ABSTRACT
Background and aim
Diabetic nephropathy (DN) is a major microvascular diabetic complication representing the key leading reason for end-stage renal disease (ESRD). The current work aimed to evaluate the value of urinary and serum NGAL (neutrophil gelatinase-associating lipocalin), as an early biomarker of DN in subjects with type-2 diabetes mellitus (T2DM).
Methods
The study included 300 adult subjects with T2DM subdivided into 3 groups: Group A: 100 subjects with normoalbuminuria. Group B: 100 subjects with moderately increased albuminuria. Group C: 100 subjects with severely increased albuminuria.
Results
Serum NGAL and urinary NGAL were significantly elevated in T2DMsubjects with albuminuria than in normoalbuminuric T2DMsubjects (p < 0.001). Serum NGAL and urinary NGAL were positively associated with albumin/creatinine Ratio, (p < 0.001). ROC curve analysis was done to discriminate among moderately/severely increased albuminuria from normoalbuminuria showed a cutoff point ≤ 10.76 for serum NGAL with AUC 0.785 (p value < 0.001) and a cutoff point ≤ 15.811 with AUC 0.687 for urinary NGAL(p < 0.001).
Conclusion
NGAL can be utilized as a biomarker for the early diagnosis of DN. Both urinary NGAL and serum NGAL may predict albuminuria and can be utilized as a noninvasive tool for diagnosing, grading, and progressions of DN.
1. Introduction
Diabetic nephropathy (DN) is an important chronic microvascular diabetic complication. Diabetic nephropathy is considered the main reason for ESRD, accounting for 35–50% per the findings of the USA renal data system [Citation1]. The mechanisms resulting in the progressions of diabetic complications are mostly attributed to poor metabolic and poor hemodynamic control [Citation2]. Among patients with diabetes, the presence of DN independently elevates the risk of cardiovascular mortality more than ten folds [Citation3]. Renal pathological variations are existing between subjects with long-standing DM before the onset of micro-albuminuria [Citation4].
Certain tubulointerstitial bio-markers- which were primarily recognized in acute kidney injuries- were stated to confer value in assessing chronic kidney disorders [Citation5]. These bio-markers comprise cystatin c, NGAL, KIM-1, periostin and MCP-1 [Citation6].
NGAL, as well named (lipocalin 2, siderocalin), is a small molecule of 178 amino acids that belongs to the lipocalin superfamily [Citation7]. NGAL is also identified as human neutrophil lipocalin; NGAL has been purified from the secondary granules of human neutrophils primarily in early 1990 by 2 non-dependent Scandinavian groups [Citation8].
NGAL is synthesized in the bone marrow throughout myelopoiesis from where it is focused and kept in the neutrophil granules. It was established that NGAL mRNA is stated in numerous non-hematopoietic tissues, like the trachea, colon, lungs and kidneys [Citation9]. In addition, higher plasma levels detected throughout conditions like acute peritonitis, acute exacerbation of obstructive pulmonic disorders and acute bacterial infections maintain their functions in tissues inflammations [Citation10]. Both serum and urinary NGALs are raised thereafter renal insults, and even though the kidneys look to be the main source of raised plasma lipocalin [Citation11].
The exact role of NGAL is not completely understood; data show that NGAL is up-regulated in cells under stressful conditions as inflammation, infections, ischemia or neoplastic transformations. Moreover, NGAL has an anti-bacterial function, as revealed by its binding to enterobactin and other siderophores [Citation12,Citation13]. Both serum NGAL and urinary NGAL are proposed to be new independent prognostic indicators of the severity of renal disorders and the progression of CKD [Citation14].
Our study aimed to assess the value of serum NGAL and urinary NGAL as early biomarkers of diabetic nephropathy in patients with type 2 diabetes.
2. Materials and methods
2.1. Study subjects
This cross-sectional research was performed on 300 subjects with T2DM, aged ranged from 30 to 70 years old, and subdivided into three groups as follows; Group A: 100 T2DM subjects with normoalbuminuria, equivalent to urine ACR (Albumin: Creatinine Ratio) less than 30 mg/g, group B: 100 T2DM subjects with moderately elevated albuminuria, equivalent to ACR 30 to 300 mg/g and group C: 100 T2DM subjects with severely elevated albuminuria, equivalent to ACR >300 mg/g to less than 2500 mg/g. Patients with a history of congestive heart failure, acute hepatitis, acute kidney injury, acute infections, inflammatory bowel disorders, chronic obstructive pulmonary disorders, malignancies, persistent hematuria or those on dialysis were excluded.
The study design was approved by the ethics committee of Alexandria University. Before any study-related procedure took place, the participating study subjects signed informed consent. The study followed the criteria set by the declaration of Helsinki.
2.2. Methods
All the study participants were subjected to full medical history, thorough clinical examination, and fundus examination. Blood samples were withdrawn from a peripheral vein after an overnight fast. Laboratory investigations were carried out for each patient including; Fasting plasma glucose, glycated Hb (HbA1c), fasting lipid profile, ALT, AST, serum albumin, serum creatinine (s.cr) and serum NGAL. The urine sample was collected from the second- morning void for the assessment of ACR (Albumin: Creatinine Ratio) and measurement of urinary NGAL. Human NGAL ELISA Kit was used: (cloud-clone corp, 2021 USA) guide [Citation15].
2.3. Statistical analysis
Statistical analysis has been done via IBM-SPSS-26 (USA). Qualitative data have been presented as numbers and percentages. The Kolmogorov-Smirnov testing has been utilized to verify normally distributed data. Quantitative data have been presented as a range (min and max), mean, SD, median and interquartile range (IQR). Significance was considered at the 5% level.
3. Results
Demographic and anthropometric parameters of the study groups are shown in There was a significant difference among the three groups regarding age, body weight, waist circumference (WC) and BMI, while there was no statistical significance regarding the sex.
Table 1. Demographics features and anthropometric measures of the study groups.
The laboratory parameters of the three groups are shown in , fasting plasma glucose (FPG), Glycosylated hemoglobin (HbA1c), serum creatinine, total cholesterol (TC), triglyceride (TG), LDL cholesterol levels were significantly elevated in T2DM subjects with albuminuria (group B and group C) in comparison to T2DM subjects with normoalbuminuria (p < 0.001). On the other hand, high-density lipoprotein (HDL) and eGFR were significantly lower in T2DM subjects with albuminuria in comparison to T2DM subjects with normoalbuminuria (P-value <0.001)
Table 2. Comparing between the study groups as regard Glycemic parameters, lipid profile, serum creatinine and GFR.
Among the three studied groups; serum NGAL in group (A) ranged between (7.79–14.22) with mean ± S.D (10.17 ± 1.72), while in group (B) ranged between (6.93–15.32) with mean ± S.D. (10.76 ± 2.0) and in Group (C) ranged between (9.15–38.81) with mean ± S.D 19.07 ± 5.0. Urinary NGAL in group (A) ranged between (9.34–20.52) with mean± S.D. 14.86 ± 2.54 while in group (B) ranged between (9.88–23.78) with mean± S.D. 15.98 ± 3.33 and in group (C) ranged between (11.68–25.28) with mean± S.D. 18.33 ± 3.24. Serum NGAL and urinary NGAL were significantly elevated in T2DM cases with albuminuria than in normoalbuminuric T2DM cases (P-value <0.001). .
Table 3. Comparing among the study groups regarding serum NGAL and urinary NGAL.
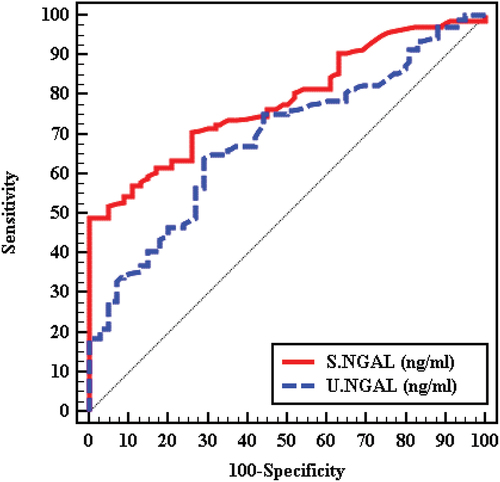
The ROC curve analysis was done to discriminate between moderately/severely albuminuria from normoalbuminuria and serum NGAL showed a sensitivity of 70.50 and specificity 74.00 at a cutoff point ≤10.76 ng/ml with (AUC 0.785 and P- value < 0.001) while urinary NGAL showed a sensitivity of 65.0 and specificity 69.00 at a cutoff point ≤15.811 ng/ml with (AUC 0.687 and P-value <0.001), which means that s-NGAL is more sensitive and more specific than u-NGAL. .
Figure 1. ROC curve for Serum NGAL (ng/ml) and urinary NGAL (ng/ml) to discriminate between moderately/severely albuminuria (group B + C) (n = 200) from normoalbuminuria (group A) (n = 100).
Correlation between ACR (Albumin: Creatinine Ratio) with serum NGAL and urinary NGAL showed a highly significant positive association between them (p < 0.001).
Univariate logistic regression analysis revealed that age, WC, BMI, triglycerides, LDL, HDL, urinary NGAL, serum NGAL and NPDR are statistically significantly related to moderately/severely increased albuminuria . Moreover, the multivariate logistic regression analyses showed that HbA1c, GFR, HDL, and serum NGAL are independently statistically significantly related to the presence of moderately/severely increased albuminuria.
Table 4. Univariate and multi-variate logistic regression analyses for the factors impacting moderately/severely albuminuria (group B+ C) (n = 200).
4. Discussion
Diabetic nephropathy is an important diabetic specific microvascular complication that is connected to the development of macro-vascular complications and all-cause mortality. Moreover, due to late diagnosing and delayed interventions, many cases reach ESRD and require renal replacement treatment [Citation16]. The detection of sensitive and precise biomarkers for renal impairment can advance management and strengthen efforts to advance reno-protective medications [Citation17].
NGAL is one of the lipocalin families that is present at a low level in different tissues and is quickly emitted from renal tubular cells in response to several renal insults. Serum NGAL and urinary NGAL levels are promising biomarkers for the early diagnosis of acute renal injuries [Citation18]. This work aimed to evaluate the value of serum NGAL and urinary NGAL, as early biomarkers of diabetic nephropathy in cases with T2DM.
This cross-sectional study included 300 cases with T2DM aged from 30 to 70 years. The studied cases were subdivided into 3 groups as follows; Group A: 100 T2DM cases with normoalbuminuria, equivalent to urine ACR (Albumin: Creatinine Ratio) less than 30 mg/g. Group B: 100 T2DM cases with moderately elevated albuminuria, equivalent to ACR 30–300 mg/g. Group C: 100 T2DM cases with severely elevated albuminuria, equivalent to ACR >300 mg/g to less than 2500 mg/g.
DN is one of the key serious microvascular DM complications. DN is marked by increased urinary albumin excretion rates, rise in BP (blood pressure), and decline in renal functions causing ESRD. Furthermore, these cases have an elevated risk of cardiovascular disorders.
In the current work, estimated GFR was significantly reduced among subjects with moderately and severely elevated albuminuria in comparison to T2DM subjects with normoalbuminuria (p < 0.001). Our findings have been supported by several studies, moreover, more reduction in the eGFR was present in the macroalbuminuric compared to the micro-albuminuric patients with diabetes [Citation19,Citation20]. However, in the study of Forghani et al. [Citation21], serum creatinine and eGFR level didn’t reveal any significant difference among normoalbuminuric and macroalbuminuric groups, confirming that abnormal albuminuria may precede deterioration in eGFR.
The present work revealed that serum NGAL and urinary NGAL were statistically significantly elevated in T2DM subjects with increased albuminuria compared to T2DM subjects with normoalbuminuric (P-value <0.001).
In the study of Kaul et al. [Citation22], they observed serum NGAL and urinary NGAL values across healthy controls and 3 groups of T2DM with normal serum creatinine, normoalbuminuria, microalbuminuria, and macroalbuminuria. A significant difference was present in median values of serum NGAL and urinary NGAL among subjects with diabetes in comparison with well-matched controls, moreover significantly higher values were detected among subjects with macroalbuminuria. These findings point to the concept that NGAL may be an early beneficial marker of DN even when serum Creatinine level is within the ordinary range. In agreement with our work, serum NGAL and urinary NGAL were statistically significantly positively correlated with albuminuria, proposing NGAL may be also a marker of the severity of renal impairment. This accomplishes that NGAL could be utilized as a biomarker to stratify DN into various grades.
Our findings were in line with previous studies that concluded the equivalent tendency for NGAL [Citation23] and other bio-markers of tubular injuries such as MCP, NAG, uLFABP and KIM-1 to detect diabetic nephropathy [Citation24]. On the contrary, Kim et al. [Citation25], revealed that there was no significant difference in neither serum NGAL nor urinary NGAL levels among normo-albuminuria and micro-albuminuria groups, thus against the use of NGAL as a biomarker of early DN.
As NGAL is a biomarker of distal tubular injuries, thus its level is independent of the presence of albuminuria or proteinuria, supporting the previous suggestion that tubular injuries may occur before glomerular injuries, thus biomarkers of tubular injuries may predict the onset of albuminuria and its future progressions [Citation26].
The ROC curve analysis was done to evaluate the diagnostic power of both serum and urinary NGAL levels as early predictors of DN. Serum NGAL showed a sensitivity of 70.50 and a specificity 74.00 at cutoff point ≤10.76 ng/ml with (AUC 0.785 and P-value <0.001), while urinary NGAL showed a sensitivity of 65.0 and a specificity 69.00 at cutoff point ≤15.811 ng/ml with (AUC 0.687 and P-value <0.001). Thus, serum NGAL is more sensitive and more specific than urinary NGAL for the prediction of diabetic nephropathy. In the study of Kaul et al. [Citation22], ROC curve analyses have been done to define the diagnostic value of serum NGAL, urinary NGAL, and urinary albumin creatinine ratio in recognizing DN. The urinary NGAL revealed a great diagnosing profile, explaining an AUC of 0.997 (95% CI: 0.99–1.000, P value < 0.001) with an optimum cutoff value of 21.31 ng/ml (sensitivity 95.1%; specificity 100%), while serum NGAL, the AUC was 0.992 (95% CI: 0.997–1.000, P value < 0.001) and an optimum cutoff value of 78.73 ng/ml (sensitivity 95.1%; specificity 100.0%).
Despite these promising findings of NGAL as an early marker of DN, the key limitation of our work was that the study was performed in one center, thus the data can’t be applied to all populations. We recommend further multicentric research to establish the value of urinary NGAL and serum NGAL assessment in patients with diabetes for early detection of DN, and confirm NGAL role in predicting and grading the severity of diabetic nephropathy.
5. Conclusion
NGAL could be utilized as an early biomarker to detect DN. Both urinary NGAL and serum NGAL could predict albuminuria and may be utilized as a noninvasive tool for diagnosing, grading, and predicting future progressions of DN.
Abbreviations
DN Diabetic nephropathy
CKD chronic kidney disease
T2DM type 2 diabetes
NGAL Neutrophil Gelatinase Associating Lipocalin
KIM-1 Kidney injury molecule-1
MCP-1 Monocyte Chemoattractant Protein-1
HbA1c glycosylated hemoglobin
BMI body mass index
WC waist circumference
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Mohamed Said Abdel salam], [Noha Gaber Amin], [Mohamed Nagy Abdel hay Ahmed Altair], and [Neveen Lewis Mikhael saad]. The first draft of the manuscript was written by [Magdy Helmy Zikry Megallaa] and [Noha Gaber Amin], and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Ethics approval
The study design was approved by the ethics committee of Alexandria University. The study followed the criteria set by the declaration of Helsinki. Confidentiality and personal privacy were respected in all levels of the study.
Informed consent
The participating study population signed an informed consent before any study related- procedure took place. Patients felt free to withdraw from the study at any time without any consequences. Participants also signed an additional consent for publication.
Consent to participate and consent to publish
Informed consent was obtained from all individual participants included in the study to participate in the study and to publish the results.
Supplemental Material
Download JPEG Image (200.5 KB)Disclosure statement
The authors have no relevant financial or non-financial interests to disclose.
The authors have no competing interests to declare that are relevant to the content of this article.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
The authors have no financial or proprietary interests in any material discussed in this article.
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20905068.2023.2230051
Additional information
Funding
References
- Lim A. Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361–381. doi: 10.2147/ijnrd.s40172
- Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24(2):302–308. doi: 10.1681/ASN.2012070718
- Robles NR, Villa J, Felix FJ, et al. Non-proteinuric diabetic nephropathy is the main cause of chronic kidney disease: results of a general population survey in Spain. Diabetes Metab Syndr. 2017; 11(Suppl 2):S777–s81. doi: 10.1016/j.dsx.2017.05.016
- Boronat M, García-Cantón C, Quevedo V, et al. Non-albuminuric renal disease among subjects with advanced stages of chronic kidney failure related to type 2 diabetes mellitus. Ren Fail. 2014;36(2):166–170. doi: 10.3109/0886022x.2013.835266
- Mancini M, Masulli M, Liuzzi R, et al. Renal duplex sonographic evaluation of type 2 diabetic patients. J Ultrasound Med. 2013;32(6):1033–1040. doi: 10.7863/ultra.32.6.1033
- Budhiraja P, Thajudeen B, Popovtzer M. Absence of albuminuria in type 2 diabetics with classical diabetic nephropathy: clinical pathological study. J Biomed Sci Eng. 2013;6:20–25. doi: 10.4236/jbise.2013.65A005
- Robles NR, Villa J, Gallego RH. Non-proteinuric diabetic nephropathy. J Clin Med. 2015;4(9):1761–1773. doi: 10.3390/jcm4091761
- Porrini E, Ruggenenti P, Mogensen CE, et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2015;3(5):382–391. doi: 10.1016/s2213-8587(15)00094-7
- Halimi JM. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab. 2012; 38(4):291–297. doi: 10.1016/j.diabet.2012.04.001
- Esteghamati A, Arefzadeh A, Zandieh A, et al. Comparison of osteoprotegerin and vascular endothelial growth factor in normoalbuminuric Type 1 diabetic and control subjects. J Endocrinol Invest. 2013;36(7):474–477. doi: 10.1007/bf03347110
- Lewis JR, Lim WH, Ueland T, et al. Elevated circulating osteoprotegerin and renal dysfunction predict 15-year cardiovascular and all-cause mortality: a prospective study of elderly women. PLoS One. 2015;10(7):e0134266. doi: 10.1371/journal.pone.0134266
- Brachemi S, Bollée G. Renal biopsy practice: what is the gold standard? World J Nephrol. 2014;3(4):287–294. doi: 10.5527/wjn.v3.i4.287
- Satirapoj B, Nast CC, Adler SG. Novel insights into the relationship between glomerular pathology and progressive kidney disease. Advances in chronic kidney disease. 2012;19(2):93–100.
- Koye DN, Magliano DJ, Reid CM, et al. Risk of Progression of nonalbuminuric CKD to end-stage kidney disease in people with diabetes: the CRIC (chronic renal insufficiency cohort) study. Am J Kidney Dis. 2018;72(5):653–661. doi: 10.1053/j.ajkd.2018.02.364
- Cloud-clone Corp. Human neutrophil gelatinase-associated lipocalin (NGAL) ELISA kit guide. 2021.
- Nazar CM. Diabetic nephropathy; principles of diagnosis and treatment of diabetic kidney disease. J Nephropharmacol. 2014;3(1):15–20.
- Peres LA, da Cunha AD Jr., Assumpção RA, et al. Evaluation of the cisplatin nephrotoxicity using the urinary Neutrophil Gelatinase-associated LipocaliN (NGAL) in patients with head and neck cancer. J Brasileiro de Nefrologia: ‘Orgao Oficial de Sociedades Brasileira E Latino-Americana de Nefrologia. 2014;36(3):280–288. doi: 10.5935/0101-2800.20140041
- Tkaczyk M, Tomczyk D, Jander A, et al. Glomerular filtration decrease after diagnostic cardiac catheterisation in children with congenital cardiac malformation - the role of serum creatinine, cystatin C, neutrophil gelatinase and urine output monitoring. Postepy w kardiologii interwencyjnej = Advances in interventional cardiology. 2018;14(1):67–74. doi: 10.5114/aic.2018.74357
- Mahfouz MH, Assiri AM, Mukhtar MH. Assessment of Neutrophil gelatinase-associated lipocalin (NGAL) and retinol-binding protein 4 (RBP4) in Type 2 diabetic patients with nephropathy. Biomark Insights. 2016;11:31–40. doi: 10.4137/bmi.s33191
- Siddiqui K, Joy SS, George TP, et al. Potential role and excretion level of urinary transferrin, KIM-1, RBP, MCP-1 and NGAL markers in diabetic nephropathy. diabetes, metabolic syndrome and obesity: targets and therapy. 2020;13:5103–5111. doi: 10.2147/dmso.s282166
- Forghani MS, Khezrian F, Khezrian S, et al. Urinary Neutrophil Gelatinase-associated LipocaliN in early detection of diabetic nephropathy; a pilot study. J Renal Injury Prev. 2020;9(3):e23. doi: 10.34172/jrip.2020.23
- Kaul A, Behera MR, Rai MK, et al. Neutrophil Gelatinase-associated lipocalin: as a predictor of early diabetic nephropathy in type 2 diabetes mellitus. Indian J Nephrol. 2018;28(1):53–60. doi: 10.4103/ijn.IJN_96_17
- Fathimah M, Alicezah MK, Thevarajah M. Neutrophil Gelatinase-Associated Lipocalin (NGAL): an early marker for diabetic nephropathy. Int J Diabetes Dev Ctries. 2012;32(1):19–24. doi: 10.1007/s13410-011-0061-z
- Morii T, Fujita H, Narita T, et al. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications. 2003;17(1):11–15. doi: 10.1016/s1056-8727(02)00176-9
- Kim SS, Song SH, Kim IJ, et al. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract. 2012;97(2):251–257. doi: 10.1016/j.diabres.2012.02.019
- Nielsen SE, Sugaya T, Hovind P, et al. Urinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care. 2010;33(6):1320–1324. doi: 10.2337/dc09-2242
