ABSTRACT
Objectives
Type 2 diabetes (T2DM) has been associated with several mental disorders including depression. Brain-derived neurotrophic factor (BDNF) has been suggested as a mediator between T2DM and depression. We investigated the association between BDNF and depression in T2DM patients in a primary health facility in Ghana.
Methods
In a case-control study design, depression was assessed using the Patient’s Health Questionnaire (PHQ-9) in 200 T2DM patients and 160 non-diabetic controls. A structured questionnaire was used to collect data on lifestyle, socio-demography and medical history. Fasting blood samples were collected from each participant to measure plasma lipid profile and serum BDNF levels.
Results
T2DM patients had higher levels of depression (31.5% vs 10.6, p < 0.001) and lower serum levels of BDNF (22.8 ± 4.6 vs 34.6 ± 10.6, p < 0.001) compared to non-diabetic controls. In non-diabetic controls, those with depression had low levels of serum BDNF compared to their non-depressed counterparts (38.9 ± 10.1 vs 32.3 ± 9.9 ng/ml, p = 0.038), but in T2DM patients, no difference in serum BDNF levels was observed in those with and without depression. Severe depression in T2DM patients [OR (95% CI): 0.81 (0.57–0.97), p = 0.042] and mild depression in non-diabetic controls [0.84 (0.6–0.95), p < 0.001] were associated with reductions in serum BDNF levels.
Conclusions
In our study sample, T2DM patients had a high burden of depression and low levels of BDNF compared to the non-diabetic controls. T2DM with severe depression and non-diabetic controls with mild depression had significant reduction of serum BDNF levels.
1. Introduction
Type 2 diabetes (T2DM) is a debilitating chronic disease that has widespread systemic complications, particularly, in the cardiovascular and nervous system, leading to a pronounced deterioration of quality of life, excessive cost of treatment and a high burden on the under-resourced healthcare systems [Citation1]. Of all the complications of T2DM, mental health disorders like depression are usually neglected by health systems in sub-Saharan Africa [Citation2]. Depression is more frequent in T2DM patients than in the general population, associated with poor diabetic control [Citation3] and higher levels of diabetic complications [Citation4,Citation5]. Diabetes and depression have a bidirectional relationship [Citation5] with the two diseases sharing common risk factors and complications. For instance, low birth weight [Citation6], obesity [Citation7], fatty diet [Citation8] and physical inactivity [Citation7] have been reported to be associated with the development of depression and diabetes. In addition, the neurotrophic hypothesis links diabetes and depression by postulating that insulin dysfunction and hyperglycemia in diabetes cause a proinflammatory milieu that reduces the expression of neurotrophins such as BDNF, leading to alteration in mood and development of depressive symptoms [Citation9,Citation10].
Brain-derived neurotrophic factor (BDNF) is the most commonly studied neurotrophic growth factor of the central nervous system that regulates the survival, development, and differentiation of neurons [Citation11]. Some studies have reported that dysregulation of circulating BDNF levels can affect cognitive functions through its capacity to modulate neurite outgrowth, neuronal differentiation, survival, and growth [Citation12,Citation13]. Furthermore, BDNF has been reported to regulate tissue metabolism through its central and peripheral influence on various enzymes that regulate intermediary metabolism, resulting in dysglycemia and dyslipidaemia [Citation14]. Some studies have reported alteration in circulating BDNF levels in patients with diabetes [Citation15,Citation16], depression [Citation17,Citation18] and mood disorders [Citation19] suggesting that BDNF may be an important psychophysiological biomarker of metabolic and mental disorders. Furthermore, some studies have reported racial variations in circulating BDNF levels among pregnant women [Citation20] and HIV patients [Citation21] with people of African descent having higher BDNF levels compared to Caucasians. There is a paucity of data on the levels of BDNF in T2DM patients with depression in the sub-Saharan African population. This study aimed to investigate the association between serum BDNF levels and depression in T2DM patients from a primary health facility in Ghana. We hypothesize that the high burden of depression in T2DM patients may be associated with a reduction of serum BDNF levels.
2. Methods
2.1. Setting, design and participants
This study was a case-control design, conducted at the Sunyani Regional Hospital in Ghana within the period February 2020 through August 2021 as reported previously [Citation3]. The Sunyani Regional Hospital is a primary healthcare facility that serves patients from the middle belt of Ghana. The hospital has a diabetes clinic that provides ambulatory medical care and consultation, dietetics, diabetes education and eye services for persons with diabetes. We recruited 200 T2DM patients, aged between 30 and 65 years, into the study by systematic random sampling of every third consenting patient visiting the diabetic clinic. Afterward, 160 non-diabetic controls were conveniently invited from the surrounding communities to join the study. T2DM status was determined clinically as patients diagnosed with diabetes after 30 years of age and were managed initially on lifestyle modification or antidiabetic drugs. Patients with type 1 diabetes, pregnant women and those under 30 years of age at diagnosis or patients older than 65 years of age were excluded from the study. In addition, patients with other causes of depression such as bereavement within the past 2 months and those with medication/history of depression or manic/hypomanic episodes were excluded from the study.
2.2. Procedure and instruments for data collection
Hardcopies of structured questionnaires were administered by trained research assistants to all the study participants to collect data on socio-demographic and clinical characteristics such as age, gender, education, employment, alcohol and smoking status. Smoking status was classified as never, previous (smoking cessation since more than 1 year before the survey) or current smoking, and alcohol intake was classified as drinkers and nondrinkers. We also collected information on the duration of diabetes and the use of insulin and other oral hypoglycemic agents to manage the diabetes. The oral hypoglycemic agents may include metformin and other medications that lower the blood glucose in different ways and they do not cause hypoglycemia except if combined with sulphonylureas or insulin.
Depression was screened using the Patient Health Questionnaire-9 (PHQ-9). PHQ-9 is an instrument that has been validated in the Ghanaian population [Citation22,Citation23] and patients with diabetes [Citation24] for the detection of major depressive symptoms. This PHQ-9 is made up of 9 items, with each item scored from 0 (not at all) to 3 (nearly every day) and culminating in a severity score of 0–27. The recommended cut points of 5, 10, and 15 were applied for the identification of mild, moderate, and severe levels of depressive symptoms, respectively; a cut point of 10 or higher was used for the diagnosis of major depression [Citation23,Citation25].
We measured the blood pressure in patients in a seated position after 5 minutes of rest using an automated digital blood pressure monitor (Omron 907XL pro, Healthcare, Inc., Vernon Hills, IL), and body weight and height were measured with a Seca 740 scale and a stadiometer respectively. Body mass index (BMI) was computed using the formula: weight in kilograms divided by the height in meters squared. Blood samples were collected before 9 a.m. after 8–12 hours of overnight fasting into appropriate sample tubes and stored at −80°C until analysis. The plasma levels of glucose and lipid profile were assayed using a biochemical analyzer (BC300, Contec Medical Systems, China) and commercial reagents (Randox Laboratory Reagents, UK).
Serum levels of BDNF were measured by enzyme-linked immunosorbent assay (ELISA) according to the procedures supplied by the manufacturer (DuoSet, R&D Systems, Minneapolis, MN, USA). BDNF assays were performed in triplicates and averaged for analysis. To reduce the impact of diurnal variability and storage effect on BDNF levels, fasting blood samples were collected early in the morning before 9 a.m. and stored at −80°C, with the assays performed within 6 months. The storage of serum samples at this temperature has been reported to have no significant effect on BDNF levels in the healthy population [Citation26]. The lower detection limit was 5 pg/ml. Concentrations were expressed as nanograms per milliliter (ng/ml). The inter- and intra-assay coefficients of variation were less than 5%.
2.3. Ethical considerations
The study was conducted in conformity with the Helsinki Declaration on Human Experimentation, 1964, with subsequent revisions, latest Seoul, October 2008. Ethical approval was obtained from the Ethics and Protocol Review Committee of the College of Health Sciences of the University of Ghana (Protocol ID number: CHS-Et/M.2–4.11/2018–2019) and each study participant provided written voluntary informed consent after the rationale and procedure of the study were thoroughly explained. Patients found to be depressed were referred to the Psychiatry Unit for further assessment and possible management.
2.4. Sample size calculation
The sample size required for this study was calculated based on the pilot data of BDNF levels from 25 T2DM patients and 25 nondiabetic controls (25.7 ± 12.1 vs 29.5 ± 11.4 ng/ml) in two stages. A minimum of 152 participants were required in each group to achieve a power of 80% at a 95% significance level. In addition, a regression model of the pilot data with two test predictors and a total of 8 predictors yielded an adjusted R2 value of 0.17, which implied that we needed a minimum of 327 participants to achieve a power of 80% at a 95% significance level. Therefore, 200 T2DM patients and 160 nondiabetic controls were recruited for the study.
2.5. Statistical analysis
Data were analyzed using Jamovi 3.28 software. Data were presented as mean with standard deviation for continuous variables and counts and percentages for categorical variables. Differences between the T2DM patients and non-diabetic control concerning their socio-demographic, clinical and biochemical variables were analyzed using a chi-square (χ2) test for categorical variables and a Student t-test for continuous variables. The distribution of data was checked using Shapiro-Wilk’s test to ensure that all the variables were normally distributed before incorporating them into the regression analyses. Logistic regression analyses were performed to determine the association between serum BDNF and depression and multiple linear regression analysis was used to determine the predictors of BDNF in study participants. The level of significance was set at p < 0.05.
3. Results
3.1. General characteristics of participants
In this study, T2DM patients were older, with a high prevalence of previous smokers and alcohol drinkers compared to non-diabetes controls. As expected, T2DM patients had higher BMI, indices of blood pressure (heart rate, systolic, diastolic, mean and pulse BPs), and biochemical parameters (fasting plasma glucose, triglycerides, total and LDL cholesterols) but low HDL cholesterol compared to nondiabetic controls. The distribution of gender, marital status and those living with relatives were not associated with diabetes status. Employment and educational levels were associated with diabetes status. The mean PHQ-9 score and prevalence of depression were higher in T2DM patients compared to non-diabetic controls. T2DM patients had lower levels of BDNF compared to non-diabetic controls (). The median duration of diabetes in the T2DM patients was 7.3 years (range: 0.1–21 years), with 93 (46.5%), 74 (37%) and 33 (16.5%) patients having a duration of T2DM with <5 years, 5–10 years and >10 years respectively. Concerning diabetes treatment, eight (4%) patients were on lifestyle management, 122 (61%) patients were on oral hypoglycemic agents and 70 (35%) patients were on insulin and oral hypoglycemic agents.
Table 1. General characteristics of the study participants.
3.2. Levels of depression and serum BDNF
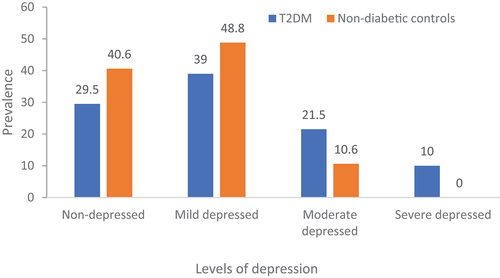
The levels of depression were associated with diabetes status with more T2DM patients having moderate depression compared to non-diabetic controls. None of the non-diabetic controls had severe depression (). In non-diabetic controls, serum BDNF levels were reduced in participants with depression compared to their non-depressed counterparts. However, there was no difference in BDNF levels between T2DM patients with and without depression (). In correlational analysis, BDNF negatively correlated with age, systolic BP, plasma triglycerides and LDL cholesterol levels, and correlated positively with plasma total and HDL cholesterol levels in the entire study participants. In T2DM patients, BDNF negatively correlated with age, systolic BP, fasting plasma glucose, and plasma triglyceride levels while correlated positively with total and LDL cholesterol levels. In non-diabetic patients, BDNF positively correlated with HDL cholesterol levels ().
Figure 2. Serum levels of BDNF among study participants by diabetes and depression status. Depression is defined as a PHQ-9 score >10.
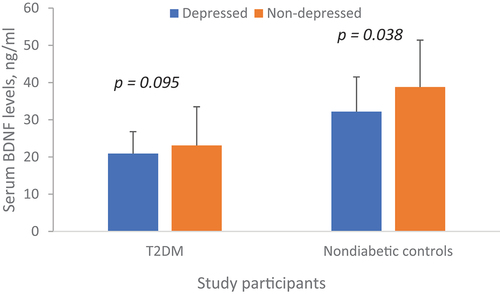
Table 2. Correlation between serum BDNF levels and participant characteristics.
3.3. Association between depression and serum BDNF
In both unadjusted and adjusted logistic regression models, a unit increase in serum BDNF level was associated with decreased odds of severe depression in all participants and T2DM patients, as well as decreased odds of mild depression in non-diabetic controls (). The main predictors of BDNF variation in the entire study participants were severe depression, diabetes status, insulin treatment, smoking, plasma total cholesterol and triglyceride levels. The major predictors of BDNF in T2DM patients were severe depression, insulin treatment, previous smoking and plasma total cholesterol levels. In non-diabetic controls, the major predictors were mild depression, alcohol intake and hypertension ().
Table 3. Associated between serum BDNF and levels of depression from logistic regression models.
Table 4. Predictors of serum BDNF levels in study participants from multiple regression analysis.
4. Discussion
In this study, compared to non-diabetic controls, T2DM patients had a higher prevalence of depression and low levels of serum BDNF. Increased serum BDNF levels were associated with decreased odds of severe depression in T2DM patients and mild depression in non-diabetic controls. Variations in BDNF levels were associated with T2DM status, insulin treatment, smoking, triglyceride, and total cholesterol levels. In this study, we found the prevalence of depression to be 31.5% and this has been reported in association with diabetic control elsewhere [Citation3].
We found that, in both depressed and non-depressed individuals, serum BDNF levels were significantly lower in T2DM patients compared to their non-diabetic counterparts. This is consistent with a study by Krabbe et al., who reported that plasma BDNF levels were reduced in diabetes patients compared to non-diabetic controls, and even in healthy individuals, hyperglycemia reduces circulating BDNF [Citation16]. Furthermore, in Chinese T2DM patients, serum levels of BDNF levels were lower compared to non-diabetic controls [Citation27,Citation28]. Contrary to our findings, some studies have reported high BDNF levels in patients with T2DM compared to non-diabetic individuals [Citation29–31]. These conflicting reports on the levels of BDNF in diabetes patients may be due, at least in part, to ethnic differences [Citation20,Citation21] and the duration of diabetes [Citation9]. Another plausible reason for the lower BDNF in the T2DM patients may be a result of the significantly lower BMI and waist circumference in the control group, compared to the T2DM patients [Citation31]. BDNF has been shown to have an anti-diabetic effect by increasing insulin secretion and sensitivity in peripheral tissues, as well as decreasing blood glucose through insulin-independent mechanisms. For example, intraventricular administration of BDNF can mitigate the levels of hyperglycemia by reducing hepatic glucose output through the normalization of glucagon secretion and hepatic expression of gluconeogenic enzyme synthesis [Citation32].
We found that the reduction in BDNF in depressed individuals was more significant in non-diabetic controls than in T2DM, and this is consistent with previous studies that reported low levels of BDNF in depressed patients [Citation33–35]. In T2DM patients, unlike non-diabetic individuals, other factors such as derangement of metabolic parameters due to insulin resistance may mask the relationship between circulating levels of BDNF and depression [Citation36–40]. We noted from the multiple regression analysis that BDNF levels were reduced in T2DM patients with severe depression, but not in those with mild and moderate depression. This observation is consistent with the findings that a reduction in BDNF was associated with the severity of depression [Citation36]. There have been several studies that have reported that, in comparison to the depressive patient without suicidal attempts or non-depressed controls, individuals who attempted suicide, an indication of severe depression, had significantly reduced circulating BDNF levels and brain BDNF mRNA expression [Citation33,Citation34,Citation41]. The mechanism linking depression with decreased circulating BDNF levels is not fully understood, but it is believed that circulating BDNF levels may have originated mainly from the brain biosynthesis of BDNF [Citation42], which is reduced in patients with neuropsychiatric disorders. It has also been demonstrated that the anti-depressive effect of pharmacological interventions [Citation18] and non-pharmacological interventions such as diet and exercise [Citation12,Citation43] improves the levels of circulating BDNF. In non-diabetic controls in our study, those with mild depression had a reduction in BDNF levels, indicating that the impact of depression on BDNF levels may be more pronounced in the absence of diabetes in our study population. However, our findings are inconsistent with the findings of a meta-analysis that reported that there is no significant correlation between BDNF expression and the severity of depression in antidepressant-free individuals [Citation35].
In this study, serum BDNF was negatively associated with high blood pressure and positively with indices of plasma lipid profiles except LDL cholesterol, similar to what was reported in the Baltimore Aging Study [Citation44] and patients with schizophrenia [Citation45]. The mechanism associating circulating BDNF with blood pressure and lipid profile is yet to be fully unraveled. Concerning the association between BDNF and high blood pressure, it has been demonstrated in spontaneously hypertensive rats that oxidative stress upregulates BDNF synthesis before a rise in blood pressure is observed [Citation46]. Similarly, BDNF was associated with hypertrophic remodeling of the carotid artery in the SAPBA study [Citation47], indicating that BDNF affects the atherogenic process in humans. Concerning plasma lipid profile, BDNF is reported to have a role in cholesterol biosynthesis in the liver and adipose tissue. The adipose tissue expresses BDNF and its receptor, TrkB, but proinflammatory condition increases the expression of BDNF while reducing that of TrkB [Citation48]. Additionally, in human neurons and astrocytes, BDNF has been reported to regulate HDL cholesterol by modulating the synthesis of apolipoprotein E synthesis [Citation49]. However, since peripheral cholesterol biosynthesis is separated from central nervous cholesterol biosynthesis, more studies may be needed to investigate the role of BDNF in circulating cholesterol levels.
The interpretation of the findings of this study has some limitations. The data were collected at one time in a single facility, limiting the inference of causality and generalization to the entire Ghanaian population. Furthermore, we measured circulating levels of BDNF in the serum, which may differ from plasma and cerebrospinal BDNF levels [Citation13]. The concentration of BDNF in serum has been reported to be 50 times higher than that of plasma. This is due to the ability of platelets to absorb BDNF produced by the brain and release them into serum during the coagulation process of processing blood samples [Citation50]. This may explain the observation of a moderate correlation between plasma BDNF and hippocampal BDNF as reported in a previous study [Citation11]. Another source of error in our analysis was the ELISA method we used to assay serum levels of BDNF, which has been reported to capture mature BDNF and proBDNF forms [Citation51]. We, however, expected the effect of this error to be negligible due to the adequate sample size and the use of non-diabetic controls. We also did not assess other factors that may affect BDNF levels such as physical activity, neuropsychiatric risks and comorbidities. A multicenter longitudinal study design that can characterize the psychiatric status of the patients in detail may be used to study the role of BDNF in the development of neuropsychiatric complications in diabetes patients in Ghana.
5. Conclusions
In our study population, T2DM patients had a high burden of depression and low levels of BDNF compared to the non-diabetic controls. In T2DM patients, having severe depression was associated with a significant reduction in BDNF levels. Further studies should be conducted to investigate the possible roles of BDNF in the development of depression in T2DM patients and how some pharmacologic and non-pharmacological interventions can help to boost BDNF and mental health in T2DM patients. In addition, the effects of various psychotherapies such as cognitive behavior therapy, behavioral activation therapy, interpersonal psychotherapy, problem-solving therapy, and non-directive counseling on depression and BDNF levels in T2DM patients may require further investigation.
Abbreviations
BDNF, Brain-derived neurotrophic factor; BMI, body mass index; BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PHQ, Patient Health Questionnaire; T2DM, Type 2 diabetes.
Authors’ contributions
KY conceptualized the study, analyzed the data and drafted the manuscript. TG collected the data and revised the manuscript. JAA analyzed the data and made scientific contributions to the manuscript. All authors approved the content of the manuscript.
Availability of data
A dataset supporting the conclusions of this paper is available and can be requested from the corresponding author.
Acknowledgments
We would like to thank all the participants at the Sunyani Regional Hospital who voluntarily took part in this study. We are grateful to the community health volunteers and healthcare providers at the Diabetic clinic for their overwhelming support during the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Kwame Yeboah
Dr Kwame Yeboah is a senior lecturer and Departmental Chair at the Department of Physiology, University of Ghana Medical School.
Thomas Gyamfi
Mr Thomas Gyamfi is a senior nurse at the Children’s Block, 37 Military Hospital, Accra, Ghana.
Jennifer Adjepong Agyekum
Ms Jennifer Adjepong Agyekum is a Biomedical scientist at the Laboratory Unit, Mamprobi Hospital, Ghana Health Service.
References
- Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA. 2014;312(7):691–692. doi: 10.1001/jama.2014.8040
- Gaynes BN, Akiba CF, Hosseinipour MC, et al. The sub-saharan Africa regional partnership (SHARP) for mental health capacity-building scale-up trial: study design and protocol. Psychiatric Serv. 2021;72(7):812–821. doi: 10.1176/appi.ps.202000003
- Agyekum JA, Gyamfi T, Yeboah K. Depression, poor sleep quality, and diabetic control in type 2 diabetes patients at Sunyani Regional Hospital, Ghana: a case–control study. Middle East Current Psychiatry. 2023;30(1):45. doi: 10.1186/s43045-023-00317-1
- Chen S, Zhang Q, Dai G, et al. Association of depression with pre-diabetes, undiagnosed diabetes, and previously diagnosed diabetes: a meta-analysis. Endocrine. 2016;53(1):35–46. doi: 10.1007/s12020-016-0869-x
- Khaledi M, Haghighatdoost F, Feizi A, et al. The prevalence of comorbid depression in patients with type 2 diabetes: an updated systematic review and meta-analysis on huge number of observational studies. Acta Diabetol. 2019;56(6):631–650. doi: 10.1007/s00592-019-01295-9
- Paile‐Hyvärinen M, Räikkönen K, Forsén T, et al. Depression and its association with diabetes, cardiovascular disease, and birth weight. Ann Med. 2007;39(8):634–640. doi: 10.1080/07853890701545722
- Huang B, Huang Z, Tan J, et al. The mediating and interacting role of physical activity and sedentary behavior between diabetes and depression in people with obesity in United States. J Diabetes Complications. 2021;35(1):107764. doi: 10.1016/j.jdiacomp.2020.107764
- Daneshzad E, Keshavarz S-A, Qorbani M, et al. Dietary total antioxidant capacity and its association with sleep, stress, anxiety, and depression score: a cross-sectional study among diabetic women. Clin Nutr ESPEN. 2020;37:187–194. doi: 10.1016/j.clnesp.2020.03.002
- Rozanska O, Uruska A, Zozulinska-Ziolkiewicz D. Brain-derived neurotrophic factor and diabetes. Int J Mol Sci. 2020;21(3):841. doi: 10.3390/ijms21030841
- Jaggar M, Fanibunda SE, Ghosh S, et al. Chapter 6 - the neurotrophic hypothesis of depression revisited: new insights and therapeutic implications. In: Quevedo J Carvalho A, editors. Neurobiology of depression. Zarate CA: Academic Press; 2019. p. 43–62.
- Klein AB, Williamson R, Santini MA, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14(3):347–353. doi: 10.1017/S1461145710000738
- Kallies G, Rapp MA, Fydrich T, et al. Serum brain-derived neurotrophic factor (BDNF) at rest and after acute aerobic exercise in major depressive disorder. Psychoneuroendocrinology. 2019;102:212–215. doi: 10.1016/j.psyneuen.2018.12.015
- Morichi S, Kashiwagi Y, Takekuma K, et al. Expressions of brain-derived neurotrophic factor (BDNF) in cerebrospinal fluid and plasma of children with meningitis and Encephalitis/Encephalopathy. Int J Neurosci. 2012;123(1):17–23. doi: 10.3109/00207454.2012.721829
- Wang C-P, Lorenzo C, Habib SL, et al. Differential effects of metformin on age related comorbidities in older men with type 2 diabetes. J Diabetes Complications. 2017;31(4):679–686. doi: 10.1016/j.jdiacomp.2017.01.013
- Davarpanah M, Shokri-Mashhadi N, Ziaei R, et al. A systematic review and meta-analysis of association between brain-derived neurotrophic factor and type 2 diabetes and glycemic profile. Sci Rep. 2021;11(1):13773. doi: 10.1038/s41598-021-93271-z
- Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia. 2007;50(2):431–438. doi: 10.1007/s00125-006-0537-4
- Cavaleri D, Moretti F, Bartoccetti A, et al. The role of BDNF in major depressive disorder, related clinical features, and antidepressant treatment: insight from meta-analyses. Neuroscience & Biobehavioral Reviews. 2023;149:105159. doi: 10.1016/j.neubiorev.2023.105159
- Mondal AC, Fatima M. Direct and indirect evidences of BDNF and NGF as key modulators in depression: role of antidepressants treatment. Int J Neurosci. 2019;129(3):283–296. doi: 10.1080/00207454.2018.1527328
- Iannitelli A, Tirassa P, Fiore M, et al. Gender differences in ultradian serum levels of NGF and BDNF correlate with psychophysical traits in healthy humans. Riv Psichiatr. 2021;56(6):314–320. doi: 10.1708/3713.37045
- Christian LM, Mitchell AM, Gillespie SL, et al. Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: associations with race, depressive symptoms, and low birth weight. Psychoneuroendocrinology. 2016;74:69–76. doi: 10.1016/j.psyneuen.2016.08.025
- Fazeli PL, Woods SP, Lambert CC, et al. Differential associations between BDNF and Memory Across Older black and white adults with HIV disease. J Acquir Immune Defic Syndr. 2022;89(2):129–135. doi: 10.1097/QAI.0000000000002831
- Weobong B, Akpalu B, Doku V, et al. The comparative validity of screening scales for postnatal common mental disorder in Kintampo, Ghana. J Affective Disorders. 2009;113(1):109–117. doi: 10.1016/j.jad.2008.05.009
- Makhubela M, Khumalo IP. Psychometric evaluation of the PHQ-9 in university students: factorial validity and measurement equivalence across three African countries. Current Psychol. 2022;42(21):18061–18069. doi: 10.1007/s12144-022-02997-0
- Cichoń E, Kiejna A, Kokoszka A, et al. People with diabetes need a lower cut-off than others for depression screening with PHQ-9. PLoS One. 2020;15(10):e0240209. doi: 10.1371/journal.pone.0240209
- Mitchell AJ, Yadegarfar M, Gill J, et al. Case finding and screening clinical utility of the patient health questionnaire (PHQ-9 and PHQ-2) for depression in primary care: a diagnostic meta-analysis of 40 studies. BJPsych Open. 2016;2(2):127–138. doi: 10.1192/bjpo.bp.115.001685
- Naegelin Y, Dingsdale H, Säuberli K, et al. Measuring and validating the levels of brain-derived neurotrophic factor in human serum. eNeuro. 2018;5(2):ENEURO.0419–17.2018. doi: 10.1523/ENEURO.0419-17.2018
- Li B, Lang N, Cheng Z-F. Serum levels of brain-derived neurotrophic factor are associated with diabetes risk, complications, and obesity: a cohort study from Chinese patients with type 2 diabetes. Mol Neurobiol. 2016;53(8):5492–5499. doi: 10.1007/s12035-015-9461-2
- He M, Wang J. Decreased serum brain-derived neurotrophic factor in Chinese patients with type 2 diabetes mellitus. Acta Biochim Biophys Sin. 2014;46(5):426–427. doi: 10.1093/abbs/gmu008
- Dias BG, Banerjee SB, Duman RS, et al. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45(4):553–563. doi: 10.1016/S0028-3908(03)00198-9
- Berton O, McClung CA, DiLeone RJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972
- Boyuk B, Degirmencioglu S, Atalay H, et al. Relationship between levels of brain-derived neurotrophic factor and metabolic parameters in patients with type 2 diabetes mellitus. J Diabetes Res. 2014;2014:1–6. doi: 10.1155/2014/978143
- Yamanaka M, Itakura Y, Tsuchida A, et al. Comparison of the antidiabetic effects of brain-derived neurotrophic factor and thiazolidinediones in obese diabetic mice. Diabetes Obesity Metab. 2007;9(6):879–888. doi: 10.1111/j.1463-1326.2006.00675.x
- Lee B-H, Kim Y-K. Reduced platelet BDNF level in patients with major depression. Prog Neuro Psychopharmacol Biol Psychiatry. 2009;33(5):849–853. doi: 10.1016/j.pnpbp.2009.04.002
- Lee B-H, Kim Y-K. BDNF mRNA expression of peripheral blood mononuclear cells was decreased in depressive patients who had or had not recently attempted suicide. J Affective Disorders. 2010;125(1):369–373. doi: 10.1016/j.jad.2010.01.074
- Molendijk ML, Spinhoven P, Polak M, et al. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry. 2014;19(7):791–800. doi: 10.1038/mp.2013.105
- Lee B-H, Kim Y-K. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investigation. 2010;7(4):231. doi: 10.4306/pi.2010.7.4.231
- Gonul AS, Akdeniz F, Taneli F, et al. Effect of treatment on serum brain–derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):381–386. doi: 10.1007/s00406-005-0578-6
- Karege F, Bondolfi G, Gervasoni N, et al. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biological Psychiatry. 2005;57(9):1068–1072. doi: 10.1016/j.biopsych.2005.01.008
- Karege F, Perret G, Bondolfi G, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/S0165-1781(02)00005-7
- Emon MPZ, Das R, Nishuty NL, et al. Reduced serum BDNF levels are associated with the increased risk for developing MDD: a case–control study with or without antidepressant therapy. BMC Res Notes. 2020;13(1):1–6. doi: 10.1186/s13104-020-04952-3
- Lee B-H, Park Y-M, Hwang J-A, et al. Variable alterations in plasma erythropoietin and brain-derived neurotrophic factor levels in patients with major depressive disorder with and without a history of suicide attempt. Prog Neuro Psychopharmacol Biol Psychiatry. 2021;110:110324. doi: 10.1016/j.pnpbp.2021.110324
- Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512
- Kackley ML, Buga A, Crabtree CD, et al. Influence of nutritional ketosis achieved through various methods on plasma concentrations of brain derived neurotropic factor. Brain Sci. 2022;12(9):1143. doi: 10.3390/brainsci12091143
- Golden E, Emiliano A, Maudsley S, et al. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: data from the Baltimore longitudinal study of aging. PLoS One. 2010;5(4):e10099. doi: 10.1371/journal.pone.0010099
- Kulaksizoglu S, Kulaksizoglu B. The relationship between metabolic syndrome, BDNF, and vitamin D in patients with schizophrenia. Neurochem J. 2017;11(1):104–111. doi: 10.1134/S1819712417010056
- Amoureux S, Lorgis L, Sicard P, et al. Vascular BDNF expression and oxidative stress during aging and the development of chronic hypertension. Fundam Clin Pharmacol. 2012;26(2):227–234. doi: 10.1111/j.1472-8206.2010.00912.x
- Smith AJ, Malan L, Uys AS, et al. Attenuated brain-derived neurotrophic factor and hypertrophic remodelling: the SABPA study. J Hum Hypertens. 2015;29(1):33–39. doi: 10.1038/jhh.2014.39
- Nakagomi A, Okada S, Yokoyama M, et al. Role of the central nervous system and adipose tissue BDNF/TrkB axes in metabolic regulation. NP J Aging Mech Dis. 2015;1(1):15009. doi: 10.1038/npjamd.2015.9
- Spagnuolo MS, Donizetti A, Iannotta L, et al. Brain-derived neurotrophic factor modulates cholesterol homeostasis and apolipoprotein E synthesis in human cell models of astrocytes and neurons. J Cell Physiol. 2018;233(9):6925–6943. doi: 10.1002/jcp.26480
- Fujimura H, Altar CA, Chen R, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost. 2002;87(4):728–734. doi: 10.1055/s-0037-1613072
- Michalski B, Fahnestock M. Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Mol Brain Res. 2003;111(1):148–154. doi: 10.1016/S0169-328X(03)00003-2