality with period time of less than 6 months ()
The accuracy of PD-L1 expression in predicting mortality in less than 6 months was further tested by ROC curve analysis in order to detect a cutoff point of which mortality is predicted. A cut off value of 2 was diagnostic of predicting mortality within period of less than 6 months with a sensitivity of 65%, specificity of 63%, and accuracy 64%.
7. Discussion
Immune response is the corner stone in tumor development and progression. The balance between tumor progression, immunosuppression and effective antitumor responses, depends on the tumor microenviroment (TME) [Citation8]. Tian C et al. [Citation15] suggested CD8 + T lymphocytes harbor cytotoxic activity against tumor cells, consequently these T cells may have a major role in antitumor immunity. In the present work, CD8 expression was detected in 77.8% of NSCLC cases.
In the current study, there was no statistically significant relation between any of patients’ age, gender, or smoking status on one hand and CD8 expression on the other hand. This was in concert with WEI Z et al [Citation16] findings.
The present study showed that CD8 expression differed significantly among NSCLC histopathologic variants, with the highest expression seen in adenocarcinoma cases (p = 0.05). This may suggest more indolent nature of adenocarcinoma compared to other NSCLC histopathologic types.
In the current study, the expression of CD8 correlated significantly with advanced TNM stage at diagnosis (p = 0.042), moreover CD8 expression was significantly higher in patients with nodal metastasis and in patients with distant metastasis. In the same context Hiroka K et al [Citation17] stated that expression of CD8 correlates with staging of the NSCLC and documented significant positive correlations with the clinical stage, on the contrary Kilvaer TK et al [Citation18] & Ye SL et al [Citation12] stated that higher expression of CD8 negatively correlated with increased staging of the NSCLC. This discrepancy between CD8 expression results (favorable prognostic marker) and its association with advanced stage, nodal and distant metastasis was explained in the literature by these CD8 + T cells are anergic and cannot lyse tumor cells. Moreover, that CD8 + T cells [Citation19] in the TME were not properly activated and have no ability to mount an antitumor immune response.
As well known; the antitumor effect of CD8 T cells could be evaded by variable ways in the tumor cells. Tumor cells may acquire the capability to evade immune surveillance through different ways, including a lack of adequate T-cell co-stimulation [Citation20] dysregulation of cell-surface MHC class I expression [Citation21], release of immunosuppressive factors, as transforming growth factor-b [Citation22] and non-function of Fas (CD95/APO1)-mediated apoptosis [Citation23].
The current study showed that higher CD8 expression was significantly correlated with improved progression free survival (PFS). Mean PFS was 11, 6 and 5 months in patients with high, low and no CD8 expression, respectively (P = 0.009). These findings were in parallel with previous study of ye SL et al. [Citation12]
Several previous studies were in general agreement with the current results as they revealed that CD8 was associated with better prognosis and improved patients’ progression free survival [Citation24–29].
CTLA4 (also known as CD152) plays a major role in immune regulation by causing negative feedback stimulation of T-cells upon activation of an immune response, thus its expression in tumor microenvironment leads to tumor immune evasion by down-regulation of CD4 + T effector (Teff) cells and the activation of Treg cell activity [Citation30].
Immune checkpoints are inhibitory pathways [Citation10]. One of the mostly studied checkpoint pathways are, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1) pathways which are expressed on T cells inhibit the activation of cytotoxic T cell to function.
In the present work, CTLA4 expression was detected in 68.9% of NSCLC cases; there was no statistically significant relation between any of patients’ age, gender, or smoking status on one hand and CTLA4 expression on the other hand. This was in concert with Liu Z et al findings et al [Citation31].Considering expression grade of CTTLA4 among the different NSCLC histopathologic variants, the results of the present study showed a non- significant difference between variable NSCLC histopathologic variants (p = 0.322). This finding was identical to that of Paulsen EE et al [Citation32]
In the current study, the expression of CTLA4 showed no significant correlation with patient’s clinical stage (p = 0.294), in the same context Paulsen EE et al [Citation32] stated that expression of CTLA4 didn’t correlate significantly with the clinical stage. The expression of CTLA4 was not significantly higher in the nodal and distant metastasis group compared to the non-metastatic group (p = 0.698 &0.123 respectively). These results were in contrast with Paulsen EE et al [Citation32] who reported that CTLA4 expression was positively correlated with nodal metastasis.
The current study showed that CTLA4 expression showed statistically significant negative correlation with progression free survival (P = 0.0018). These findings were in parallel with Deng L et al. [Citation33]
Several previous studies were in general agreement with the current study as they showed that CTLA4 was associated with worse prognosis and decreased patients’ overall survival [Citation34–38].
In the TME, PD-1 and its ligand PD-L1 perform a great role in tumor progression by evading the tumor neutralizing immune surveillance. Through PD-1 expression on a variety of immune cells and PDL-1 being expressed on tumor cells and antigen presenting cells (APCs), consequently their interaction will lead to T cell dysfunction and interleukin-10 (IL-10) production in the tumor [Citation39]. So, the PD-L1 immune checkpoint inhibitors, pembrolizumab and nivolumab, showed significant improvement in the survival of patients with advanced NSCLC [Citation40–42].
In the present work, PD-L1 was expressed in 75.6% of the studied NSCLC cases. These results were in close to those of Vranker M et al [Citation43] who reported that positive PD-L1 expression was in 68.4% of their cases.
In the present study, no statistically significant correlation was found between PD-L1 expression in NSCLC cases with respect to patient age, gender, smoking status and histopathological subtype and nodal metastasis (P < 0.05). This finding was identical to that of Rashed HE et al [Citation44] and WEI Z et al. [Citation16]
In the present study, the expression of PD-L1 showed no significant correlation with patient’s TNM stage (p = 0.222). In the same context, Dix Junqueira Pinto G et al [Citation45] & Paulsen EE et al [Citation46] stated that higher expression of PD-L1 didn’t show significant positive correlations with the clinical stage. The expression of PD-L1 in group with distant metastasis was statistically significantly higher than the group having no distant metastasis (p = 0.037). This finding was identical to that of Chen Qet al. [Citation47]
The current study showed that PD-L1 expression showed statistically significant negative correlation with progression free survival (P = 0.002), These findings were in parallel with previous study of Rashed HE et al [Citation44] & Tokito T et al. [Citation48]
Several previous studies were in general agreement with the current results as they reported that PD-L1 was associated with worse prognosis and decreased patients’ survival [Citation49–52].
It was found that the cut off value for CD8 expression for the predicting mortality in less than 6 months was at a score of 8 with sensitivity 75%, specificity 70% and accuracy 72%. Accordingly, cases that showed a CD8 score above 8 displayed a survival more than 6 months. Conversely, those with a score equal or less than 8 had mortality in less than 6 months
Moreover, It was found that the highest diagnostic accuracy for CTLA4 expression for the predicting mortality in less than 6 months was at a score 2b with sensitivity 65%, specificity 61%, and accuracy 63%. Accordingly, cases that showed a CTLA4 score below 2b displayed a survival more than 6 months. Conversely, those with a score equal to 2b had mortality in less than 6 months
In the same context, the highest diagnostic accuracy for PD-L1 expression for the predicting mortality in less than 6 months was at a score equal 2 with sensitivity 65%, specificity63% and accuracy 64%. Accordingly, cases that showed a PD-L1 score below 2 displayed a survival more than 6 months. Conversely, those with a score equal 2 had mortality in less than 6 months.
In the present study, investigating the immunohistochemical expression of CD8, CTLA4 and PD-L1 in NSCLC could be considered as the first step to highlight the important role of these biological markers in NSCLC. This could contribute to the development of prognostic markers and more therapeutic targets for NSCLC.
8. Conclusions
Immunostaining for CD8, CTLA4 and PD-L1 may play an important role in anticipating the biological behavior of NSCLC cases, regardless of their smoking status. Moreover, these results enhance our knowledge of the mechanisms underlying tumor growth and possible aggressive behavior of NSCLC and could lead to the development of prognostic markers and potential therapeutic targets for NSCLC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289.
- Gkretsi V, Stylianou A, Papageorgis P, et al. Remodeling components of the tumor microenvironment to enhance cancer therapy. Fron Oncol. 2015;5. 214.
- Youlden DR, Cramb SM, Baade PD. The international epidemiology of lung cancer: geographical distribution and secular trends. J Thorac Oncol. 2008;3(8):819–831.
- Sasco A, Secretan M, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45:S3–S9.
- Proctor RN. Tobacco and the global lung cancer epidemic. Nat Rev Cancer. 2001;1(1):82–86.
- Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8(4):605–615.
- Kelly RJ, Gulley JL, Giaccone G. Targeting the immune system in non–small-cell lung cancer: bridging the gap between promising concept and therapeutic reality. Clin Lung Cancer. 2010;11(4):228–237.
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. nature. 2008;454(7203):436–444.
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252.
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–1260.
- Ye SL, X-Y L, Zhao K, et al. High expression of CD8 predicts favorable prognosis in patients with lung adenocarcinoma: a cohort study. Medicine (Baltimore). 2017;96(15):e6472.
- Kassardjian A, Shintaku PI, Moatamed NA. Expression of immune checkpoint regulators, cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1), in female breast carcinomas. PloS One. 2018;13(4):e0195958.
- Au N, Cheang M, Huntsman D, et al. Evaluation of immunohistochemical markers in non‐small cell lung cancer by unsupervised hierarchical clustering analysis: a tissue microarray study of 284 cases and 18 markers. J Pathol. 2004;204(1):101–109.
- Tian C, Lu S, Fan Q, et al. Prognostic significance of tumor-infiltrating CD8+ or CD3+ T lymphocytes and interleukin-2 expression in radically resected non-small cell lung cancer. Chin Med J. 2015;128(1):105–110.
- Wei Z, Zhan X, Fan L, et al. Programmed death-ligand 1 expression and CD8+ tumor-infiltrating lymphocytes in advanced non-small cell lung cancer treated with microwave ablation and chemotherapy. Int J Hyperthermia. 2018;35(1):591–598.
- Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94(2):275–280.
- Kilvaer TK, Paulsen -E-E, Andersen S, et al. Digitally quantified CD8+ cells: the best candidate marker for an immune cell score in non-small cell lung cancer? Carcinogenesis. 2020;41(12):1671–1681.
- Trojan A, Urosevic M, Dummer R, et al. Immune activation status of CD8+ T cells infiltrating non-small cell lung cancer. Lung Cancer. 2004;44(2):143–147.
- Melero I, Shuford WW, Newby SA, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3(6):682–685.
- Turner JG, Rakhmilevich AL, Burdelya L, et al. Anti-CD40 antibody induces antitumor and antimetastatic effects: the role of NK cells. J Immunol. 2001;166(1):89–94.
- Inge TH, Hoover SK, Susskind BM, et al. Inhibition of tumor-specific cytotoxic T-lymphocyte responses by transforming growth factor β1. Cancer Res. 1992;52(6):1386–1392.
- Hahne M, Rimoldi D, Schröter M, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274(5291):1363–1366.
- Guo M, Yuan F, Qi F, et al. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8+ T cells in hepatocellular carcinoma using multiplex quantitative analysis. J Transl Med. 2020;18(1):1–13.
- Oshi M, Asaoka M, Tokumaru Y, et al. CD8 T cell score as a prognostic biomarker for triple negative breast cancer. Int J Mol Sci. 2020;21(18):6968.
- Nobari N, Niknejad N, Poorolajal J, et al. Clinicopathologic features and prognostic role of CD3, CD8, and PD-1 positive tumor-infiltrating lymphocytes and the association with COX-2 overexpression in endometrial carcinoma. Int J Cancer Manag. 2020;13(4):e99210.
- Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104(10):3967–3972.
- Schumacher K, Haensch W, Röefzaad C, et al. Prognostic significance of activated CD8+ T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61(10):3932–3936.
- Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–3494.
- Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287.
- Liu Z, Pei -M-M, Liu J-X, et al. The expressions and significance of B7-H3 and CTLA-4 in the clinical stages of non-small-cell lung cancer. Int J Clin Exp Pathol. 2019;12(8):3032–3041.
- Paulsen -E-E, Kilvaer TK, Rakaee M, et al. CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: diverging prognostic impact in primary tumors and lymph node metastases. Cancer Immunol Immunother. 2017;66(11):1449–1461.
- Deng L, Gyorffy B, Na F, et al. Association of PDCD1 and CTLA-4 gene expression with clinicopathological factors and survival in non–small-cell lung cancer: results from a large and pooled microarray database. J Thorac Oncol. 2015;10(7):1020–1026.
- Zhang X-F, Pan K, Weng D-S, et al. Cytotoxic T lymphocyte antigen-4 expression in esophageal carcinoma: implications for prognosis. Oncotarget. 2016;7(18):26670.
- Kahlmeyer A, Stöhr CG, Hartmann A, et al. Expression of PD-1 and CTLA-4 are negative prognostic markers in renal cell carcinoma. J Clin Med. 2019;8(5):743.
- Omura Y, Toiyama Y, Okugawa Y, et al. Prognostic impacts of tumoral expression and serum levels of PD-L1 and CTLA-4 in colorectal cancer patients. Cancer Immunol Immunother. 2020;69(12):2533–2546.
- Yu H, Yang J, Jiao S, et al. Cytotoxic T lymphocyte antigen 4 expression in human breast cancer: implications for prognosis. Cancer Immunol Immunother. 2015;64(7):853–860.
- Santoni G, Amantini C, Morelli MB, et al. High CTLA-4 expression correlates with poor prognosis in thymoma patients. Oncotarget. 2018;9(24):16665–16677.
- Sun Z, Fourcade J, Pagliano O, et al. IL10 and PD-1 cooperate to limit the activity of tumor-specific CD8+ T cells. Cancer Res. 2015;75(8):1635–1644.
- Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550.
- Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–3933.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833.
- Vrankar M, Kern I, Stanic K. Prognostic value of PD-L1 expression in patients with unresectable stage III non-small cell lung cancer treated with chemoradiotherapy. Radiat Oncol. 2020;15(1):247.
- Rashed HE, Abdelrahman AE, Abdelgawad M, et al. Prognostic significance of programmed cell death ligand 1 (PD-L1), CD8+ tumor-infiltrating lymphocytes and p53 in non-small cell lung cancer: an immunohistochemical study. Turk Patoloji Derg. 2017;1(1):211–222.
- Junqueira Pinto G D, de Souza Viana L, Scapulatempo Neto C, et al. Evaluation of PD-L1 expression in tumor tissue of patients with lung carcinoma and correlation with clinical and demographic data. J Immunol Res. 2016;2016:9839685.
- Paulsen -E-E, Kilvaer TK, Khanehkenari MR, et al. Assessing PDL-1 and PD-1 in non–small cell lung cancer: a novel immunoscore approach. Clin Lung Cancer. 2017;18(2):220–233.
- Chen Q, Y-Y F, Yue Q-N, et al. Distribution of PD-L1 expression and its relationship with clinicopathological variables: an audit from 1071 cases of surgically resected non-small cell lung cancer. Int J Clin Exp Pathol. 2019;12(3):774–786.
- Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14.
- Litvin IE, Paganella MP, Wendland EM, et al. Prognosis of PD-L1 in human breast cancer: protocol for a systematic review and meta-analysis. Syst Rev. 2020;9(1):66.
- Abdel-Wahab SA, El Mahdy MM, Baioumy WA-M, et al. Prognostic value of PD-l1 tumor expression in egyptian patients with epithelial bladder cancer. Ain Shams Med J. 2019;70(10, 11 & 12):715–728.
- Gao H-L, Liu L, Z-H Q, et al. The clinicopathological and prognostic significance of PD-L1 expression in pancreatic cancer: a meta-analysis. Hepatobiliary Pancreat Dis Int. 2018;17(2):95–100.
- Li Y, He M, Zhou Y, et al. The prognostic and clinicopathological roles of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. Front Pharmacol. 2019;10:139.
