ABSTRACT
Background
Left ventricular diastolic dysfunction (LVDD) is the first preclinical sign of diabetic cardiomyopathy. Nonalcoholic fatty liver disease (NAFLD) is linked to morbidity and mortality in Type 2 diabetes mellitus (T2DM) and increases the chance of developing cardiovascular disease, the primary cause of death.
Aim
This work aimed to identify the association of LVDD and NAFLD in T2DM.
Methods
We recruited 40 patients with T2DM (20 with NAFLD and 20 without NAFLD). Laboratory investigations and abdominal ultrasonography were carried out. The degree of hepatic steatosis was measured by the hepatorenal index (HRI). The LVDD was assessed by echocardiography and tissue doppler imaging.
Results
The Left atrial volume, left ventricular volume index, and left ventricle filling pressure index (E/é) were higher in the NAFLD group (P < 0.05). The E/é index was correlated with HRI and gamma-glutamyl transpeptidase (P < 0.05). Hepatic steatosis by HRI was the only independent variable associated with LVDD.
Conclusion
NAFLD is associated and correlated with an increased risk of left ventricular diastolic dysfunction in patients with T2DM regardless of ventricular systolic function.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a growing worldwide pandemic hand in hand with the rising prevalence of obesity, sedentary life, and metabolic syndrome. It is the commonest cause of chronic liver disease in patients with type 2 diabetes mellites (T2DM), affecting up to 70% of these patients. In addition, the association of NAFLD with T2DM increases the likelihood of having NAFLD in a more severe form, such as nonalcoholic steatohepatitis (NASH), advanced fibrosis, or cirrhosis [Citation1,Citation2].
The current evidence suggests that NAFLD is linked to not just morbidity and mortality associated liver disease, but also to the high risk of developing cardiovascular disease, which is the major cause of death among T2DM patients [Citation3,Citation4].
NAFLD has been related to an increased risk of coronary artery disease and subclinical atherosclerosis, which manifests as an increase in carotid wall thickness and a reduction in endothelial flow-mediated vasodilation. Additionally, NAFLD patients had altered left ventricular (LV) geometrical alterations, as well as early signs of LV diastolic dysfunction which can be detected by echocardiography [Citation5,Citation6].
T2DM is associated with higher risk of heart failure, left ventricular diastolic dysfunction and LV hypertrophy, which are all described as diabetic cardiomyopathy [Citation7]. NAFLD, especially NASH are prevalent in persons with T2DM. However, if NAFLD or NASH in T2DM patients carries an independent risk for heart failure (away from the consequences of DM and insulin resistance), is an area of research interest. In addition, the effect of hepatic steatosis grade, and fibrosis stage on the cardiac changes are attractive points to investigate. In the current study, we thought to investigate the association between NAFLD and LV function (as measured by echocardiography), and the severity of hepatic steatosis (as evaluated by the hepato-renal index).
2. Patients
2.1. Patient inclusion and setting
The recruitment process is shown in . In a case-control design, the study included 40 patients with type T2DM. They were divided into two groups; group I: 20 patients with T2DM and with NAFLD, and group II: 20 patients with T2DM without NAFLD. The patients were drawn from the internal medicine department, Alexandria Main University hospital, as well as the diabetes clinic at Damanhour Medical Institute. Protocol serial number: 0201107. Informed consent was obtained from all patients for being included in the study. The diagnosis of DM was depending on an 8 hours overnight fasting plasma glucose ≥126 mg/dL, or 2-h post-prandial glucose ≥200 mg/dL during oral glucose tolerance test, or hemoglobin A1C ≥ 6.5% [Citation8]. The anti-diabetic agents used by the patients were shown in . The diagnosis of NAFLD was dependent on hepatic steatosis by ultrasound abdomen with exclusion of other causes of chronic liver disease [Citation9].
Figure 1. The study recruitment steps. eGFR: estimated glomerular filtration rate; NAFLD: nonalcoholic fatty liver disease.
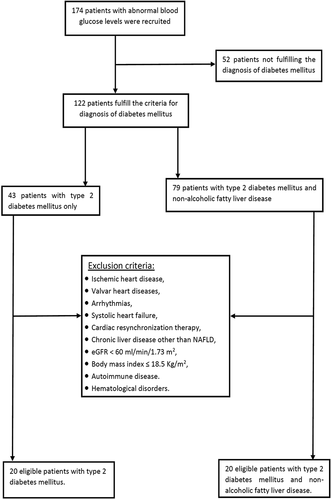
Table 1. Types of anti-hyperglycemic agents.
2.2. Exclusion criteria
Patients with ischemic heart disease, acquired or congenital valvar diseases, chronic AF, systolic heart failure, patients receiving cardiac resynchronization therapy, patients having left bundle branch block, chronic liver disease (Non-NAFLD cirrhosis, drug-induced liver disease, hemochromatosis, autoimmune and viral liver disease), Alcoholics, glomerular filtration rate˂60 ml/min/1.73 m2, body mass index ≤ 18.5 Kg/m2,autoimmune disease, a recent history of severe infection at the study time and hematological disorders or malignancy.
3. Methods
All patients were subjected to demographic data collection and physical examination including waist circumference (WC), and body mass index (BMI) calculation. Neurological examination for detection of diabetic peripheral neuropathy was done. Laboratory investigations including full blood counts, fasting and post-prandial blood glucose, glycated hemoglobin A1c (HbA1c), fasting insulin for calculation of Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), lipid profile, serum urea, serum creatinine, uric acid, urinary albumin/creatinine ratio (uACR), glomerular filtration rate estimation (eGFR), serum aminotransferases, and gamma-glutamyl transpeptidase (GGT).
A cardiac assessment was done for all participants. A 12-lead standard resting electrocardiogram was done to exclude silent ischemia or cardiac arrhythmias. Echocardiography was done using ultrasound equipment (GE-VingMed System FiVe with a 3.5 MHz transducer, General Electric-Ving Med Sound AB, Horten, Norway). This was done on the same day of abdominal ultrasound examination.
Transthoracic echocardiography was utilized to determine LV diameters, wall thickness, and mass. At rest, LV end-diastolic volume (EDV), LV end-systolic volume (ESV), and EF were assessed at the apical 4-chamber and 2-chamber perspectives (by modified Simpson rule). The maximum volume of the left atrium was measured at the end of LV systole using the apical 4-chamber and 2-chamber perspectives. When appropriate, measurements were correlated to body surface area. Trans-mitral peak early diastolic velocity (E), peak late diastolic velocity (A), and E-wave deceleration time were measured using pulsed-wave Doppler [Citation10].
A Doppler trans-mitral flow-velocity curve was obtained utilizing the volume at the mitral tips to evaluate the peak early (E) and late (A) diastolic flow velocities. A tissue Doppler imaging (TDI) of mitral annular motion at the septum was performed to evaluate LVDD. The early (e’) diastolic annular velocities’ peak values were calculated using the TDI. These measurements were used to calculate the mitral E to TDI e’ ratio (E/e’) which served as a measure of the LV filling pressure [Citation11,Citation12].
Abdominal ultrasonography was done for all patients by a single expert blinded radiologist. Features of hepatic steatosis were identified (e.g. diffuse hyper-echogenicity of the liver compared to the kidneys, ultrasound beam attenuation, and poor visualization of intrahepatic artery boundaries and diaphragm). The liver’s and kidneys’ echogenicity was assessed using a grayscale histogram, and the hepatorenal index (HRI) was calculated using the mean results with a dedicated software. The degree of hepatic steatosis was semi-quantitatively graded as follows: healthy liver (HRI = 1.00 − 1.04), mild steatosis (HRI = 1.05–1.24), moderate steatosis (HRI = 1.25–1.64), and severe steatosis (HRI ≥ 1.65) [Citation13].
3.1. Statistical analysis
Statistical analysis was conducted using the Statistical Package for Social Sciences (SPSS version 20.0) software. Normality for the data was assessed. The data were expressed as mean ± SD, median (minimum-maximum), or proportions as appropriate. The unpaired Student’s t-test for normally distributed data was used to compare two means or the Mann-Whitney U-test for non-normally distributed quantitative variables as appropriate. The Chi-square (χ2) test was used to compare between proportions or Fisher’s Exact test (FET). Univariate and multivariate analysis were done. Statistical significance was estimated at P ≤ 0.05. All determined P values were two-tailed.
4. Results
shows the baseline clinical and biochemical characteristics of the study groups. The duration of DM was higher in group I compared to group II (P = 0.001). The median duration of DM in patients in groups I and II were 10, and 4 years respectively. There was no difference between the two groups as regards the eGFR.
Table 2. Comparison between the study groups regarding the baseline characteristics and laboratory findings.
There was no statistical difference between the studied groups according to their EF, LVM, LVED volume and peak early diastolic flow velocity (E) (P > 0.05). Patients with T2DM and NAFLD, in comparison to patients with T2DM only, had higher peak late diastolic flow velocities (A), higher left atrial volume, higher peak early/late diastolic flow velocities (E/A), lower peak early diastolic annular velocities (e’), and higher left ventricle filling pressure index (E/e’) (P = 0.05, P = 0.04, P < 0.001, and P < 0.001, respectively). Echocardiographic findings are shown in .
Table 3. Comparison between studied groups regarding echocardiography findings.
4.1. Hepatic steatosis and echocardiography parameters
Based on HRI classification of steatosis grade, there was a significant difference between different steatosis grades as regards LAV, LVVI, peak early diastolic annular velocities (e’), and left ventricle filling pressure index (E/e’) (P < 0.005). Patients with severe steatosis had significantly higher left ventricle filling pressure index (E/e’) compared to patients with mild, or moderate steatosis, and compared to normal subjects (P < 0.001), ()
Table 4. Comparison between different grades of hepatic steatosis as regards the echocardiographic parameters.
4.2. Correlations
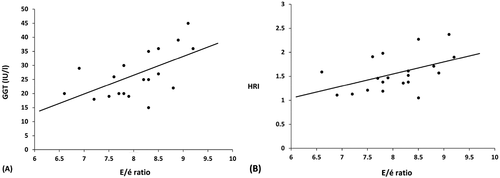
Among patients with T2DM and NAFLD, the left ventricle filling pressure index (E/e’) was positively correlated with the HRI (P = 0.008, r = 0.58), and serum GGT (P = 0.035, r = 0.47). However, there was no correlation between the (E/e’) ratio and the other parameters ().
4.3. Regression analysis
A univariate analysis was done. The duration of DM, presence of HTN, HOMA-IR, serum GGT, and hepato-renal index were significant predictors for the presence of LVDD. However, a multivariate regression analysis using the non-parametric ANCOVA (Quade’s test) showed that the HRI was the only significant predictor for LVDD (P < 0.001, OR = 30.24), ().
Table 5. Univariate and multivariate analysis (ANCOVA) for predictors of LVDD among the study population.
5. Discussion
Obesity and T2DM have become epidemic proportions throughout the world. Early mortality is associated with T2DM, which is also a significant risk factor for cardiovascular illness, particularly ischemic heart disease and chronic heart failure. Early cardiac functions alternation can also be seen as aberrant diastolic dysfunction, which over time may result in loss of contractile function [Citation14].
Nonalcoholic fatty liver disease is the most prevalent cause of chronic liver disease. When NAFLD is associated with T2DM. This will raise the chance of developing cardiovascular disease, the leading cause of death, in addition to the morbidity and mortality related to the liver. It has a high correlation with cardiac dysfunction, particularly LVDD [Citation15].
In the current study, patients with T2DM plus NAFLD had significantly lower peak early diastolic annular velocities (é), higher LVED volume, and higher left ventricle filling pressure index (E/é) compared with T2DM patients without NAFLD. However, these findings were detected while the left ventricular EF was normal. These parameters reflect the presence of early left ventricular diastolic dysfunction among T2DM patients who had NAFLD even with preserved LV ejection fraction.
These results are consistent with Saluja M et al., who found that NAFLD patients had significantly lower E’ tissue velocity, higher E/e’ ratio, higher LVend-diastolic pressure, and higher LV end-diastolic pressure/end-diastolic volume LV ratio. They concluded that in patients with T2DM and NAFLD, even if the LV morphology and systolic function are preserved, early features of LV diastolic dysfunction were detected [Citation16].
In our study, the left ventricle filling pressure index positively correlated with the HRI, and serum GGT (as a reflection of steatosis severity). In a study by Bonapace S et al., there was an incremental pattern of the E-to-e′ ratio across subgroups according to the steatosis severity [Citation17]. This comes in agreement with our findings, however; they graded the steatosis by a subjective evaluation, unlike our methodology which used a dedicated objective software for steatosis severity evaluation.
In the current study, we found a significant difference between different grades of steatosis as measured by HRI as regards the (e’) peak early diastolic annular velocities, (E/e’) left ventricle filling pressure index. The greater the steatosis, the higher the peak early diastolic annular velocities, left ventricle filling pressure index (i.e. more LVDD). In addition, the multivariate analysis showed that HRI is the only independent variable associated with LVDD in patients with T2DM and NAFLD.
Our results were following that of Mantovani A et al. as they reported that after adjusting for cardiometabolic risk confounders, NAFLD was associated with three-fold increased odds of mild and/or moderate LVDD. They concluded that in T2DM patients with intact systolic function, NAFLD is independently associated with early LVDD [Citation18]. Similarly, Lee H et al. found in their study LV DD was substantially more frequent in the NAFLD versus non-NAFLD group (59.7% vs. 49.0%, P = 0.011). Additionally, they revealed that liver fibrosis was independently associated with diastolic dysfunction (odds ratio = 1.58) after considering insulin resistance and cardiovascular risk variables into account [Citation19].
The pathophysiological link between NAFLD and LVDD is not fully understood. Whether NAFLD is just a marker of cardiometabolic risk and ectopic fat deposition or is an independent risk factor for cardiac abnormalities, is a debatable topic. Recently, growing evidence suggested that NAFLD is a main contributor in the pathogenesis of both structural and functional myocardial defects in patients with NAFLD and diabetes [Citation20,Citation21].
A pathogenic ‘cross-talk” between the liver and the malfunctional adipose tissue has been discussed. The ectopic adipose tissue including visceral fat produces a lot of free fatty acids, hormones and pro-inflammatory adipokines, that lead to insulin resistance. In this context, the liver is both a target for these adipokines, and is a source for various hepatokines that may amplify the myocardial and vascular damages [Citation22].
In addition, NAFLD, especially necro inflammatory phase (NASH), produces proinflammatory cytokines (e.g. interleukin 6, tumor necrosis factor-α, monocyte chemoattractant protein 1, etc.), procoagulants, and adhesion molecules which may be implicated in the myocardium oxidative stress, endothelial dysfunction and atherogenic dyslipidemia [Citation4]. Changes in the myocardial tissue, such as the accumulation of advanced glycation end products, increased resting tension in cardiomyocytes and fibrosis which can end by LV diastolic stiffness [Citation23]. Indeed, the link between NAFLD and myocardial dysfunction is an interesting area in the recent research, especially the conception of a pathophysiological continuous sequence between NAFLD and heart failure with preserved ejection fraction (HFpEF). NAFLD has been suggested to drive three major HFpEF phenotypes (metabolic, advanced liver disease phenotype and obstructive), that indicate a spectrum ranging from mild form to severe disease with varying patterns across various patients [Citation24].
The main limitation of our study is the small number of patients. Also, a liver biopsy which is the gold standard to assess the severity of liver damage was not performed. However, our study still has a point of strength. To the best of our knowledge, this is the first study which correlate the severity of hepatic steatosis with the LVDD using a noninvasive, quantitative, and approved tool for measuring the hepatic steatosis; the hepatorenal index.
In conclusion, our results confirm that NAFLD is associated with LVDD even with preserved LV systolic functions and can be an independent risk factor for early alterations in LV diastolic function in T2DM patients. This also highlights the need for future studies to investigate the value of preemptive NAFLD management among T2DM patients to prevent cardiac dysfunction.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Lee BW, Lee YH, Park CY, et al. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: a position statement of the fatty liver research group of the Korean diabetes association. Diabetes Metab J. 2020;44(3):382–401.
- Vanjiappan S, Hamide A, Ananthakrishnan R, et al. Nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and its association with cardiovascular disease. Diabetes Metab Syndr. 2018;12(4):479–482.
- Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–2224.
- Tana C, Ballestri S, Ricci F, et al. Cardiovascular risk in non-alcoholic fatty liver disease: mechanisms and therapeutic implications. Int J Environ Res Public Health. 2019;16(17):3104.
- Gholoobi A, Gifani M, Gholoobi A, et al. Relationship between the prevalence and severity of non-alcoholic fatty liver disease and coronary artery disease: findings from a cross-sectional study of a referral center in northeast Iran. JGH Open. 2022;6(5):330–337.
- Jaruvongvanich V, Chenbhanich J, Sanguankeo A, et al. Increased arterial stiffness in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2017;29(9):e28–e35.
- Marwick TH, Ritchie R, Shaw JE, et al. Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. J Am Coll Cardiol. 2018;71(3):339–351.
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–s33.
- Paul J. Recent advances in non-invasive diagnosis and medical management of non-alcoholic fatty liver disease in adult. Egypt Liver J. 2020;10(1):37.
- Othman F, Abushahba G, Salustri A. Adherence to the American society of echocardiography and European association of cardiovascular imaging recommendations for the evaluation of left ventricular diastolic function by echocardiography: a quality improvement project. J Am Soc Echocardiogr. 2019;32(12):1619–1621.
- Dong Y, Huang D, Sun L, et al. Assessment of left ventricular function in type 2 diabetes mellitus patients with non-alcoholic fatty liver disease using three-dimensional speckle-tracking echocardiography. Anatol J Cardiol. 2020;23(1):41.
- Mauermann E, Bouchez S, Bove T, et al. Assessing left ventricular early diastolic velocities with tissue doppler and speckle tracking by transesophageal and transthoracic echocardiography. Anesth Analg. 2021;132(5):1400–1409.
- Stahlschmidt FL, Tafarel JR, Menini-Stahlschmidt CM, et al. Hepatorenal index for grading liver steatosis with concomitant fibrosis. PLoS One. 2021;16(2):e0246837.
- VanWagner LB, Wilcox JE, Ning H, et al. Longitudinal association of non-alcoholic fatty liver disease with changes in myocardial structure and function: the CARDIA study. J Am Heart Assoc. 2020;9(4):e014279.
- Yamazaki H, Tsuboya T, Tsuji K, et al. Independent association between improvement of nonalcoholic fatty liver disease and reduced incidence of type 2 diabetes. Diabetes Care. 2015;38(9):1673–1679.
- Saluja M, Kumar K, Swami YK, et al. Association between non- alcoholic fatty liver disease and left ventricular diastolic dysfunction in patients of type 2 diabetes. J Assoc Physicians India. 2019;67(8):20–24.
- Bonapace S, Perseghin G, Molon G, et al. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35(2):389–395.
- Mantovani A, Pernigo M, Bergamini C, et al. Nonalcoholic fatty liver disease is independently associated with early left ventricular diastolic dysfunction in patients with type 2 diabetes. PLoS One. 2015;10(8):e0135329.
- Lee H, Kim G, Choi YJ, et al. Association between non-alcoholic steatohepatitis and left ventricular diastolic dysfunction in type 2 diabetes mellitus. Diabetes Metab J. 2020;44(2):267–276.
- Petta S, Argano C, Colomba D, et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62(4):928–933.
- Byrne CD, Targher G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: implications for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34(6):1155–1161.
- Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169(3):166–176.
- Nikolajević Starčević J, Janić M, Molecular Mechanisms ŠM. Responsible for diastolic dysfunction in diabetes mellitus patients. Int J Mol Sci. 2019;20(5):1197.
- Salah HM, Pandey A, Soloveva A, et al. Relationship of nonalcoholic fatty liver disease and heart failure with preserved ejection fraction. JACC Basic Transl Sci. 2021;6(11):918–932.