ABSTRACT
Aim of the Work
Aim of the work is to evaluate the effect of intracavernosal injection of platelet-rich plasma (PRP) for the treatment of arteriogenic erectile dysfunction (ED).
Methods
This study was conducted up on 25 men complaining of mild-to-moderate ED, who were in a stable relationship for more than 1 year.
Results
After 6 months of intracavernosal PRP injection, there was a statistically significant improvement of the International Index of Erectile Function-Erectile Function domain score, which then declines after 1 year of the treatment. No adverse effects were observed during the study.
Conclusions
PRP intracavernosal injections may be a promising option for the management of arteriogenic ED. But further studies are needed to confirm these findings.
1. Introduction
The therapeutic use of platelet-rich plasma (PRP) in different medical specialties has grown steadily after its introduction in 1987 [Citation1–4]. One of the most widely used platelet-based therapies is autologous PRP [Citation5].
PRP is an autologous product which is prepared from whole blood. PRP contains high concentration of multiple platelet growth factors. The presumed mechanism of the action of these growth factors is that they act locally, recruiting undifferentiated cells to the site of injury, which trigger mitosis in these cells, and to induce angiogenesis [Citation6].
Across multiple specialties, PRP has been used both as a sole treatment modality and also as a supplement to other therapies to improve wound healing, tissue regeneration, and angiogenesis. Although most of the studies discussing PRP therapies have been relatively small and heterogenous, they particularly support the safety and the efficacy of these therapies. Moreover, the concept of autologous treatment may particularly be attractive to some patients [Citation7].
The intracavernosal injection of PRP is a newly emerging treatment for erectile dysfunction (ED) that warrants awareness among primary care physicians and urologists alike. Currently, there is a trend toward the worldwide use PRP as a new modality of treatment for ED; however, despite the introduction and commercialization of this therapy, there is a lack of evidence to guide the clinician in the decision-making and to support its use [Citation8].
The PRP therapy is provided to patients in several clinics worldwide and marketed as “P-shot” [Citation9]. Many of these clinics are cosmetic or naturopathic clinics under the supervision of cosmetic surgeons or the general practitioners [Citation10]. Most of these clinics instruct the patients to repeat the procedure to achieve the optimal outcome [Citation11].
2. Methods
This study was conducted up on 25 men suffering from mild-to-moderate arteriogenic ED (peak systolic velocity less than 35 ml/sec on penile Doppler and a score of [11–25] in the International Index of Erectile Function-Erectile Function (IIEF-EF) domain) who were in a stable relationship for more than 1 year and not receiving any treatment for ED. If the patients were previously taking any commercially available treatment for ED such as oral therapy, injection therapy, topical applications, herbal, vacuum constriction devices or alternative medicines, such treatments should have been discontinued 2 weeks before the enrollment in the study and should never been used during the study period [Citation12,Citation13].
Patients with the history of penile fracture, priapism, peyronie’s disease, significant penile curvature, or any other structural anomaly affecting erectile function were excluded from the study. In addition, those with previous (major pelvic surgery, major penile surgery or pelvic radiation) were also excluded. Other exclusion criteria include (i) morning serum testosterone less than 300 ng/dL; (ii) diabetics; (iii) and those with psychogenic ED.
After taking a well-informed written consent from all patients to be enrolled in this study, they underwent (i) detailed medical, surgical, sexual and drug history; (ii) complete general, neurological, and genital examination; (iii) penile Doppler; (iv) complete blood count, glycated hemoglobin Hb A1C, and hormonal analysis (serum prolactin and serum total testosterone).
Two participants did not proceed for the evaluation due to the pandemic of COVID 19, and one participant was excluded after being enrolled due to the use of other medication for ED (phosphodiesterase inhibitor) during the study period.
About 50 cc of the patient’s own blood were obtained in a sterile vacutainer. For 10 min, the blood was centrifuged using a “soft” spin (250 g) (Hettichzentrifugenportofix 32 A, Germany). The obtained plasma containing platelets was transferred into another sterile tube which undergo another centrifugation cycle for another 10 min at a higher speed (300 g) to obtain a platelet-rich plasma. The obtained plasma is rich in platelets which contain multiple growth factors.
Before injection, the calcium chloride solution was added to the plasma derived in a ratio of 1:10 to activate the platelets. A very small needle (1/2-inch-long, 30 gauge) was used for the injection. The topical anesthetic cream was used prior to the injection. The procedure was done once, and the patients were reevaluated after 6 and 12 months, using IIEF-EF questionnaire.
2.1. Statistical analysis
The statistical analysis of the data was done using the package for Social Sciences (SPSS ver.25 Chicago, IL, USA). The K-S test of normality was used to assess the normality of quantitative variables, and it revealed the normally distributed data, so the data were described using mean and SD. The quantitative variables pre-intervention and postintervention were compared using paired t-test. The level of significance of .05 were used in all statistical tests, below which the results were considered to be statistically significant.
3. Result
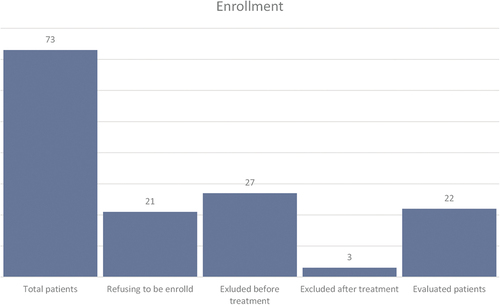
From January 2021 till November 2022, 73 patients with mild-to-moderate ED were evaluated at the outpatient clinic of urology department; 21 patients refused to be enrolled in the study; 27 patients were excluded from the current study, as they were not meeting the inclusion criteria; 25 patients were treated using PRP. After excluding 3 patients (two participants did not proceed for the evaluation due to the pandemic of COVID 19, and one participant was excluded after being enrolled due to the use of other medication for ED during the study period), 22 patients were re-evaluated after 6 months and 1 year ().
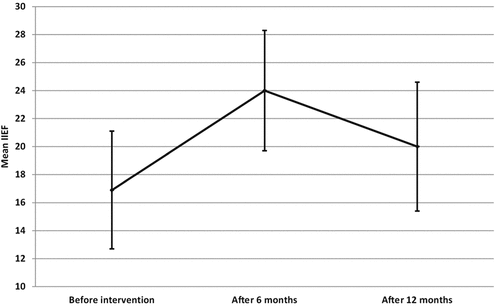
The mean age of the patients was 44.9 ± 11.3, and the mean peak systolic velocity was 25.9 ± 2.4 . After 6 months, there was a statistically significant improvement of IIEF-EF domain score (p < .001*), which then significantly declines after 1 year of the treatment (p < .001*) but still higher than the pre-treatment score (, ).
Table 1. The mean age and peak systolic velocity of the patients included in the study.
Table 2. IIEF before, after 6 months and after 12 months of intracavernosal injection of PRP.
4. Discussion
One of the new treatment options for ED is the use of restorative therapy, aiming not only to treat symptoms but also to restore the structure and function of the cavernous tissue. Among these restorative approaches, PRP has been studied in different urologic conditions [Citation14]. PRP is an autologous plasma that has a supraphysiologic concentrations of the activated platelets (approximately 4–6 folds the concentration of platelet normally present in the serum) [Citation15].
Platelets have a very important role in the coagulation cascade which promote wound healing following injury as they contain multiple growth factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), transforming growth factor (TGF)-b, epithelial growth factor (EGF), and insulin-like growth factor 1. These growth molecules have an integral role in the process of cellular regeneration as they recruit stem cells, modulate the inflammatory responses, and stimulate new angiogenesis [Citation16].
Some of these active biological growth factors have been found to improve erection in both preclinical and clinical studies [Citation17]. VEGF modulate the penile vascularity and improve the erectile response through the promotion of endothelial cell proliferation and angiogenesis. These effects of VEGF are largely dependent on endothelial nitric oxide synthase eNOS activation [Citation18].
Epifanova et al. used flow cytometry to document the increased concentrations of some growth factors, such as FGF, PDGF and VEGF, in ED and control groups. These results support the hypothesis that these growth factors are responsible for erectile function recovery [Citation16].
To our knowledge, very few studies have attempted to illucidate the effect of PRP on the recovery erectile function. In these studies, PRP was shown to increase maximal intracavernosal pressures after cavernous nerve stimulation, improve cavernous nerve myelination, and decrease the intracorporeal expression of TGF-beta 1 when compared to placebo [Citation6,Citation19,Citation20].
Platelet-rich fibrin matrix injection was found to be safe, feasible, and a well-tolerated treatment option in the management of patients with multiple urologic conditions, with reported improvement in the IIEF score after injection [Citation21].
In Greece, Evangelos et al. assessed the safety and efficacy of PRP injections in patients with mild-to-moderate ED [Citation22]. This study was the first double-blind, randomized, placebo-controlled trial, which has been conducted upon 60 sexually active patients. They reported clinically significant improvement in the IIEF-EF of the PRP group than placebo.
The limitations of these studies are the small sample sizes, the different methods of PRP preparation and the lack of standardization of PRP concentrations.
Further studies should aim to standardize PRP preparation protocols, dosage and frequency of administration. Additionally, well-conducted molecular and animal studies may further clarify the pathophysiological mechanisms that contribute to the improvement of erectile function after PRP injection in patients with vasculogenic ED.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Sameh Nassar
Sameh Mohamed Abd Elwahab Nassar, received his M.B.B.ch from Faculty of Medicine, Alexandria University in November 2009, M.Sc. Urology, Faculty of Medicine Alexandria University in May 2016, Egyptian Fellowship in Urology From 2017 to 2022, now he works as assistant lecturer of urology in damanhour medical national institute, Egypt and as a fellower in the urology department faculty of medicine, Alexandria university.
Hussein M Abdeldaeim
Hussein Mamdouh Abdeldaeim, received his M.B.B.C.H degree at Alexandria University, Faculty of medicine in 1998, his Master degree of Urology, from Alexandria University in 2003 and earning his PHD From Alexandria University in 2003, worked as a resident at Urology department, Alexandria university 2000-2003, Assistant lecturer of Urology, Alexandria University 2004-2009, Lecturer of Urology, Alexandria University since 2009-2015, Associate Professor of Urology 2015 -2022 and now he is a professor of urology at Alexandria university since 2022.
Mostafa Said
Mostafa Said Taha, received his Bachelor of Medicine and Surgery - Faculty of Medicine Alexandria University – Egypt in 2008, his Master degree of Urology, from Alexandria University in 2014 and earning his PHD From Alexandria University in 2021, worked as a resident at Urology department, Alexandria university 2010-2014, Assistant lecturer of Urology, Alexandria University 2014-2021, and now he is a lecturer of Urology, Alexandria University since 2021.
Mostafa A Sakr
Mostafa Abdelmoneim Mohamed Sakr, he received his M.B.B.ch, from Faculty of Medicine Alexandria University, November, 1986, his M.Sc. Urology, Faculty of Medicine Alexandria University, May 1991 and he gained MD degree of from Urology department, Faculty of Medicine Alexandria University, November in 1998. He also had the Fellowship in Urology Department, Tulane University, Louisiana State USA From 28/2/1994 to 31/1/1996. He worked as a house officer in Alexandria Main University Hospitals and affiliated ministry of health Hospitals from 1987 to 1988, Resident in Urology, Urology Department, Faculty of Medicine, Alexandria University, Alexandria - Egypt from 1988 to1991, Assistant Lecturer of Urology, Faculty of Medicine, Alexandria University, from 1991 to1998, Lecturer of urology, Faculty of Medicine, Alexandria University from1998 to 2003, Associate professor, Department of Urology, Faculty of Medicine, Alexandria University, from 2003 till 2009, Professor of Urology, Faculty of Medicine, Alexandria University from 30-9-2009 so far. And he worked as a director of Endourology Unit, Urology department, Faculty of Medicine, Alexandria University from November 2017 until December 2019.
Akram Eldeghiedy
Akram Abdel Moneim Deghady, gained his MB BCh from the Faculty of Medicine, University of Alexandria 1984, Master degree (MS) in Clinical Pathology from the Faculty of Medicine, University of Alexandria 1991, And achieve his doctor degree (MD) in Clinical Pathology from the Faculty of Medicine, University of Alexandria 1997. He worked as a house officer in the Alexandria University Hospitals 1985-1986, Physician in the Ministry of health hospitals March 1986- May 1986, Resident in the Clinical Pathology Dept. Alexandria University Hospitals May 1986- May 1989, Demonstrator in the Clinical Pathology Dept. Faculty of Medicine Alexandria University from 1989-1991, Assistant Lecturer in the Clinical Pathology Dept. Faculty of Medicine Alexandria University 1991-1997, Lecturer in the Clinical Pathology Dept. Faculty of Medicine Alexandria University 1997-2002, Assistant Professor in the Clinical Pathology Dept. Faculty of Medicine, Alexandria University 2002-2007, and now he wors as a Professor of Clinical Pathology Clinical Pathology Dept. Faculty of Medicine, Alexandria University 2007 till now.
Abdel Rahman Zahran
Abdel Rhman Zahran is a Current professor of Urology & Head of Endourology & Andrology unit, faculty of Medicine, Alexandria University, EGYPT. He has been elected as a board member of the Egyptian Urological association (EUA) in 2022 for 4 years. He completed his medical school at the Alexandria University School of Medicine in EGYPT the year 1991, urology residency at the Urology department at the main university hospital (1993-1996), and specialty fellowship at the University of Mc gill CANADA (1996-2000). Then he had two another fellowships with Dr. Tom Lue at the UCSF in the year 2005 &2006 as well as in Cleveland clinic with Dr. Drogo Montague in the year 2006 Dr. He was the He was the president of the section of the male sexual dysfunction at the Egyptian urological association also he is the past secretary general of the Middle East society of sexual medicine & he is currently the secretary general of the Egyptian society of sexual medicine surgery (ESSMS).
References
- Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721–730. doi: 10.1038/nrrheum.2013.141
- Patel AN, Selzman CH, Kumpati GS, et al. Evaluation of autologous platelet rich plasma for cardiac surgery: outcome analysis of 2000 patients. J Cardiothorac Surg. 2016;11(1):62. doi: 10.1186/s13019-016-0452-9
- Liu Z, Yuan X, Fernandes G, et al. The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects. Stem Cell Res Ther. 2017;8(1):122. doi: 10.1186/s13287-017-0574-6
- Strazzulla LC, Avila L, Lo Sicco K, et al. An overview of the biology of platelet-rich plasma and microneedling as potential treatments for alopecia areata. J Investig Dermatol Symp Proc. 2018;19(1):S21–s4. doi: 10.1016/j.jisp.2017.10.002
- Xie X, Zhang C, Tuan RS. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. 2014;16(1):204. doi: 10.1186/ar4493
- Wu CC, Wu YN, Ho HO, et al. The neuroprotective effect of platelet-rich plasma on erectile function in bilateral cavernous nerve injury rat model. J Sex Med. 2012;9(11):2838–2848. doi: 10.1111/j.1743-6109.2012.02881.x
- Weiss RA. Autologous cell therapy: will it replace dermal fillers? Facial Plast Surg Clin North Am. 2013;21(2):299–304. doi: 10.1016/j.fsc.2013.02.008
- Scott S, Roberts M, Chung E. Platelet-rich plasma and treatment of erectile dysfunction: critical review of literature and global trends in platelet-rich plasma clinics. Sex Med Rev. 2019;7(2):306–312. doi: 10.1016/j.sxmr.2018.12.006
- Priapus Shot. Priapus shot directory; 2023 [cited 2023 Jun]. Available from: http://www.priapusshot.com/members/directory/
- U.S. Patent and Trademark Office. Available from: https://www.uspto.gov
- Britt D, Blankstein U, Lenardis M, et al. Availability of platelet-rich plasma for treatment of erectile dysfunction and associated costs and efficacy: a review of current publications and Canadian data. Can Urol Assoc J. 2021;15(6):202–206. doi: 10.5489/cuaj.6947
- Fisher WA, Gruenwald I, Jannini EA, et al. Standards for clinical trials in male and female sexual dysfunction: III. Unique aspects of clinical trials in male sexual dysfunction. J Sex Med. 2017;14(1):3–18. doi: 10.1016/j.jsxm.2016.08.016
- Nascimento B, Miranda EP, Terrier JE, et al. A Critical Analysis of Methodology Pitfalls in Duplex Doppler Ultrasound in the evaluation of patients with erectile dysfunction: technical and interpretation deficiencies. J Sex Med. 2020;17(8):1416–1422. doi: 10.1016/j.jsxm.2020.05.023
- Milenkovic U, Campbell J, Roussel E, et al. An update on emerging drugs for the treatment of erectile dysfunction. Expert Opin Emerg Drugs. 2018;23(4):319–330. doi: 10.1080/14728214.2018.1552938
- Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3–4):165–174. doi: 10.1007/s12178-008-9032-5
- Epifanova MV, Chalyi ME, Krasnov AO. Investigation of mechanisms of action of growth factors of autologous platelet-rich plasma used to treat erectile dysfunction. Urologiia. 2017;4:46–48. doi: 10.18565/urology.2017.4.46-48
- Campbell JD, Burnett AL. Neuroprotective and nerve regenerative approaches for treatment of erectile dysfunction after cavernous nerve injury. Int J Mol Sci. 2017;18(8):1794. doi: 10.3390/ijms18081794
- Musicki B, Palese MA, Crone JK, et al. Phosphorylated endothelial nitric oxide synthase mediates vascular endothelial growth factor-induced penile erection. Biol Reprod. 2004;70(2):282–289. doi: 10.1095/biolreprod.103.021113
- Ding XG, Li SW, Zheng XM, et al. The effect of platelet-rich plasma on cavernous nerve regeneration in a rat model. Asian J Androl. 2009;11(2):215–221. doi: 10.1038/aja.2008.37
- Wu YN, Wu CC, Sheu MT, et al. Optimization of platelet-rich plasma and its effects on the recovery of erectile function after bilateral cavernous nerve injury in a rat model. J Tissue Eng Regen Med. 2016;10(10):E294–e304. doi: 10.1002/term.1806
- Matz EL, Pearlman AM, Terlecki RP. Safety and feasibility of platelet rich fibrin matrix injections for treatment of common urologic conditions. Investig Clin Urol. 2018;59(1):61–65. doi: 10.4111/icu.2018.59.1.61
- Mykoniatis I, Pyrgidis N, Zilotis F, et al. Platelet-rich plasma (PRP) improves erectile function: a double-blind, randomized, placebo-controlled clinical trial. J Sex Med. 2022;19:S157–S8. doi: 10.1016/j.jsxm.2022.08.146