ABSTRACT
Introduction
Systemic Lupus Erythematosus, an autoimmune disease, can target various organs of the human body. Lupus nephritis (LN) is a major complication affecting almost half of all SLE patients, with classes “III and IV” being the most aggressive. The severity and prognosis of LN can be influenced by different biomarkers, some of which are used as therapeutic targets. One such target is the B-cell activating factor (BAFF), a key figure in the pathophysiology of SLE. New add-on treatments have emerged in the treatment of LN targeting BAFF as well as other pathways. Assortment of patients to suitable treatment combinations needs more precision using guiding markers.
Methods
This prospective study aims to explore BAFF-expression in patients with active-proliferative LN and its possible association with the response after induction therapy. Peripheral blood mononuclear cells (PBMC) and renal tissue samples (if applicable) were collected from participants before the initiation of induction treatment. RNA was extracted, and the expression levels of BAFF-mRNA were measured using quantitative real-time polymerase chain reaction. Clinical and laboratory data, including disease activity indices and renal functions, were recorded at baseline and 6-months after the commencement of the induction treatment.
Results
The study included 24 patients,14 responders and 10 non-responders. At baseline, median PBMC BAFF-mRNA expression was nearly identical in responders and non-responders. Renal BAFF-expression was found to be higher in non-responders, although non-significant (p = 0.21). Contrary to PBMC BAFF expression, renal BAFF-mRNA was negatively correlated with baseline glomerular filtration rate and with the primary endpoint of response, which was reduction of proteinuria to≥50% of baseline (p = 0.07).
Conclusions
Renal BAFF expression could be a better representative of severity and therapeutic response than blood expression. We acknowledge that the use of BAFF expression as a predictive for early refractoriness to therapy in LN is not yet conclusive.
1. Background
Systemic lupus erythematosus (SLE) is a multifaceted heterogeneous disorder of immunity that can impact musculocutaneous, renal, nervous and other systems. The prevalence of SLE differs in different populations, with a reported prevalence of up to 43 per 100,000 persons in some areas [Citation1]. An estimated prevalence of 1.2/100,000 males and 11.3/100,000 females was reported among the Egyptian population [Citation2]. Around half of SLE-affected individuals present with lupus nephritis (LN) either early or late in the course of the disease [Citation3]. Lupus nephritis causes long-term kidney damage, especially if not treated early and can be a significant cause of mortality among lupus patients [Citation4,Citation5]. Lupus nephritis was classified in 2003 by the International Society of Nephrology/Renal Pathology Society (ISN/RPS) into 6 classes. Class 3 and 4 were considered active and proliferative, needing intense immunosuppressive therapy [Citation6].
Lupus nephritis diagnosis and response evaluation are established mainly by measuring urinary protein excretion and monitoring changes in renal function parameters. Other clinical and molecular parameters can change the course of LN and alter the response to therapy. Part of these parameters are considered routine workup in the assessment of LN and many others are still under investigation [Citation7].
The B-cell activating factor (BAFF) is one of the cytokine networks involved in the immune pathogenesis of lupus and LN [Citation8]. Belimumab is an immunoglobulin G biologic therapy that was developed to neutralize BAFF and decrease cell activity and maturation. Belimumab was subsequently approved by the Food and Drug Administration in the treatment of autoantibody-positive lupus (excluding nephritis and neuropsychiatric lupus) [Citation9]. Recently, promising response improvements were achieved by adding belimumab to standard therapy in trials involving active proliferative LN patients [Citation10–12].
Our prospective study aim was to explore whether peripheral mononuclear cell (PBMC) or tissue BAFF expression could aid in assessing the severity of early active proliferative LN and in predicting early response to standard induction therapy. Our study seeks to contribute to the existing body of knowledge regarding the potential role of BAFF in the pathogenesis of proliferative LN and related kidney damage.
2. Patients and methods
2.1. Study population
Our prospective study was conducted on patients recruited from the lupus clinic; this was initiated in May 2021 and completed in October 2022. Sample size was calculated using Epi Info-7 program [Citation13] by adjusting power at 80%, confidence level 95.0%. The minimum estimated sample size was 15 patients. It is recommended to increase the sample size by 10% (2 patients). The total estimated sample size = 17 patients.
The following formula was used:
S = Z2 × P × (1−P)/M2
Where;
• S = sample size for infinite population
• Z = Z score(1.96)
• p = population proportion
• M = Margin of error (0.05)
Out of 39 recently diagnosed LN patients who sought advice at the lupus clinic of Alexandria’s main university hospital during the study period, 24 patients who had active proliferative LN were recruited for this study.
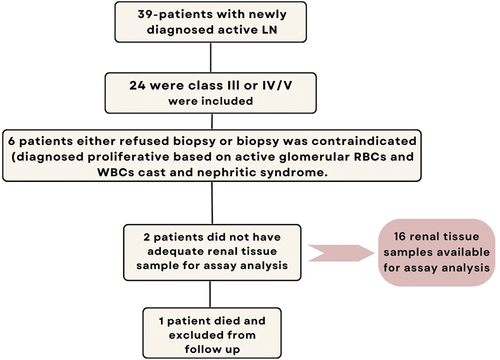
The diagnosis of SLE was made by applying the classification criteria established by the “Systemic Lupus International Collaborating Clinics (SLICC)” [Citation14]. A diagnosis of LN was made following the detection of urinary protein of more than 0.5 g/mg creatinine by urinary protein creatinine ratio (u-PCR) quantification method in addition to a confirmatory renal biopsy, or in case renal biopsy could not be done, the presence of red blood cells (RBCs) or white blood cell (WBCs) casts. Renal biopsy was not done for 6 patients because they either refused a kidney biopsy or a biopsy was contraindicated. These patients were presumed to have proliferative LN based on the presence of red blood cells (RBCs) and white blood cells (WBCs) casts and symptoms of nephrotic syndrome. Two renal tissue samples were insufficient for assay analysis. Ultimately, 16 renal tissue samples remained available for assay analysis and were used in the study. One patient was excluded from the follow-up response analysis due to death ().
Plasma samples were stored at -80 and renal biopsy samples were kept in paraffin blocks until used for analysis. Each participant provided written informed consent after being informed about the nature of the study.
2.2. Data collection
Demographics, the presence of hypertension (HTN) and edema, the onset of SLE, past and present lupus activity, previous medications, the SLE disease activity index (SLEDAI-2K) [Citation15], the renal SLEDAI (rSLEDAI), laboratory results (such as a complete blood count (CBC), u-PCR, creatinine, urea, glomerular filtration rate estimation (eGFR) using the “CKD-Epi (chronic kidney disease epidemiology)” equation [Citation16], uric acid, and urine analysis were among the data collected at baseline. We additionally reported abnormalities in complement 3 and 4 (c3, c4), anti-double stranded deoxyribonucleic acid (anti-dsDNA), and renal histopathologic features.
All patients were treated by standard induction protocols for treatment of class III or IV LN, which included “pulse steroid therapy (500 mg-1 g daily for 3 days) followed by mycophenolate-mofetil (MMF) 2–3 g per day or monthly cyclophosphamide INH protocol (0.5–1 g/m2 for 6 months) or Eurolupus protocol (500 mg every 2 weeks for 6 doses).” Maintenance therapy was constituted of either azathioprine 2–2.5 mg/kg daily or MMF 1–2 mg daily [Citation17].
On follow-up, six months after the initiation of induction therapy, variations in the previously reported clinical findings, laboratory parameters, SLEDAI-2K, and rSLEDAI were recorded. Renal response was defined as “the reduction of u-PCR by 50% or more and a normal or nearly-normal GFR (or, if previously abnormal, within 10% of the range of normal GFR)” [Citation18].
2.3. Peripheral blood sample collection
Samples of seven milliliters of whole blood were collected from subjects and divided into two vacutainer tubes: 3 ml in BD-vacutainer® red-top tubes (BD, USA) to obtain serum for chemical and serological analysis and 4 ml in BD-vacutainer® lavender-top tubes (BD, USA) for mononuclear cell separation for estimation of gene expression. A morning urine sample was collected for measurement of urinary protein creatinine ratio.
2.4. Renal biopsy sample collection
Adequate (≥9 glomeruli) renal core biopsies which were formalin-fixed, paraffin-embedded (FFPE) from LN patients were taken before induction treatment. 3 to 5 mm thick FFPE sections were prepared with hematoxylin and eosin, Masson’s trichrome, periodic acid-Schiff, and periodic acid-silver methenamine stains for light microscopy examination and were assessed by 2 pathologists.
Lupus nephritis classification, as well as chronicity and activity indices (out of 12 and 24, respectively), were determined in accordance to “the revised 2018 ISN/RPS classification for LN;” the activity index was measured by adding the scores of; “endocapillary proliferation (out of 3), fibrinoid necrosis (out of 6), cellular or fibro-cellular crescents (out of 6), neutrophilic infiltration and karyorrhexis (out of 3), hyaline thrombi (out of 3) and interstitial inflammation (out of 3).” The index of chronicity was estimated by adding the semi-quantitative scores of; “glomerular sclerosis (out of 3), fibrous crescents (out of 3), interstitial fibrosis (out of 3), and tubular atrophy (out of 3)” [Citation6].
For BAFF expression analysis, FFPE blocks were extracted from the pathology department archives at the faculty of medicine at Alexandria University. For each paraffin block, three sections were discarded and then eight sections of 5 µm thicknesses were cut using a scalpel and transferred into a sterile microcentrifuge tube.
2.5. Laboratory investigations
Serum creatinine, urinary protein, and urinary creatinine were measured using the chemistry analyzer Dimension RxL Max (Siemens Healthineers, Germany). The estimated glomerular filtration rate (eGFR) was recorded using the 2021 CKD-EPI equation. Complements 3 and 4 were measured using BN ProSpec system (Siemens Healthineers, Germany). Anti-dsDNA was measured using BIO-FLASH chemiluminescent analyzer (INOVA Diagnostics, USA).
2.6. Relative quantification of blood and tissue gene expression of BAFF using quantitative real‐time polymerase chain reaction (qRT-PCR)
2.6.1. RNA extraction
Total RNA was extracted from PBMCs and FFPE renal tissues, respectively by using RNeasy Mini-Kit and RNeasy FFPE Kit (Qiagen, USA), according to the manufacturer’s manual. The RNA concentration and purity were assessed by Nano-drop 2000/2000c spectro-photometer (ThermoFisher Scientific, USA).
2.6.2. Reverse transcription (RT)
Genomic RNA was transformed into complementary DNA (cDNA) using Revert-Aid First Strand cDNA Synthesis kit (ThermoFisher Scientific, USA). One μg of total RNA was added to the RT reaction mix in a total volume of 20 μL. Twenty microliters of RT reaction mix were prepared by adding one μg of the total RNA. This mixture was incubated in Arktik thermal cycler (ThermoFisher Scientific, USA) at 25°C for 5 minutes, 42°C for 60 minutes, and finally 70°C for 5 minutes.
2.6.3. Quantitative RT-PCR
Relative quantification of PBMCs and renal tissue BAFF transcript was conducted by Maxima-SYBR Green qPCR Master-Mix (2X) (ThermoFisher Scientific, USA, Cat. No. K0251) using Rotor-Gene qRT-PCR System (QIAGEN, Germany) according to manufacturer instructions. The PCR reactions were carried out in duplicate with a final volume of 25 μL. The reaction mixture included 12.5 μL of Maxima-SYBR Green/ROX qPCR Master Mix (2X), 1 μL of forward primer (10 pmole), 1 μL of reverse primer (10 pmole), 0.05 μL of 10× diluted ROX solution, 200 ng of cDNA and nuclease-free water to make up the final volume of 25 μL. The reaction protocol included initial denaturation for 10 min at 95°C (one cycle), subsequent denaturation for 15 seconds at 95°C (40 cycles), 30 second-annealing at 55°C and extension for another 30 seconds at 72°C. The process of amplifying the cDNA of both BAFF and GAPDH as a housekeeping gene involved using custom-made primers supplied by Applied Biosystems, USA. These primers specifically targeted the forward and reverse sequences of the cDNA strands. Four unlabeled sequence-specific primers were used to ensure accurate and efficient amplification of the desired cDNA sequences. The sequence of the primers was as follows: BAFF forward primer “5ʹ-GGG-AGC-AGT-CAC-GCC-TTAC-3ʹ” and reverse “5ʹ-GAT-CGG-ACA-GAG-GGG-CTTT-3ʹ;” GAPDH forward primer “5ʹ-CCA-CTC-CTC-CAC-CTT-TGA-CG-3ʹ” and reverse “5ʹ-CCA-CCA-CCC-TGT-TGC-TGT-AG-3ʹ.” Relative expression was calculated using the 2–ΔΔCT method [Citation19].
2.7. Statistical analysis of the data
The data collected was analyzed via the IBM SPSS software package (version 25.0). The qualitative data were illustrated in terms of numbers and percentages to give a clear overview of the data set. To verify the normality of the data distribution, the Kolmogorov-Smirnov test was applied. The quantitative data was displayed using the range (minimum and maximum), mean, median, and standard deviation. A significant result was determined if p was equal to or less than 0.05, indicating a high level of confidence in the results.
The tests used were:
Mann-Whitney test: A non-parametric test employed to compare quantitative variables between two independent groups.
Chi-square
Fisher’s exact significance was calculated when the expected count in more than 25% of cells is less than 5 for 2 × 2 tables.
For C X R tables, the Monte Carlo value of significance was calculated when the expected count in over 25% of cells is below 5.
3. Spearman Rho correlation coefficient: Non-parametric correlation between two quantitative variables.
3. Results
The median age of the study group was 24.5 (IQR: 20–30); 21 were females; 14 were responders and 10 were non-responders. The difference in main baseline characteristics according to response is summarized in . The only significant different parameter was the nature of disease activity before the onset of LN, where it shows less previous disease activity in the responders group.
Table 1. Baseline demographic and clinical characteristics of the studied active proliferative LN patients.
3.1. Baseline clinical data and laboratory measures of the studied LN patients
We found no difference in the percentage of patients with edema and HTN between responders and non-responders (p = 0.341, p = 1.00, respectively).
Most of the measured parameters did not significantly differ between the two groups, with the exception of u-PCR, which showed a higher median in responders compared to Non-responders (p = 0.04**)
The median of baseline Renal SLEDAI was higher in non-responders with a P-value of 0.10, which may indicate a trend toward significance, .
Table 2. Baseline clinical data and laboratory measures of the studied LN patients.
3.2. Histopathological characteristics of the study group (18 patients)
provides histopathological data comparing responders and non-responders, which shows:
A statistically significant difference in chronicity index was found between responders and non-responders, with responders having a median score of 2.5 and non-responders having a median score of 5; the p-value is 0.04.
There was a trend toward significant differences in the degree of interstitial inflammation between both groups, with a p-value of 0.06.
Other histological features showed no statistically significant differences between the two groups, as indicated by their respective p-values (all above 0.05).
Table 3. Histopathological characteristics of the taken renal biopsies at baseline (n = 18).
3.3. Peripheral blood and tissue BAFF gene expression
and Present comparative data on BAFF expression levels in PBMCs and renal tissues, distinguishing between responders and non-responders.
Figure 1. Difference in BAFF expression levels in PBMCs and renal tissues in responders and non-responders.
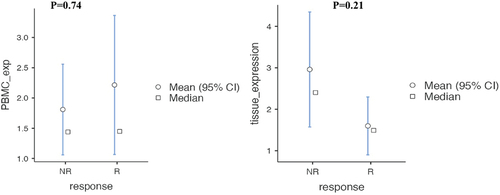
Table 4. Difference in BAFF expression levels in PBMCs and renal tissues in responders and non-responders.
For PBMC B-cell activating factor expression:
Responders (14) had a median expression level of 1.45, with a range from 0.74 to 1.83.
Non-responders (10) had a nearly identical median expression level of 1.44 but with a slightly wider interquartile range from 0.922 to 1.97.
The statistical comparison between these two groups yielded a p-value of 0.747, suggesting no significant difference in PBMC BAFF expression between responders and non-responders.
For renal BAFF expression:
Responders (9) showed a median expression level of 1.49 with a range from 1.07 to 2.42.
Non-responders (7) had a substantially higher median expression level of 2.4, with a range from 2.16 to 3.82.
The p-value for the comparison between renal BAFF expression in responders and non-responders was 0.210, indicating that, while there is a trend toward higher expression in non-responders, the difference did not reach statistical significance.
4. Correlation of BAFF mRNA with 6-month parameters
At baseline, PBMCs` BAFF was not correlated with any of the other assessed laboratory parameters, while tissue BAFF expression was only negatively correlated with eGFR (r = -0.530, p = 0.035) and positively correlated with high uric acid (r = 0.644, p = 0.007).
At 6 months, PBMC expression was not correlated with the u-PCR reduction. However, PBMC mRNA quantification showed a near-significant correlation with follow-up creatinine and eGFR and a positive significant correlation with the number of urinary leucocytes, renal SLEDAI and SLEDAI-2K. On the other hand, tissue BAFF expression showed a near significant negative correlation with the degree of reduction in u-PCR (-0.482, p = 0.071*), which may indicate a trend to poor early response in patients with high intra-renal BAFF expression and more correlation to lack of response in comparison to PBMC expression of BAFF, .
Table 5. Correlation between both PBMC and tissue BAFF expression and 6-month follow-up parameters.
5. Discussion
Treatment options for LN have evolved considerably over the past years, resulting in decreased burden of the disease. However, the lack of validated severity stratifying predictors can lead to the unguided choice of treatment options and subsequently increase the chance of no or delayed response, which may increase the possibility of residual renal damage. Besides blood pressure measurements, proteinuria and renal function assessment, renal core biopsy is still considered the gold standard for judging the diagnosis, kidney inflammation and damage and predicting response [Citation20]. Two previous studies highlighted some inadequacies of renal biopsy in evaluating LN; the earlier one revealed that 17% of SLE patients with silent LN had active proliferative LN despite the absence of active urinary sediment or decline in renal function, and the later one indicated that proteinuria persisted in 29% of patients who had biopsy-confirmed remission after treatment [Citation21,Citation22]. In addition to being invasive and potentially under-representative, renal biopsy may not consistently correlate with clinical indicators, limiting its ability to accurately assess the activity, classify LN severity or predict treatment outcomes. Several blood, urinary and tissue biomarkers have been studied for predicting the severity of LN and therapeutic response, but there is currently no reliable predictor or severity stratification tool [Citation20]. Therefore, most of the treatment choices still rely on renal biopsy results in combination with early clinical and laboratory renal parameters.
The involvement of BAFF and A Proliferation-Inducing Ligand (APRIL) in SLE pathogenesis has been established through various research studies [Citation23,Citation24,Citation25–30]. Nonetheless, the application of BAFF and APRIL as biomarkers for evaluating the severity and forecasting the course of SLE and LN remains debated. Research has yielded conflicting results; certain studies indicate a correlation between elevated levels of BAFF and the intensity of the disease, while others have been unable to confirm such a relationship. Investigations have revealed that murine models of lupus with heightened renal susceptibility to APRIL/BAFF-targeted treatments exhibited increased serum concentrations of APRIL and BAFF proteins. Furthermore, it has been noted that mice deficient in BAFF develop LN and interstitial inflammation; however, they do not typically advance to renal failure, likely due to reduced damage to endothelial cells and the renal tubule [Citation31]. These observations contribute to a deeper understanding of how BAFF influences the development and severity of LN.
Research conducted by Zollars and colleagues indicates a specific correlation between BAFF protein levels and the presence of anti-ds DNA antibodies only but not overall activity, whereas they found BAFF-mRNA expression to be a reliable indicator of both present and future SLE activity [Citation30]. Furthermore, in the context of phase III clinical trials for belimumab, it was observed that initial serum concentrations of BAFF protein did not correlate with the prognostic outcomes for patients treated with standard protocols or therapies targeting BAFF [Citation32]. The fluctuation in BAFF levels was also suggested to be influenced significantly by the degree of proteinuria [Citation33]. These insights have provided substantial support to our investigation of the efficacy of BAFF-mRNA, rather than BAFF protein levels, as a reliable biomarker for judging disease severity and therapeutic response in patients with active LN.
Regarding the Correlation between peripheral blood BAFF expression with LN severity and response, several studies have reported that the expression of the BAFF gene expression was higher in SLE patients than in healthy controls [Citation30,Citation34,Citation35], but contrary to the results of Zollar et al. which reported a correlation with SLE activity [Citation30], Eilertesen et al. found that BAFF expression did not correlate with SLE or LN severity or renal biopsy findings [Citation35]. Our results were consistent with these findings, as we found that the peripheral monocyte BAFF-mRNA did not correlate with clinical, laboratory, or histopathological activity markers at baseline nor with the response to conventional therapy in class III/IV LN patients.
When it comes to the Correlation of renal tissue BAFF expression with LN severity and response, few immunohistochemical analyses have previously demonstrated elevated intrarenal levels of B BAFF in cases of proliferative LN [Citation36–39]. Neusser et al. described the increased expression of BAFF and APRIL-mRNA in patients exhibiting active proliferative LN in contrast to those with membranous LN and healthy control kidney tissues [Citation36]. Additional research has identified enhanced immunohistochemical expression of BAFF and its receptors, specifically in class III and IV LN when compared to class II and class V LN [Citation37,Citation40]. Schwarting et al.’s investigation showed a significant association between elevated BAFF expression in renal tubules and the severity of LN in both murine models and human kidney biopsy samples of proliferative LN [Citation38].
In parallel, a study conducted in Thailand reported that patients with LN who did not respond to treatment exhibited higher baseline levels of intrarenal APRIL and BAFF-mRNA, as assessed RT-PCR, compared to those who exhibited early treatment response, despite employing different response endpoints than those in our research [Citation29]. Surprisingly, a more recent investigation reported a positive prognosis correlating with early high BAFF expression in renal tissue. However, this study’s cohort consisted of only 17 patients [Citation41]. These cumulative findings advance the understanding of the prognostic implications of BAFF and APRIL expression in LN, suggesting their potential as biomarkers for disease activity and treatment response.
In our study, while there was a trend toward higher renal tissue expression in non-responders, the difference did not reach statistical significance (p = 0.210). Furthermore, an analysis of baseline parameter measurements and tissue BAFF gene expression revealed no statistically significant correlation. The small sample size may likely have contributed to the lack of statistical significance. It is also possible that variations in research populations or test sensitivity are the reasons for the disparities observed in the studies.
The correlation between BAFF biomarkers and SLE disease activity indices remains inconclusive. An early study found no correlation between serum BAFF and SELENA-SLEDAI score of disease activity but reported an association between SELENA-SLEDAI and patient global assessment scores with blood BAFF-mRNA in SLE patients [Citation30]. The following studies similarly failed to establish a conclusive connection between BAFF protein level and disease activity as evaluated by SLEDAI-2k and rSLEDAI in patients with lupus kidney disease [Citation27,Citation29,Citation32].
6. Conclusion
The current study aimed to investigate whether BAFF expression could be used as a marker of early response to treatment in active proliferative LN. Our findings did not establish a clear association between peripheral and renal BAFF gene expression and early response to induction treatment in an Egyptian cohort of patients with proliferative LN. Although we observed a trend toward higher BAFF expression in renal tissue samples of non-responders, it did not reach statistical significance. Additionally, high renal BAFF-mRNA levels correlated to poor response better than PBMC expression. However, this needs more investigation in future research. Hence, we could conclude that BAFF expression alone may not be a reliable indicator to predict early non-response. The small sample sizes of all relevant studies, including ours, may explain inconsistent results, lack of reproducibility and inability to prove an association. Identifying an ideal predictive marker for LN treatment has been proven to be difficult due to inconsistent relationships between treatment and various cytokine concentrations. Therefore, the European League Against Rheumatism recommends the use of add-on therapies such as Belimumab for all patients with class III or IV LN. Belimumab targets BAFF and aims to intensify the induction protocol and prevent early damage [Citation42].
7. Limitations
The main limitations of our study, as well as previously published comparable research, were the relatively small sample size and retrospective data collection. Some patients received immunosuppression for other prior lupus activities, which may have affected the assay result. Furthermore, some patients had no or insufficient renal biopsy samples for parallel tissue and peripheral analysis of all study participants.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Asmaa Beltagy
Dr. Asmaa Beltagy is a lecturer of Internal Medicine, Rheumatology Unit, Internal Medicine Department, Alexandria University.
Raghda Saad Zaghloul Taleb
Dr. Raghda Saad Zaghloul Taleb is a lecturer of clinical and Chemical Pathology at the Department of Clinical and Chemical Pathology, Faculty of Medicine, Alexandria University, Egypt.
Maram Allam
Dr. Maram Allam is a lecturer of Pathology at the Department of Pathology, Faculty of Medicine, Alexandria University, Egypt.
Ragaa Abd El-Kader
Dr. Ragaa Abd El-Kader is a Professor of Internal Medicine, Rheumatology Unit, Internal Medicine Department, Alexandria University.
Amira Al-Girby
Dr. Amira Al-Girby is a Professor of Internal Medicine, Rheumatology Unit, Internal Medicine Department, Alexandria University.
Abeer Abdelati
Dr. Abeer Abdelati is an assistant professor of Internal Medicine, Rheumatology Unit, Internal Medicine Department, Alexandria University.
References
- Tian J, Zhang D, Yao X, et al. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheumatic Dis. 2023;82(3):351–356. doi: 10.1136/ard-2022-223035
- El Hadidi KT, Medhat BM, Abdel Baki NM, et al. Characteristics of systemic lupus erythematosus in a sample of the Egyptian population: a retrospective cohort of 1109 patients from a single center. Lupus. 2018 May;27(6):1030–1038. doi:10.1177/0961203317751856 PubMed PMID: 29431056. Epub 2018/02/13. eng.
- Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol (Hoboken, NJ). 2016;68(6):1432–1441. eng. doi: 10.1002/art.39594
- Moghazy A, Ibrahim AM. Mortality in a cohort of Egyptian systemic lupus erythematosus patients: retrospective two-center study. Egypt Rheumatol Rehabil. 2021 2021/02/22;48(1):14. doi: 10.1186/s43166-021-00062-5
- Arkema EV, Saleh M, Simard JF, et al. Epidemiology and damage accrual of systemic lupus erythematosus in Central Sweden: a single-center population-based cohort study over 14 years from Östergötland county. ACR Open Rheumatol. 2023 Aug;5(8):426–432. doi: 10.1002/acr2.11585 PubMed PMID: 37469135. PMCID: PMC10425583. Epub 2023/07/20. eng.
- Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the international society of nephrology/renal pathology society classification for lupus nephritis: clarification of definitions, and modified national institutes of health activity and chronicity indices. Kidney Int. 2018 Apr;93(4):789–796. doi: 10.1016/j.kint.2017.11.023 PubMed PMID: 29459092. Epub 2018/02/21. eng.
- Parodis I, Tamirou F, Houssiau FA. Prediction of prognosis and renal outcome in lupus nephritis. Lupus Sci Med. 2020;7(1):e000389. doi:10.1136/lupus-2020-000389 PubMed PMID: 32153796. PMCID: PMC7046967. Epub 2020/03/11. eng.
- Möckel T, Basta F, Weinmann-Menke J, et al. B cell activating factor (BAFF): structure, functions, autoimmunity and clinical implications in systemic lupus erythematosus (SLE). Autoimmun Rev. 2021 Feb;20(2):102736. doi:10.1016/j.autrev.2020.102736 PubMed PMID: 33333233. Epub 2020/12/18. eng.
- Blair HA, Duggan ST. Belimumab: a review in systemic lupus erythematosus. Drugs. 2018 Mar;78(3):355–366. doi: 10.1007/s40265-018-0872-z PubMed PMID: 29396833. Epub 2018/02/06. eng.
- Furie R, Rovin BH, Houssiau F, et al. Safety and efficacy of belimumab in patients with lupus nephritis: open-label extension of BLISS-LN study. Clin J Am Soc Nephrol. 2022 Nov;17(11):1620–1630. doi: 10.2215/CJN.02520322 PubMed PMID: 36302567. PMCID: PMC9718049. Epub 2022/10/28. eng.
- Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of Belimumab in lupus nephritis. N Engl J Med. 2020 Sep 17;383(12):1117–1128. doi: 10.1056/NEJMoa2001180 PubMed PMID: 32937045. Epub 2020/09/17. eng.
- Joy A, Muralidharan A, Alfaraj M, et al. The role of Belimumab in systemic lupus erythematosis: a systematic review. Cureus. 2022 Jun;14(6):e25887. doi: 10.7759/cureus.25887 PubMed PMID: 35844357. PMCID: PMC9277571. Epub 2022/07/19. eng.
- Dean AG, Arner TG, Sunki GG, et al. Epi Info™, a database and statistics program for public health professionals. Atlanta (GA) (USA): CDC; 2011.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012 Aug;64(8):2677–2686. doi: 10.1002/art.34473 PubMed PMID: 22553077. PMCID: PMC3409311. Epub 2012/05/04. eng.
- Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheumatol. 1992;35(6):630–640. doi: 10.1002/art.1780350606
- Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021 Nov 4;385(19):1737–1749. doi:10.1056/NEJMoa2102953 PubMed PMID: 34554658. PMCID: PMC8822996. Epub 2021/09/24. eng.
- El Miedany Y, Kamel NS, Abu-Zaid MH, et al. Egyptian evidence-based consensus on clinical practice recommendations for the management of lupus nephritis. Egypt Rheumatol Rehabil. 2022;49(1):48. doi: 10.1186/s43166-022-00146-w 2022/09/13.
- Rovin BH, Adler SG, Barratt J, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 2021 Oct;100(4):753–779. doi: 10.1016/j.kint.2021.05.015 PubMed PMID: 34556300. Epub 2021/09/25. eng.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262 2001/12/01/.
- Mok CC. Prognostic stratification of lupus nephritis: the importance of renal histology. J Rheumatol. 2023 Jun 1;50(9):1095–1096. doi: 10.3899/jrheum.2023-0381 PubMed PMID: 37263655. Epub 2023/06/02. eng.
- Ishizaki J, Saito K, Nawata M, et al. Low complements and high titre of anti-Sm antibody as predictors of histopathologically proven silent lupus nephritis without abnormal urinalysis in patients with systemic lupus erythematosus. Rheumatol (Oxford). 2015 Mar;54(3):405–412. doi: 10.1093/rheumatology/keu343 PubMed PMID: 25183834. Epub 2014/09/04. eng.
- Malvar A, Pirruccio P, Alberton V, et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant. 2017 Aug 1;32(8):1338–1344. doi: 10.1093/ndt/gfv296 PubMed PMID: 26250434. PMCID: PMC5837387. Epub 2015/08/08. eng.
- Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999 Dec 6;190(11):1697–1710. doi: 10.1084/jem.190.11.1697 PubMed PMID: 10587360. PMCID: PMC2195729. Epub 1999/12/10. eng.
- Moore PA, Belvedere O, Orr A, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999 Jul 9;285(5425):260–263. doi: 10.1126/science.285.5425.260 PubMed PMID: 10398604. Epub 1999/07/10. eng.
- Vincent FB, Saulep-Easton D, Figgett WA, et al. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 2013 Jun;24(3):203–215. doi: 10.1016/j.cytogfr.2013.04.003 PubMed PMID: 23684423. PMCID: PMC7108297. Epub 2013/05/21. eng.
- Vincent FB, Morand EF, Schneider P, et al. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. 2014 Jun;10(6):365–373. doi: 10.1038/nrrheum.2014.33 PubMed PMID: 24614588. Epub 2014/03/13. eng.
- Vincent FB, Northcott M, Hoi A, et al. Association of serum B cell activating factor from the tumour necrosis factor family (BAFF) and a proliferation-inducing ligand (APRIL) with central nervous system and renal disease in systemic lupus erythematosus. Lupus. 2013 Aug;22(9):873–884. doi: 10.1177/0961203313496302 PubMed PMID: 23846230. Epub 2013/07/13. eng.
- Sari S, Cinar S, Yalcinkaya Y, et al. The relationship between serum a proliferation-inducing ligand and B-cell activating factor levels with disease activity and organ involvement in systemic lupus erythematosus. Lupus. 2022 Apr;31(5):555–564. doi: 10.1177/09612033221086123 PubMed PMID: 35249405. Epub 2022/03/08. eng.
- Treamtrakanpon W, Tantivitayakul P, Benjachat T, et al. APRIL, a proliferation-inducing ligand, as a potential marker of lupus nephritis. Arthritis Res Ther. 2012 Nov 21;14(6):R252. doi: 10.1186/ar4095 PubMed PMID: 23171638. PMCID: PMC3674621. Epub 2012/11/23. eng.
- Zollars E, Bienkowska J, Czerkowicz J, et al. BAFF (B cell activating factor) transcript level in peripheral blood of patients with SLE is associated with same-day disease activity as well as global activity over the next year. Lupus Sci Med. 2015;2(1):e000063. doi: 10.1136/lupus-2014-000063 PubMed PMID: 26113988. PMCID: PMC4477150. Epub 2015/06/27. eng.
- Kang S, Fedoriw Y, Brenneman EK, et al. BAFF induces tertiary lymphoid structures and positions T cells within the glomeruli during lupus nephritis. J Immunol. 2017 Apr 1;198(7):2602–2611. doi: 10.4049/jimmunol.1600281 PubMed PMID: 28235864. PMCID: PMC5360485. Epub 2017/02/27. eng.
- Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012 Jul;64(7):2328–2337. doi: 10.1002/art.34400 PubMed PMID: 22275291. PMCID: PMC3350827. Epub 2012/01/26. eng.
- Aringer M, Costenbader K, Daikh D, et al. European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019 [2019 Sep];71(9):1400–1412. doi: 10.1002/art.40930 PubMed PMID: 31385462. PMCID: PMC6827566. Epub 2019/08/07. eng.
- Marín-Rosales M, Cruz A, Salazar-Camarena DC, et al. High BAFF expression associated with active disease in systemic lupus erythematosus and relationship with rs9514828C>T polymorphism in TNFSF13B gene. Clin Exp Med. 2019;19(2):183–190. doi: 10.1007/s10238-019-00549-8 2019/05/01.
- Eilertsen GØ, Ghelue M, Nossent JC. BAFF expression is increased in patients with lupus nephritis and associated with antinucleosome antibodies, C1 inhibitor, Α-1-acid-glycoprotein and endothelial activation markers. J Data Mining Genomic Proteomic. 2012;3(01). doi: 10.4172/2153-0602.1000113
- Neusser MA, Lindenmeyer MT, Edenhofer I, et al. Intrarenal production of B-cell survival factors in human lupus nephritis. Mod Pathol. 2011 Jan;24(1):98–107. doi: 10.1038/modpathol.2010.184 PubMed PMID: 20890272. Epub 2010/10/05. eng.
- Marín-Rosales M, Palafox-Sánchez CA, Franco-Topete RA, et al. Renal tissue expression of BAFF and BAFF receptors is associated with proliferative lupus nephritis. J Clin Med. [2022 Dec 22];12(1). 71. doi: 10.3390/jcm12010071 PubMed PMID: 36614872. PMCID: PMC9821186. Epub 2023/01/09. eng.
- Schwarting A, Relle M, Meineck M, et al. Renal tubular epithelial cell-derived BAFF expression mediates kidney damage and correlates with activity of proliferative lupus nephritis in mouse and men. Lupus. 2018 Feb;27(2):243–256. doi: 10.1177/0961203317717083 PubMed PMID: 28659046. Epub 2017/07/01. eng.
- Sun CY, Shen Y, Chen XW, et al. The characteristics and significance of locally infiltrating B cells in lupus nephritis and their association with local BAFF expression. Int J Rheumatol. 2013;2013:1–9. doi: 10.1155/2013/954292 PubMed PMID: 23843794. PMCID: PMC3694495. Epub 2013/07/12. eng.
- Suso JP, Posso-Osorio I, Jiménez CA, et al. Profile of BAFF and its receptors’ expression in lupus nephritis is associated with pathological classes. Lupus. 2017;27(5):708–715. doi: 10.1177/0961203317739132 2018/04/01
- Nawata A, Nakayamada S, Hisano S, et al. Differential expression of IFN-α, IL-12 and BAFF on renal immune cells and its relevance to disease activity and treatment responsiveness in patients with proliferative lupus nephritis. Lupus Sci Med. 2023 Jul;10(2). e000962. doi: 10.1136/lupus-2023-000962 PubMed PMID: 37460249. PMCID: PMC10357699. Epub 2023/07/18. eng.
- Antonis F, Myrto K, Jeanette A, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheumatic Dis. 2024;83:15–29.