ABSTRACT
Background
Obesity causes changes in the production of adipose tissue secreted factors, which are responsible for metabolism modulation. One of these factors is Apelin, a small peptide that interacts with a specific cell-surface receptor to modulate various cells signaling processes. Apelin is regarded as a new player because of its powerful role in energy metabolism and improvement of insulin sensitivity.
Aim of the Work
This study investigates the plasma apelin 36 role and to what extent it can impact the insulin resistance in obese Egyptians diagnosed with type 2 diabetes (T2DM).
Subjects and Methods
The study involved 90 participants, with 30 assigned to each of the three study involved categories: obese individuals with diabetes, obese individuals without diabetes, and healthy controls (n = 30 for each group). Measurements of skin fold thickness, body mass index (BMI), waist/hip ratio, and body fat % were obtained along with a comprehensive clinical examination and history. The following were investigated: plasma insulin, Hb A1c, lipid profile, plasma apelin (ELISA), plasma leptin (ELISA), and plasma adiponectin (ELISA).
Results
The median value of serum leptin in obese subjects (groups I and II) was higher significantly than healthy control group (group III) (p = <0.001). A significant decrease in serum adiponectin could be detected in obese subjects (groups I and II) compared to control subjects (Group III) (p = 0.004). When compared to the healthy control group (group III), the mean serum apelin level was significantly higher in the obese participants (groups I and II) and much higher among obese diabetic group (p = <0.001). A statistically significant correlation (p = 0.001) was observed between fasting serum insulin and both HOMA-IR and serum apelin among the non-diabetic obese participants involved in the study.
Conclusions
Obese people have raised serum apelin levels; those with T2DM have significantly greater serum apelin levels. While it showed a positive correlation with insulin level and HOMA-IR among the obese non-diabetic individuals.
KEYWORDS:
1. Introduction
The rise in noncommunicable diseases has been related to changes in food and physical activity habits, as well as migration to urban areas and increased economic prosperity. Globally, diabetes is growing more and more common [Citation1]. In 2010, the worldwide incidence of diabetes among adults (aged 20 to 79) was 6.4%, affecting 285 million people and it is expected to rise to 7.7% and 439 million people by 2030. Between 2010 and 2030, the number of adults with diabetes will grow by 69% in developing countries and 20% in developed ones. In 2011, there were 365 million individuals with diabetes, which is expected to grow to 552 million by 2030 [Citation2]. Diabetes mellitus (DM) is a growing clinical and public health concern in Egypt. Numerous studies were conducted to find out how common diabetes risk factors, diagnosed diabetes, and previously undiagnosed diabetes were among the Egyptian community. In addition to the US Agency for International Development, the Egyptian Ministry of Health and Population also carried out these studies. According to these estimates, by 2025 there will be 8.80 million people in Egypt who have both diagnosed and undiagnosed diabetes. There will be a 3.6-fold rise in the number of individuals with diabetes who are 65 years of age or older between 1995 and 2025, from around 515,000 to 1.87 million. Diabetes will affect around 2.28 million urban dwellers, increasing by 3.2 times to 7.21 million. By 2025, diabetes will affect 13.3% of the population aged 20 and older. The elderly will account for 21% of the overall population with diabetes, while urban inhabitants will account for 82% [Citation3]. T2DM is marked by a both peripheral insulin resistance and inadequate pancreatic beta cells insulin secretion [Citation4,Citation5]. The broad definition of insulin resistance is a reduction in the tissue’s sensitivity to insulin. Clinically, it can be evaluated directly by assessing the effectiveness of a set dosage of insulin to enhance total body glucose elimination, or indirectly by monitoring fasting insulin levels. A rise in insulin levels in the presence of normal plasma glucose reveals insulin resistance [Citation6]. Adipose tissue can affect the insulin sensitivity of other tissues by producing signaling molecules called adipokines, which can either increase (adiponectin) or inhibit (TNF-α, IL-6, leptin, resistin, and others) insulin signaling locally or in distant target tissue [Citation7,Citation8]. Other compounds with either local or systemic effects are also secreted by the adipose tissue. These substances include cytokines like TNF-α, which are involved in insulin resistance and fat metabolism, and IL-6, which stimulates C-reactive protein [Citation9]. Total leptin levels in circulation are positively correlated with body mass index and adiposity extent; in obese people, leptin is free to circulate, whereas in lean people, it is bound to plasma proteins. Leptin plays several different physiological roles, one of which is that of an anti-obesity hormone. Along with its involvement in the metabolism of lipids and glucose, leptin also has a role in reproduction, controlling the proliferation and apoptosis of human ovarian cells. It raises sympathetic nerve activity in the kidney, skeletal muscle, adrenal gland, and brown adipose tissue. It plays a part in the development of cardiovascular, renal, and hypertensive disorders attributed to obesity [Citation10]. A further significant adipocytokine that has been demonstrated to play a protective effect in the pathophysiology of diseases associated to obesity is adiponectin. Peroxisome proliferator activated receptor (PPAR)-γ, a crucial transcriptional factor involved in adipocyte differentiation, is activated in response to an increase in adiponectin expression, which is then released from adipocytes. It also significantly improves insulin resistance and lowers dyslipidemia, which have additional advantages. Apart from its anti-atherogenic properties, adiponectin has a wide variety of anti-tumor effects [Citation11]. Adipocytokine resistin was initially discovered in 2001 as a gene target of thiazolidinediones, an insulin-sensitizing medication. Resistin has been associated with atherosclerosis, inflammation, obesity, and T2DM.
In 1998, researchers identified apelin, an endogenous ligand for the G-protein-coupled APJ receptor. Apelin is derived from the C-terminal of a pre-proprotein of 77 amino acids transcribed by a gene on the chromosome X q25–26.1 band. The apelin receptor (APJ) is a class A G-protein coupled receptor that has recently been matched with its endogenous ligand apelin. It is abundantly expressed in the brain and nearly all peripheral organs. Through contact with their unique cell-surface receptor, a member of the seven transmembrane G protein coupled receptor superfamily, apelin regulates a variety of cell signaling events [Citation12]. The distribution of apelin determines the physiologic functioning of the APJ system [Citation13]. Recently, apelin has been classified as an endocrine adipokine due to its production in adipocytes and its variation with insulin levels and obesity. Apelin is secreted in human adipocytes with a considerable rise in apelin levels in both plasma and adipocytes in humans with hyperinsulinemia, demonstrating that hyperinsulinemia, rather than obesity or high-calorie diet are the determining factors in elevated plasma apelin levels; while in those with low insulin levels there is reduced secretion of apelin. Like insulin, apelin expression is strongly inhibited by fasting and returns rapidly to normal after eating. These findings imply that apelin synthesis in adipocytes is directly regulated by insulin, pointing to a possible association between apelin secretion, insulin sensitivity, and obesity [Citation14]. Plasma and adipocytes apelin levels elevation was evident in obese mice with hyperinsulinaemia [Citation15].
2. Subjects and methods
In this study, 90 individuals were split up into three groups, which were as follows: Group 1 consisted of 30 obese with T2DM; Group 2 included 30 obese non-diabetic patients; and Group 3 consisted of 30 healthy individuals with normal body weight. Exclusion criteria were diabetic patients under insulin therapy, hypertension, heart failure, renal failure and chronic liver disease. Allsubjects and controls were subjected to complete history taking and thorough clinical examination. Waist/hip ratio was estimated by dividing the waist by the hip measurements, skin fold thickness was determined using a calliper, and BMI was obtained by dividing weight in kilograms by square height in meters. The WHO defines ratios of > 0.9 in men and > 0.85 in women as benchmarks for metabolic syndrome.Body fat mass percent (By bioelectric impedance) was measured using the device in Body (720). Investigations done were fasting plasma glucose, hemoglobin A1c, lipid profile(serum triglyceride, total cholesterol, LDL, HDL), plasma apelin (ELISA), plasma leptin (ELISA), plasma adiponectin (ELISA), Fasting plasma insulin (ELISA) and HOMA IR involved by multiplying fasting plasma insulin (FPI) by fasting plasma glucose (FPG), and subsequently dividing the resulting value by the constant 22.5.
3. Results
The level of fasting serum leptin in obese diabetic patients (group I) ranged from 6 to 100 ng/ml with a mean value (37.67 ± 27.93 ng/ml), in group II (obese non diabetic) it ranged from 10 to 100 ng/ml with a mean value (41.6 ± 26.3 ng/ml), while in healthy control group (group III), it ranged from 2 to 25 ng/ml with a mean value (10.02 ± 7.53 ng/ml).The median value of serum leptin in obese subjects (groups I and II) was higher significantly than among the healthy involved control group (group III). The fasting serum adiponectin level ranged from 2.5 to 30 ng/ml with a mean value (15.43 ± 7.54 ng/ml) in obese diabetic individuals (group I) and from 5.2 to 35 ng/ml with a mean value (15.02 ± 6.94 ng/ml) in obese non diabetic subjects (group II), while in control group (group III) the range was from 12 to 65 ng/ml with a mean value of (20.2 ± 9.47 ng/ml). A significant difference could be detected between obese subjects (groups I and II) in relation to control subjects (Group III). The fasting serum apelin level in obese diabetic patients (group I) ranged from 0.6 to 1.6 ng/ml with a mean value (1 ± 0.28 ng/ml). In group II (obese non diabetic), it ranged from 0.5 to 1 ng/ml with a mean value (0.75 ± 0.15 ng/ml), while in healthy control group (group III) it ranged from 0.4 to 0.9 ng/ml with a mean value (0.6 ± 0.18 ng/ml). Compared to the healthy control group (group III), the mean serum apelin level was higher significantly in the obese participants (groups I and II) and much higher particularly among the obese diabetes group. .
Table 1. Comparison between studied groups according to the different adipokines.
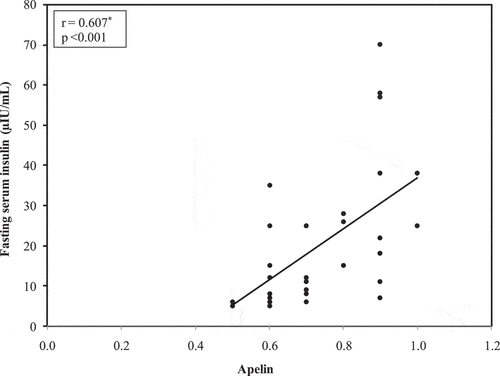
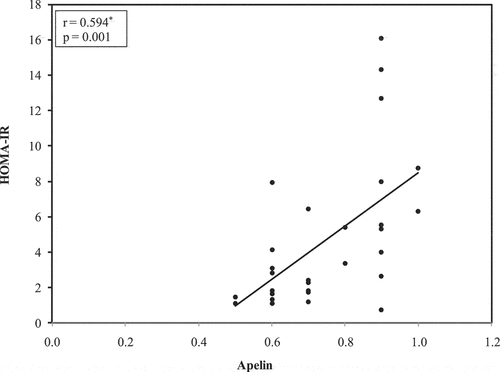
In the obese diabetic participants investigated (n = 30), there was a significant direct association statistically between both serum leptin and serum adiponectin (r = 0.537 and p = 0.002).In obese non-diabetic patients (n = 30), there was a significant direct statistical association between serum apelin and waist circumference (r = 0.382 and p = 0.037). In the same group, there was a direct significant correlation statistically between serum apelin and fasting serum insulin (r = 0.607 and p < 0.001) statistically direct significant correlation between serum apelin and HOMA-IR (r = 0.594 and p = 0.001) . Furthermore there was an inverse correlation with statistical significance between both serum apelin and adiponectin (r = 0.411 and p < 0.024), and there was a significant direct correlation statistically between serum involved leptin and adiponectin (r = 0.451 and p < 0.012).
4. Discussion
The current investigation found that leptin levels were considerably greater in both obese groups than in the control group. In our study, we observed that leptin levels were greater in (non-diabetic) group II than in (diabetic) group I. This was due to the fact that (non-diabetic) group II included more females and a higher BMI. In addition, both obtained leptin and BMI measurements were found positively correlated in group II. In agreement with our study, Mohiti et al. showed in his study that plasma leptin in obese diabetics increased more than four folds with body mass index [Citation16]. Passaro et al. in her study found no significant difference statistically among the two involved obese groups diabetic and non diabetic as regards serum leptin level and leptin level was significantly higher in females [Citation17]. Moller et al. as in our results reported that serum leptin level was higher in females too [Citation18]. In contrast to our study Williams et al. found that leptin level was unaffected by gender [Citation19]. In our study, we found that serum leptin and BMI and body fat percentage (measured by bioelectrical impedance) are positively correlated. In group II obese non-diabetic subjects in concordance with this result Masoud et al. found that serum leptin levels were greater in Omani obese group and positively correlated with body fat and obesity. This study was done on 35 obese Omani subjects [Citation20]. In our investigation, we discovered that, as compared to the control group, the blood adiponectin levels were considerably lower in both obese groups. Although adiponectin is only released by adipocytes, Arita et al.“s findings are consistent with ours in that they discovered that adiponectin was widely prevalent in healthy volunteers and that obese participants” plasma concentrations of the protein were much lower than those of non-obese people [Citation21]. Additionally, Halleux et al. observed that people with insulin resistance who are obese and have metabolic syndrome have lower levels of adiponectin [Citation22]. Serum apelin 36 levels were observed to be significantly higher in both obese groups in the current investigation compared to the healthy control group. In line with our research, Yu S et al. discovered that apelin levels were considerably greater in his study of 81 newly diagnosed obese type 2 diabetes participants than in the normal control group [Citation23]. Another study agreed with our work, Heinonen et al. [Citation24] The study conducted by Maria Gisella diabetes patients also discovered that there was no significant link between serum apelin and HOMA-IR in the obese diabetic group, and that the rise in apelin was favorably associated with insulin level [Citation25]. According to our findings, apelin and serum insulin as well as apelin and HOMA-IR were significantly positively correlated in the group of obese non-diabetics.
In contrast to our study, Yu Zhang et al. found that plasma apelin 17 were lower in diabetic group compared with control subjects [Citation26]. Explanations of the difference in the studies that found contradictory results with ours are using different types of apelin, the current study we used apelin 36 others used apelin 12 and apelin 17, also our diabetic patients were on medical treatments. Other studies were done on newly diagnosed diabetic patients. The apelin increase may be due to a so called apelin-resistance, driven by yet unknown mechanisms determining this effect [Citation26,Citation27].
5. Conclusions
Both categories of obese people had considerably higher leptin levels. In the obese non-diabetic group, leptin and BMI and percent body fat (as determined by BIA) showed positive correlations. Compared to the control group, serum adiponectin levels were considerably lower in both obese groups. Comparing both obesity groups to the healthy control group, serum apelin levels were shown to be considerably higher in both, but in the obese type 2 diabetes group, they were greater than among the obese non-diabetic group. In obese diabetic participants, there was no significant association found between the level of serum apelin and HOMA-IR; however, in obese non-diabetic subjects, there was a significant positive relationship found between serum apelin and serum insulin as well as between serum apelin and HOMA-IR.
Abbreviations
| T2DM | = | TYPE 2 Diabetes mellitus, |
| (HOMA-IR) | = | Homeostasis Model Assessment of insulin resistance index, |
| (ELISA) | = | Enzyme- linked Immunosorbent assay |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Ahmed Kamal Swidan
Ahmed Kamal Swidan is a Consultant Endocrinology and Diabetes at Dar AlShifa Hospital, Kuwait. I am an Associate Professor in Medicine and Endocrinology at Alexandria University since 2015. I am a Fellow of the Royal College of Edinburgh since 2012 and an International Examiner of the Clinical exam of MRCP (UK) (PACES) since 2016. I graduated from Alexandria University and trained in Alexandria University Hospital and then continued my training in Medicine and then Endocrinology and Diabetes in the UK. I am an active member of AACE and European Society of Endocrinology. Publications include abstracts and papers in diabetes, thyroid and andrology, in high rank Journals (Q1). I have been involved in quality and patient care as part of accreditation of the hospital. I have involved in establishing clinical guidelines and pathways. I have been web-streaming live conferences to Kuwait from the Royal College of Edinburgh. Special interests are in thyroid disorders, diabetes and pituitary disorders.
Aliaa AlAghouri
Aliaa Alaghouri Professor of Internal medicine and Endocrinology. Previous Head of The Endocrine Unit in Alexandria University. Supervised MD and MSc theses for more than 20 candidates . Special interest in thyroid diseases. Founder and President of the Thyroid forum which is run twice per year with National and International Speakers. Published over 20 articles and papers with focus on diabetes and endocrinology.
Mohammad Kamal Ghitany
Mohammad Kamal Ghitany Faculty Of Medicine, Alexandria University, Honor degree, MS-internal Medicine (1987), Alexandria University, Clinical presentations and incidence of complications in schistosmal hepatic fibrosis in the medical words. Study of the hypothalamo-pituitary gonadal axis integrity in elderly males. All pituitary, thyroid, parathyroid, adrenal disorders & disorders of puberty.
Akram Abd-Elmoneim Deghady
Akram Abd-Elmoneim Deghady Professor of Clinical Pathology, Previous head of the Clinical Pathology department. Developed and expanded the department to cope with the increased demand for the University Hospitals. Supervised more than 10 MD and MSc theses in Alexandria University. Has more than 25 publications in national and International journals . Special interest in diabetes, insulin resistance and stem cell therapy.
Mona Ziada Ibrahim Ahmed
Mona Ziada Ibrahim Ahmed graduated from Alexandria University Faculty of Medicine. Completed residency in Alexandria University Hospital , Medical department . Then I completed my MD thesis in Endocrinology Unit in Alexandria Faculty of Medicine. I joined the National Health Service in Gamal AbdelNaser Hospital and worked as a consultant in Medical department with special interest in diabetes and diabetic foot disease. I have special interest in diabetes and endocrinology. MD thesis was in diabetes and adipokines.
References
- Cheema A, Adeloye D, Sridhar D, et al. Urbanization and prevalence of type 2 diabetes in southern Asia: a systemic analysis. J Global Health. 2014 jun;4(1):016404.
- International Diabetes Federation. One adult in ten will have diabetes by 2021. Diabetes atlas. 10 th ed.
- Bos M, Chareles A. Prevalence and complications of diabetes mellitus in north Africa; a systemic review. BMC Public Health. 2013;13(1):387. doi: 10.1186/1471-2458-13-387
- Unger RH, Orci L. Of islets and paracrinopathy of diabetes. Proc Nah Academy Sci USA. 2010 Sep 14;107(37):16009–16012.
- Basha F, Lee S, Gungor N, et al. From prediabetes to type 2 diabetes in obese youth: diabetes care. Diabetes Care. 2010 Oct;33(10):2225–2231. doi: 10.2337/dc10-0004
- Lee SH, Park SA, Yim HW, et al. Insulin resistance and inflammation. Metbolism. 2010 Feb;59(2):241–246.
- Brabant G, Muller G, Horn R, et al. Hepatic leptin signaling in obesity. FASEB J. 2005 Jan;19(8):1048–1050.
- Fuke Y, Fujita T, Satoomura A, et al. Alterations of insulin resistance and serum adiponectin. Diabetes Technol Ther. 2010 May;12(5):393–398. doi: 10.1089/dia.2009.0126
- Florez H, Castillo-Florez S, Mendez A, et al. C reactive protein is elevated in obese patients with metabolic syndrome. Diabet Res Clin Pract. 2006 Jan;71(1):92–100.
- Meyers JA, Liu AY. Serum leptin concentrations and markers of immune function in overwight. J Endocrinol. 2008;199(1):51–60. doi: 10.1677/JOE-07-0569
- Matsuzawa Y. Adiponectin: identification, physiology and clinical relevance. Atherosclerosis Suppl. 2005;6(2):7–14. doi: 10.1016/j.atherosclerosissup.2005.02.003
- Kunduzora O, Alet N, Delesque T, et al. Apelin/APJ signaling system: a potential link between adipose tissue and endothelial angiogenic processes. FASEB J. 2008;22(12):4146–4153. doi: 10.1096/fj.07-104018
- Kleinz MJ, Davenport A. Emerging roles of apelinin biology and medicine. Pharmacol Ther. 2005;107(2):198–211. doi: 10.1016/j.pharmthera.2005.04.001
- Soriguer F, Garrido L, Garcia-Serrano, S et al. Apelin levels are increased in morbidly obese subjects with T2DM. Obese Sur. 2005 Nov;19(11):1574–1580.
- Dray C, Knauf C, Davioud D, et al. Apelin stimulates glucose utilization in normal and obese insulin resistant mice. Cell Metab. 2008;8(5):437–454. doi: 10.1016/j.cmet.2008.10.003
- Mohiti J, Doudi Q. Relation between leptin and insulin in patients with type 2 DM. Turk J Endocrinol Metab. 2005;2:55–58.
- Passaro A, Calzani F, Pier F, et al. Eur J Endocrinol. 2001;45:173–179. doi: 10.1530/eje.0.1450173
- Moller M, Peter O, Nair, KS et al. Disruption of the relationship between fat content and leptin levels with aging in humans. J Clin Endocrinol Metab. 1998;83(3):931–934. doi: 10.1210/jc.83.3.931
- Williams I, Sivitiz S, Wayson M. Leptin and body fat in type 2 diabetes and monodrug therapy. J Clin Endocrinol Metab. 2003;88(4):1543–1553. doi: 10.1210/jc.2002-021193
- Masoud Y, Adel A. Correlation between serum leptin levels BMI and obesity in omanis. Med J. 2006 Dec;6:27–31.
- Yukio A, Shinji K, Masahiko T, et al. Adiponectin in obesity. Bioch & Biophy Reas. 1999 April;127:79–83.
- Halleux CM, Takashi M, Detry MR, et al. Secretion of Adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem Biophys Res Commun. 2001;11(5):1102–1107. doi: 10.1006/bbrc.2001.5904
- Yu S, Zhang Y, Li R, et al. Chemerin and apelin are positively correlated with inflammation in obese type2DM. Clin Med J (Engl). 2012;125(19):40–44.
- Heinonen MV, Purhonin AK, Miettinen, P, et al. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005 Aug;130(1–2):7–13.
- Gisella M, Federico S, Slaric B. Altered glucose homeostasis is associated with increased serum apelin in T2DM. First Author Cavallo GM. 2012;7(12):e51236.
- Attane C, Daviaud D, Dray C, et al. Apelin stimulate glucose uptake. J Mol Endocrinol. 2011;26:21–28.
- Attane C, Foussal C, Le Gonidec S, et al. Apelin treatment increases fatty acid oxidation. Diabetes. 2012;61(2):310–320. doi: 10.2337/db11-0100