ABSTRACT
Introduction
Kidney damage in COVID-19 is common and may lead to CKD. Early recognition of progression is mandatory, so there is a need for sensitive markers of kidney damage. Renal functional reserve (RFR) is described as the ability of the kidneys to augment their function in special situations.
Aim of Work
Was to assess subclinical renal dysfunction post-COVID-19.
Methods
This cross-sectional study investigates 31 patients previously infected with COVID-19 within 2–3 months and 31 healthy subjects with matched age and sex. Assessment of RFR was done by calculating the estimated GFR using the CKD-EPI equation before and 2 hours after 80 g protein load by intravenous infusion.
Results
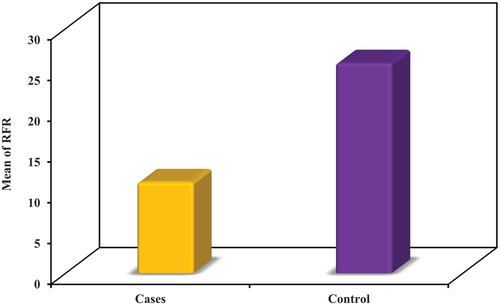
RFR was significantly lower in post-COVID-19 patients (mean of 11.19 ± 4.76) than the control (mean of 25.81 ± 4.42, p < 0.001).
Conclusion
Despite preserved eGFR, there is a reduction in RFR, indicating progressive loss of nephron mass post-COVID.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a rapidly spread infection that was first discovered in December 2019 in China by unusual cases of pneumonia and was complicated by acute respiratory distress syndrome (ARDS). In January 2020, the World Health Organization (WHO) reported that the infection resulted from a new strain of coronavirus named severe acute respiratory syndrome-coronavirus-2 (SARS-COV-2) [Citation1]. WHO stated that it is a pandemic disease and has rapid spread and transmission from person to person, which is widely recognized. There are several medical, psychological, and socioeconomic implications associated with the COVID-19 pandemic. The global burden of COVID-19 is very high [Citation2,Citation3].
Renal dysfunction in patients with COVID-19 is present and not uncommon. It can be considered a COVID-19 complication during disease progression that can affect global morbidity and mortality rates.Acute kidney injury (AKI) is the most prevalent type, but also chronic persistent injury is also present, resulting in chronic kidney disease (CKD) [Citation4]. According to a UK study, within 90 days, 16% of AKI individuals suffering from the COVID-19 infection progressed to chronic kidney disease (CKD). Moreover, after three months of COVID-19 infection, 20% of AKI patients who needed renal replacement therapy did not recover. According to a Chinese cohort study, CKD progression occurred in one-third of COVID-19-infected patients six months after hospitalization [Citation5,Citation6].
The long-term effect of COVID-19 on kidneys is still not well understood. Early recognition of renal affection is of high importance because it can prevent or delay CKD progression. Although serum creatinine and estimated glomerular filtration rate (eGFR) may get back to their usual levels after AKI recovery, the kidneys are not completely recovered. Some studies showed that there are persisting inflammation, renal fibrosis, and functional deficits after kidney injury. Also, acute COVID-19 infection can result in diabetes mellitus or cardiovascular disease that may affect the kidney in a long-term manner [Citation7,Citation8]. A US study that conducted an extensive evaluation of prolonged COVID-19 using medical records discovered that COVID-19 raised the possibility of CKD and that people with serious medical conditions were more likely to be at that vulnerability [Citation9].
Renal functional reserve (RFR) is “the kidney’s capacity to raise its function in reaction to either normal or abnormal circumstances.” RFR can be clinically evaluated following the ingestion of a protein meal via oral or intravenous amino acid infusion once the resting GFR has been established. The difference between the resting and stress GFR is identified as RFR [Citation10,Citation11]. Normally, elevated resting GFR is seen in single kidneys or individuals with hypertension, diabetes, or pregnancy. RFR can serve as a dependent and early method of evaluating the kidney’s impairment in function. The normal range of RFR is variable and differs according to age and sex. It begins to decline with progressive nephron damage and CKD progression. A study stated that the healthy control group had an average kidney reserve of 23.4%, whereas the CKD stages were 19% in stage 1, 15.4% in stage 2, 8.9% in stage 3, and 6.7% in stage 4.A decline in RFR with an apparent normal basal GFR may be considered an early sign of subclinical renal mass loss and predict CKD progression [Citation12,Citation13].
2. Aim of work
Our work aims to assess subclinical renal dysfunction in patients previously infected with COVID-19 infection using measurement of RFR.
3. Subjects
This study was carried out on 62 patients.The first 31 patients had a previous infection with COVID-19 within 2–3 months, of various severities, who attended the outpatient clinic for follow-up after treatment of COVID-19 from November 2020 to April 2021, and 31 persons were randomly selected as healthy controls with the same sex and age group for comparison of RFR.All subjects didn’t receive the COVID-19 vaccine until the start of the study.
The diagnosis of COVID-19 infection depended on:
The clinical symptoms and signs of patients suggesting infection with COVID-19.
Laboratory investigations suggest an infection with COVID-19. These investigations include a complete blood count (CBC) with absolute lymphopenia, increased serum C-reactive protein (CRP), increased serum ferritin, and increased D-dimer.
A computed tomography scan (CT) of the chest raises the probability of COVID-19 infection.
Polymerase chain reaction (PCR) positive result of COVID-19 from a nasopharyngeal or oropharyngeal swab.
The first three criteria together, or PCR positive, are used as evidence of COVID-19 infection.
3.1. Inclusion criteria
Young adult age group from 20 to 40 years.
Male sex.
Individuals with past COVID-19 disease within 3 months from the beginning of the diagnosis to the start of the research.
Individuals with past COVID-19 disease, regardless of the course of the disease.
3.2. Exclusion criteria
Preexisting chronic kidney disease.
Abnormal renal function and/or abnormal GFR.
Abnormal urinary abnormality in a complete urine analysis.
Patients with solitary kidneys or kidney donors.
Patients with a history of renal transplants.
Hypertension.
Diabetes.
All patients were asked for informed written consent.
4. Methods
Each and every patient endured the following:
1. A detailed patient history of the disease with special stress on:
• Time of infection with COVID-19, symptoms of infection, course of the infection, need for hospitalization, use of respiratory assistance, drugs used in management, and complications of infection.
2. Laboratory assessment includes:
Serum urea, serum creatinine [Citation14].
Complete urine analysis, evaluation of urinary protein\Creatinine ratio [Citation14].
One recently developed equation for estimating GFR is the CKD-EPI equation. Particularly in cases where GFR is elevated, it has been suggested to be more accurate than the Modification of Diet in Renal Disease-6 (MDRD-6) formula. Additionally, it demonstrates increased accuracy and fewer biases [Citation15].
4.1. Statistical analysis of the data
The computer received the data, and IBM SPSS software package version 20.0 was used for analysis. (IBM Corp., Armonk, NY) Numbers and percentages were used to describe the qualitative data. The Smirnov test was employed to confirm the distribution’s normality. The range (lowest and highest), mean, standard deviation, median, and interquartile range (IQR) were used to characterize quantitative information. To compare among more than two periods or phases, use an ANOVA with multiple tests for quantitative variables that are normally distributed. Student t-test for quantitative variables with a normal distribution, for comparison between the two groups under study. Paired t-test for quantitative factors with a normal distribution to compare two time frames. Mann-Whitney test for quantitative parameters with abnormal distributions in order to compare the two groups under study.Wilcoxon signed a rank test for quantitative factors with abnormal distributions to compare two time frames. The results were deemed significant at the 5% level.
5. Results
This study included 62 individuals. They were subdivided into two groups: Group I included 31 individuals who had a previous infection with COVID-19 within 2–3 months, of various severities, and who attended the outpatient clinic for follow-up after treatment with COVID-19; and Group II included 31 healthy control persons.
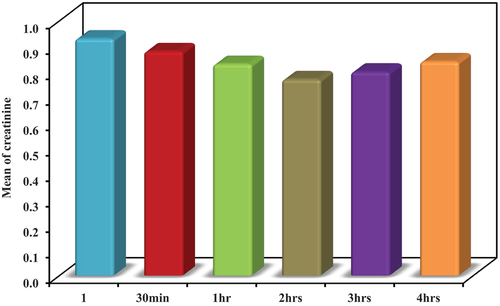
At the beginning of the study, a pilot study was done on 10 controlsinGroup II. Serum creatinine was measured prior to, 30 minutes, 1 hour, 2 hours, 3 hours, and 4 hours following the intravenous infusion of 80 g of protein.
Before infusion, serum creatinine ranged from 0.71 to 1.0 mg/dL, with a mean of 0.93 ± 0.09 mg/dL.
After 30 minutes, it ranged from 0.69 to 0.98 mg/dL, with a mean of 0.88 ± 0.09 mg/dL.
After 1 hour, it ranged from 0.64 to 0.95 mg/dL, with a mean of 0.83 ± 0.10 mg/dL.
After 2 hours, it ranged from 0.58 to 0.89 mg/dL, with a mean of 0.77 ± 0.10 mg/dL.
After 3 hours, it ranged from 0.60 to 0.91 mg/dL, with a mean of 0.80 ± 0.10 mg/dL.
After 4 hours, it ranged from 0.64 to 0.94 mg/dL, with a mean of 0.84 ± 0.09 mg/dL.
The best response of serum creatinine after protein infusion was after 2 hours. So, it was used as the marker for the assessment of RFR in the whole study. ()
Table 1. Comparison between the different studied periods according to S.Creatinine (n = 10).
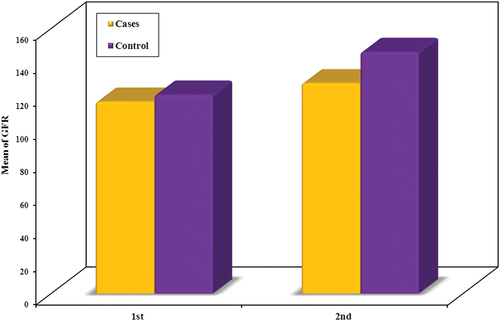
Before infusion, Group I had an estimated GFR range of 91.0 to 119.0 with a mean of 111.5 ± 7.16.Group II’s estimated GFR ranged from 103.0 to 119.0 with a mean of 114.5 ± 4.0.Between groups, there was no statistically important difference with regard to estimated GFR (U = 384.50, p = 0.174).
After protein infusion, Group I’s estimated GFR ranged from 112.0 to 145.0 with a mean of 126.58 ± 8.70.Group II had estimated GFR ranged from 140.0 to 150.0 with a mean of 145.9 ± 3.28.
There was a statistically significant difference between groups as regards the estimated GFR (U = 22.0, p < 0.001). ()
Table 2. Comparison between the two studied groups according to GFR.
Group I had an RFR range of 5.0 to 25.0 with a mean of 11.19 ± 4.76 and an RRI range of 0.52 to 0.91 with a mean of 0.74 ± 0.11.
Group II had an RFR range of 21.0 to 37.0 with a mean of 25.81 ± 4.42 and an RRI range of 0.41 to 0.79 with a mean of 0.58 ± 0.11.
The groups’ differences were of statistical importance with regard to RFR and RRI (U = 26.0, p < 0.001, t = 6.058, p < 0.001, respectively) ().
Table 3. Comparison between the two studied groups according to RFR.
6. Discussion
COVID-19 is a rapidly spreading infection of renal affection caused by various mechanisms. The most accepted one is the virus’s direct entry into the kidney by the viral spike protein, causing direct tubular injury. Other mechanisms are ischemic injury to the kidney, inflammatory injury due to circulating cytokines during infections, and drug-induced nephrotoxicity through the treatment course of the disease [Citation5,Citation8].
This research was done on 62 individuals. They were split up into two divisions: Group I included 31 people who had a previous infection with COVID-19 within 2–3 months of various severities and attended the outpatient clinic for follow-up after treatment of COVID-19, and Group II included 31 healthy control persons for the same age group and sex for comparison of RFR.
At first, we reviewed the study of Schmidt-Lauber C. et al. that was carried out on patients with an average of 9 monthspost-mild and moderate COVID-19 infection using eGFR to assess chronic post-COVID-19 renal dysfunction. This study concluded that there were no significant ongoing or progressive chronic kidney injuries after non-severe COVID-19. From this point, we started our study using RFR, aiming to detect subclinical renal dysfunction using RFR despite apparent normal GFR and so predict nephron loss [Citation16].
In the beginning, we started bya pilot study and the best response of lowering serum creatinine level 0.4–0.8 mg/dL after protein infusion with 1 gm\kg of amino acids was after 2 hours (). So, it was used as the marker for the assessment of RFR in the whole study (). It matches the study of Sharma A. et al., who found the highest GFR level is after 2 hours of protein loading. The difference is that Sharma et al. discussed RFR after stress by protein oral load. Here, we used protein load by I.V. infusion [Citation11].
After analysis of our results, between the groups, there was a statistically important distinction in terms of GFR (U = 22.0, p < 0.001) () (). This matches the study of Bilo H. et al., which showed that there was an increase in GFR after L-arginine ingestion of an average of 34 ml/min [Citation17]. Damianaki A. et al.made a study on RFR with the use of inulin clearance and contrast-induced ultrasound. It showed no significant improvement in GFR after ingestion of protein 1–1.2 g\kg. Our study used creatinine clearance for the assessment of GFR using the CKD-EPI equation.The CKD-EPI equation, according to the study of Andrew S. has lower prevalence estimates for CKD, more precise risk prediction for unfavorable outcomes, fewer false-positive CKD evaluations, and better GFR estimation than other equations [Citation18].
In our research, regarding RFR, there was a statistically significant variance between the groups. (U = 26.0, p < 0.001) () (). We assessed RFR in patients after 2–3 months of COVID-19 infection. According to the study of Cantaluppi V. et al., assessment of RFR in acute infection with COVID-19 is inappropriate and should be assessed under steady-state conditions for metabolic and hemodynamic stability [Citation12]. Many studies, such as Legrand M. et al.,discussed the impact of COVID-19 infection onAKI in hospitalized patients and its clinical features according to the pathology in the renal structure. We aimed to predict subclinical renal dysfunction in post-infection patients with apparently normal basal GFR by detecting a decreasing level of RFR for predicting worse outcome and CKD progression [Citation19].
6.1. Limitations of the study
Small sample size of subjects and selection bias of patients as the first 31 attended patients were included in the study.
7. Conclusion
We concluded that despite preserved eGFR, there is a reduction in RFR, indicating progressive loss of nephron mass post-COVID. Additional research is required to ascertain the extent of renal affection following COVID-19.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20905068.2024.2333595
References
- Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471
- Penninx B, Benros M, Klein R, et al. How COVID-19 shaped mental health: from infection to pandemic effects. Nat Med. 2022;28(10):2027–2037. doi: 10.1038/s41591-022-02028-2
- Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397
- Geetha D, Kronbichler A, Rutter M, et al. Impact of the COVID-19 pandemic on the kidney community: lessons learned and future directions. Nat Rev Nephrol. 2022;18:724–737. doi: 10.1038/s41581-022-00618-4
- Jdiaa SS, Mansour R, El Alayli A, et al. COVID-19 and chronic kidney disease: an updated overview of reviews. J Nephrol. 2022;35(1):69–85. doi: 10.1007/s40620-021-01206-8
- Yende S, Parikh CR. Long COVID and kidney disease. Nat Rev Nephrol. 2021;17:792–793. doi: 10.1038/s41581-021-00487-3
- Divyaveer S, Jha V. COVID-19 and care for patients with chronic kidney disease: challenges and lessons. FASEB Bioadv. 2021;3(8):569–576. doi: 10.1096/fba.2021-00002
- Martin de Francisco Á, FernándezFresnedo G. Enfermedad renal en la COVID-19 persistente: unobjetivoinmediatoparaNefrología [long COVID-19 renal disease: a present medical need for nephrology]. Nefrologia. 2023;43(1):1–5. doi: 10.1016/j.nefro.2022.04.004
- Fuhrman DY. The role of renal functional reserve in predicting acute kidney injury. Crit Care Clin. 2021;37(2):399–407. doi: 10.1016/j.ccc.2020.11.008
- Sharma A, Mucino MJ, Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract. 2014;127(1–4):94–100. doi: 10.1159/000363721
- Cantaluppi V, Guglielmetti G, Dellepiane S, et al. A call to action to evaluate renal functional reserve in patients with COVID-19. Am J Physiol Renal Physiol. 2020;319(5):792–795. doi: 10.1152/ajprenal.00245.2020
- Barai S, Gambhir S, Prasad N, et al. Functional renal reserve capacity in different stages of chronic kidney disease. Nephrology (Carlton). 2010;15(3):350–353. doi: 10.1111/j.1440-1797.2010.01291.x
- Lamb EJ, Price CP. Kidney function tests. In: Burtis C, Ashwood E Bruns D, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 5th ed. New York: Elservier Saunders; 2012. p. 669–708.
- Levey AS, Stevens LA. Estimating GFR using the CKD epidemiology collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337
- Schmidt-Lauber C, Hänzelmann S, Schunk S, et al. Kidney outcome after mild to moderate COVID-19. Nephrol Dial Transplant. 2023;38(9):2031–2040. doi: 10.1093/ndt/gfad008
- Bilo H, Schaap G, Blaak E, et al. Effects of chronic and acute protein administration on renal function in patients with chronic renal insufficiency. Nephron. 1989;53(3):181–187. doi: 10.1159/000185742
- Damianaki A, Brito W, Garessus J, et al. Contrast-enhanced ultrasound and protein shakes are No alternatives for inulin clearance and meat to assess renal functional reserve in humans. Kidney Blood Press Res. 2022;47(11):664–673. doi: 10.1159/000527313
- Legrand M, Bell S, Forni L, et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat Rev Nephrol. 2021;17(11):751–764. doi: 10.1038/s41581-021-00452-0