ABSTRACT
Background
Acute kidney injury (AKI) in the ICU is one of the life-threatening situations that may need renal replacement therapy (RRT). Survival benefit of early initiation of RRT is still not proven. There is still a debate whether early or late RRT significantly affects morbidity and mortality.
Methods
This is a randomized controlled trial (RCT) that was conducted on 94 critically ill subjects with stage 2 AKI, according to KDIGO classification, who were on invasive mechanical ventilation. Recruited subjects were selected only if they failed to respond to furosemide stress test after volume status optimization. Subjects were randomly assigned into two groups according to timing of RRT initiation. In the early group, RRT was initiated without the presence of urgent indications for hemodialysis, whereas in the late group, RRT was implemented only in the presence of one or more of urgent indications such as severe metabolic acidosis or hyperkalemia. Primary outcomes of the study were ICU mortality and length of stay. Secondary outcomes included recovery of renal function in addition to duration of mechanical ventilation.
Results
There was no statistically significant difference in the primary outcomes, namely ICU mortality and ICU length of stay. About 51.1% of the subjects survived in the early RRT group, whereas 46.8% survived in the late group (p = .680). Although duration of mechanical ventilationwas not significantly different (p = .486), recovery of renal functionand less dependency on RRT after discharge, were significantly higher in the early RRT group (p = < .001).
Conclusion
Even after choosing AKI patients who failed to respond to furosemide stress test, timing of RRT initiation did not affect survival, ICU length of stay or duration of mechanical ventilation. However, early RRT may have positive effect on RRF for survived patients.
1. Introduction
Acute kidney injury (AKI) is a frequently encountered complication in patients admitted for acute illnesses, with its prevalence on the rise [Citation1–3]. This condition is generally characterized by a sudden decline in kidney function, disrupting metabolic, electrolyte, and fluid balance over a period ranging from hours to days [Citation4]. The spectrum of AKI varies widely, encompassing subtle changes in biochemical markers of kidney function to severe kidney failure necessitating the initiation of renal replacement therapy (RRT) to treat hazardous consequences such as severe metabolic acidosis, serious electrolyte disturbance as hyperkalemia, or pulmonary edema as a consequence of hypervolemia. Compelling evidence from observational studies suggests an increasing incidence of AKI, accompanied by a simultaneous decrease in mortality [Citation5,Citation6]. It is estimated that up to 50% of the patients may develop AKI during their ICU stay [Citation7–10].
There is still a persistent debate regarding the optimal time for starting RRT for those who develop serious complications of AKI. Theoretically speaking, early RRT would better correct metabolic derangements although it may be associated with secondary complications such as arrhythmia, hypotension, coagulopathy, or infection [Citation8,Citation11]. However, cautious postponement of RRT may give a chance for improvement of renal function and metabolic abnormalities without the risk of hemodialysis complications. The results of prior observational and randomized controlled studies on timing of RRT initiation were controversial and inconclusive. That might be attributed to the choice of patients [Citation12–15]. Therefore, more studies are still needed to confirm, disprove, or find a significant relationship between the timing of RRT and improvement of morbidity or mortality.
A trial furosemide stress test may be used before initiation of RRT. However, this should only be done after volume status optimization. The test can serve as a tool for identifying individuals with a high risk of requiring RRT. It has been employed in decision-making processes to inform the timing of RRT initiation, especially in clinical trials aimed at improving the enrollment of participants based on RRT timing [Citation16]. This will reserve RRT for patients who are more likely to benefit from it. In addition, it will also allow time for spontaneous recovery for patients who are responsive to furosemide with adequate urine output.
1.1. Study design and approval
This is a randomized controlled trial (RCT) that was conducted on 94 critically ill subjects with AKI, who were on invasive mechanical ventilation, to assess the impact of implementation of early RRT compared to late RRT on subjects’ outcome.
Approval of the medical ethics committee of Faculty of Medicine was obtained before conducting the study (serial number 0201633). The trial is also registered in Clinicaltrials.gov identifier NCT05382598. Available at https://clinicaltrials.gov/study/NCT05382598. An informed written consent was obtained from the subject’s next of kin before their enrollment in the study.
2. Subjects
After statistical analysis of a previous study addressing a similar research issue, we used Mann–Whitney U test for non-parametric adjustment at level of significance (0.05) to calculate the required minimal sample size, which was found to be 90 subjects (45 per group) [Citation17]. This was to achieve 80% power to detect difference in the mean ICU stay between early RRT group (11 ± 4 days) and delayed RRT group (13.5 ± 4 days). This sample size was calculated using PASS 12 program [Citation18].
The selection of participants for this study adhered to specific inclusion and exclusion criteria. Inclusion criteria encompassed adult patients aged above 18 years who had stage 2 AKI according to KDIGO classification [Citation19]. Subjects were on invasive mechanical ventilation at the initiation of RRT. The study comprised individuals who either presented with AKI upon their ICU admission or developed AKI during their ICU stay.
Notably, only subjects failing to respond to a furosemide stress test after volume status optimization were included. This was done through the administration of a bolus of 1 mg/kg furosemide (provided that the patient was furosemide naive) or 1.5 mg/kg furosemide otherwise. If more than 200 ml urine was produced within two hours that implied positive (successful) test (patient was excluded from the study). The presence of a negative furosemide stress test (less than 200 ml produced urine) was used as a prerequisite for subjects’ recruitment [Citation16]. Before the trial of furosemide stress test, clinical and ultrasonographic signs were checked to exclude hypovolemia. Central venous pressure measurements should be more than 8 mmHg if PEEP is ≥ 5. In addition, inferior vena cava diameter >1.5 cm with collapsibility index < 50% [Citation20].
Conversely, exclusion criteria were meticulously defined to exclude individuals with specific characteristics. Subjects known to have grade 5 CKD according to KDIGO classification were excluded, while those with CKD grades 1 to 4 were included only if they developed or presented with stage 2 AKI on top of their existing CKD grade [Citation19,Citation21]. Furthermore, individuals with AKI resulting from obstructive or traumatic causes were excluded. The study also excluded patients with septic shock who were on high doses of vasopressors or inotropes (norepinephrine infusion more than 1 mcg/kg/minute, dopamine, or dobutamine infusion more than 5 mcg/kg/minute). Lastly, pregnant female subjects were also excluded from the study.
3. Methods
3.1. Subdivision of patients and randomization technique
After evaluation of 143 subjects for eligibility criteria, especially failure to respond to furosemide stress test, 94 subjects were included in our study. Demographic data, such as age and gender, were recorded. In addition, medical comorbidities, causes of ICU admission, indications of mechanical ventilation and the reasons for AKI were all documented.
Subjects were randomized into two groups, each one consisted of 47 subjects. Computer-based simple randomization technique was used to identify which of the 94 subjects would receive either early or late RRT.
In the early RRT group, RRT was initiated once the mechanically ventilated subject presented with or developed stage 2 AKI, without waiting for presence of urgent indications of hemodialysis. While in the late RRT group, subjects received RRT only after developing any of the urgent indications necessitating hemodialysis. These indications included severe hyperkalemia (>6.5 mEq/L) and oliguria with an inadequate response to diuretics, particularly in the presence of life-threatening pulmonary edema requiring elevated ventilator settings (i.e. PEEP > 10 and FiO2 > 50%). Additionally, patients exhibiting severe metabolic acidosis with a pH below 7.15, along with those presenting with uremic pericarditis, encephalopathy, or coagulopathy, were deemed eligible for RRT.
All subjects received RRT in the form of intermittent hemodialysis (IHD) using average blood pump flow of 200–250 ml/minute. Average duration of each hemodialysis session was around 4 – 6 hours per session. Average amount of ultrafiltration per session was around 2.2–2.3 liters per session.
3.2. Outcome measures
The primary endpoints included ICU mortality at 28 days as well as ICU length of stay. The secondary endpoints encompassed the duration of mechanical ventilation for surviving subjects, levels of urea and creatinine at discharge from the ICU, recovery of renal function (RRF), and RRT dependency on discharge. Additionally, the study delved into respiratory parameters, examining average lung compliance both before and after dialysis sessions. Moreover, the average hypoxic index (PaO2/FiO2) was reported before and after dialysis sessions. Average daily urine output (UOP) was also recorded.
As regards RRF and RRT dependency after discharge, we considered full recovery from AKI as the absence of AKI criteria. Partial recovery was considered to be a fall in AKI stage. Non-recovery had the same meaning as RRT dependency, which denoted the need for RRT twice or more per week after discharge [Citation22,Citation23].
3.3. Statistical analysis
The statistical analysis of the data was conducted utilizing IBM SPSS software package version 20.0 (Armonk, NY: IBM Corp) [Citation24]. Qualitative data were presented using numbers and percentages, while the normality of distribution for quantitative data was assessed through the Shapiro–Wilk test. Descriptive statistics for quantitative data included the range (minimum and maximum), mean, standard deviation, median, and interquartile range (IQR). The significance of the obtained results was evaluated at the 5% level. Various statistical tests were employed based on the nature of the data: the Chi-square test for categorical variables to compare between different groups, the Student t-test for normally distributed quantitative variables to compare between two studied groups, and the Mann–Whitney test for non-normally distributed quantitative variables when comparing between two studied groups.
4. Results
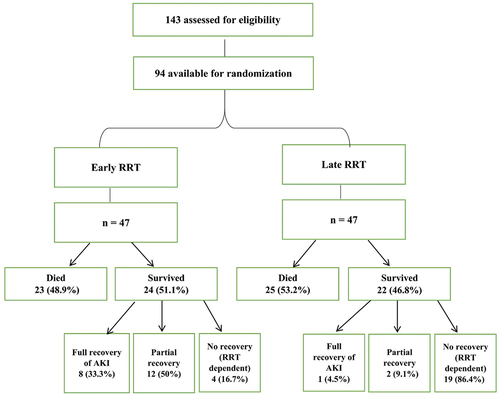
A total of 143 subjects were evaluated for the study. About 49 subjects were excluded after they showed positive (successful) response to furosemide stress test. The remaining 94 subjects were randomized into two groups according to timing of RRT initiation ().
4.1. Baseline data
Both groups were comparable in terms of their demographic variables, i.e. age and gender. In addition, there was no significant discrepancy in the incidence of different medical co-morbidities, causes of ICU admission or indications for invasive mechanical ventilation between the two groups (). Sepsis was the most common cause of AKI in both groups. In addition, causes of AKI did not differ significantly in both groups. There was no significant difference of the incidence of complications during RRT sessions in both groups (). Severe metabolic acidosis was the most common indication for RRT in the late group (61.7%), followed by life-threatening hyperkalemia (19.1%).
Table 1. Comparison between the two studied groups according to baseline parameters.
Table 2. Comparison between the two studied groups according to the cause of AKI, complications during RRT, lung compliance, and hypoxic index after hemodialysis.
4.2. Primary outcomes
As shown in and , ICU mortality and ICU length of stay were comparable in both groups. 48.9% of the early RRT group died, whereas the mortality rate in the late group was 53.2% (p = .680). The average ICU length of stay for survived patients in the early group was 8.5 days compared to 6 days in the late group (p = .457).
Table 3. Comparison between the two studied groups according to outcomes.
4.3. Secondary outcomes
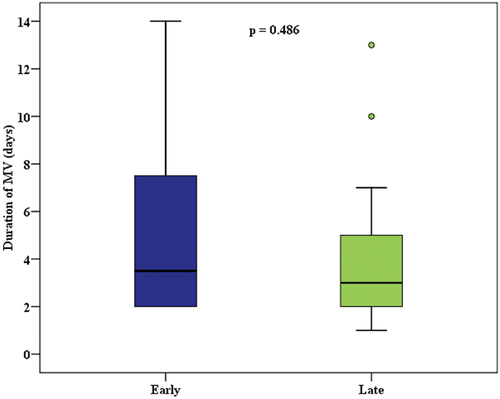
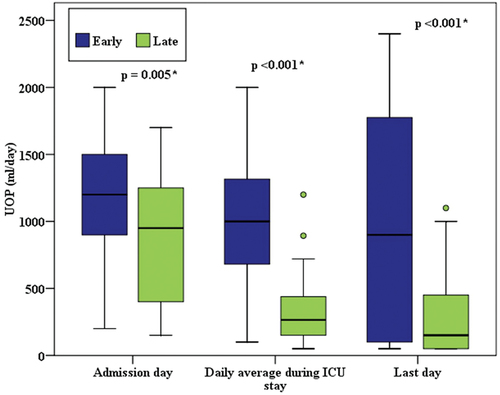
Duration of mechanical ventilation, average lung compliance, and hypoxic index before and after hemodialysis did not differ significantly in both groups ( and ). However, a significantly higher rate of renal function recovery and a lower incidence of RRT dependency were observed in the early group (p = <.001). 33.3% of the survived patients of the early group had full recovery of AKI compared to 4.5% in the late group (). 86.4% of the late RRT group were RRT dependent after discharge from ICU compared to 16.7%of the early group. Early RRT group had significant improvement of the amount of urine output (), as well as significantly lower levels of urea and creatinine on discharge from ICU and ().
5. Discussion
This RCT was conducted on 94 mechanically ventilated patients who had AKI. After volume status optimization, we included patients who failed to respond to a trial of furosemide stress test. This was a unique feature of our study. This test was performed to predict which type of AKI patients might benefit from RRT initiation, and it allowed those with adequate response to diuretics to avoid RRT hazards and continue conservative measures. It also predicted who are less likely to recover from AKI and might eventually need RRT. Our hypothesis was that its addition to the evaluation may lead to better selection of patients leading to better outcomes in the early group. However, its use did not lead to improved mortality, length of stay, or duration of mechanical ventilation in the early group, but better outcome was noticed in recovery of renal function in the survived patients.
Regarding primary outcomes, we found that there was no statistically significant difference in mortality or length of stay between early and late RRT (). These outcomes are similar to many previous trials which were reported in several systematic reviews and meta-analyses [Citation12,Citation14,Citation15,Citation25–28].
A recent study had used similar approach of subjects’ selection by using furosemide stress test to exclude responsive subjects from randomization. The criteria for RRT initiation in their study was slightly different from ours. They initiated RRT in the early group after six hours of randomization whereas the other group of patients were subjected to RRT when any of the following conditions were met: blood urea nitrogen levels equal to or exceeding 100 mg/dL, serum potassium surpassing 6 mmol/L, serum bicarbonate falling below 12 mmol/L or pH dropping below 7.15, PaO2/FiO2 ratio falling below 200, or the chest radiograph showing signs consistent with pulmonary edema. However, there were no discernible distinctions in 28-day mortality rates (62.1% versus 58.3%, p = .68) or the RRT dependency by day 28. In addition, no significant difference was identified in terms of ICU length of stay or mechanical ventilation free days [Citation29].
Castro et al., conducted a meta-analysis which involved 13 different trials on 5193 ICU patients. The combined mortality rates were 37.18% for early RRT and 37.15% for late RRT. No statistically significant difference was observed between the two groups (RR 1.00; 95% CI 0.89 to 1.12; p = 1.00) [Citation25].
Another meta-analysis, by Gaudry et al., retrieved data from nine studies (2083 patients). No significant differences were found between the two groups as regards mortality or duration of hospital stay [Citation12]. In addition, neither Besen et al. nor Yang et al., in their systematic reviews, have detected any significant difference between early and late RRT groups in terms of mortality or ICU length of stay [Citation14,Citation15].
A combined examination of well-conducted observational studies and RCTs reveals comparable outcomes in terms of mortality. The advantage of decreased mortality associated with the early commencement of RRT appears to be constrained to studies of lower quality. It is crucial to note that these findings are contingent upon significant variations and potential publication bias, particularly within studies characterized by a high risk of bias [Citation14].
Zarbock’s study (The ELAIN-Trial) stands out among randomized studies with a low risk of bias, demonstrating a decrease in mortality, reduced time on mechanical ventilation, and shorter hospital length of stay. However, a notable drawback is the baseline imbalance between the study groups [Citation26]. Additionally, the study protocol outlined an adjusted mortality comparison using a Cox model, but this analysis was not presented. The study’s fragility index is 3, indicating that only 3 events influenced the final outcome [Citation14]. Ultimately, the ELAIN trial’s results could be attributed to the higher baseline severity of the late initiation group for continuous renal replacement therapy (CRRT) [Citation27].
In a recent systematic review, which investigated four studies [Citation28,Citation30–32] that addressed the duration of ICU length of stay, combined findings revealed no statistically significant variances in the length of ICU stay between the initiation of early RRT and delayed RRT (mean difference [MD] — 0.94; 95% confidence interval [CI] —2.43—0.55; p = .22; I2 = 0%). This suggests that the timing of commencing RRT was not associated with the duration of ICU stay [Citation33].
The duration of intensive care unit (ICU) stay is impacted by a confluence of medical, social, psychological, and institutional determinants. Specifically, within the realm of medical considerations, a prolonged reliance on mechanical ventilation within the initial 24 hours following admission to the ICU, irrespective of the existence of infection, was indicative of an extended hospitalization period. Moreover, the severity of the underlying disease was identified as an additional factor influencing the length of stay in the ICU [Citation34–36].
Regarding the secondary endpoints, we did not detect any statistically notable difference in the impact of RRT timing on duration of mechanical ventilation (). Multiple systemic reviews of a variety of studies have also failed to provide significant evidence on the effect of timing of RRT on weaning off mechanical ventilation [Citation12,Citation14,Citation15]. Previous studies has demonstrated that achieving a negative volume balance may be associated with a higher likelihood of successful weaning [Citation37]. However, the length of mechanical ventilation depends mainly on the initial diagnosis and the extent of physiological abnormalities [Citation38]. This is why timing of RRT might not affect the duration of the mechanical ventilation.
A prospective cohort study was conducted on mechanically ventilated patients with AKI, to assess for impact of hemodialysis on static lung compliance and hypoxic index (PaO2/FiO2 ratio). It was revealed that hemodialysis has significantly improved lung mechanics and oxygenation (p < .001). However, timing of RRT initiation was not investigated [Citation39]. In our study, there was no statistically significant difference in these post-dialysis parameters between early and late groups (). This might also explain why there was no significant difference between both groups in terms of duration of mechanical ventilation.
As an indicator of potential benefit from early RRT, we found that early RRT has a statistically remarkable impact on RRF and less incidence of RRT dependency after discharge from ICU (p = 0.003 and p = < .001 respectively) (). This was contradictory to the previous findings of the meta-analysis conducted by Bhatt et al. which noted that incidence of RRT dependency on day 90 was significantly higher in the early RRT group (RR: 1.55; 95% CI: 1.15, 2.09; moderate certainty of evidence) [Citation40]. The authors of the STARRT-AKI trial proposed that prolonged exposure to RRT in individuals with preexisting conditions, coupled with adverse events like hypotension and electrolyte imbalance during RRT, were significant factors contributing to the delay in renal recovery and the restoration of renal functions in early RRT group. Additionally, the inclusion of a substantial proportion of patients with CKD (approximately 44%) in the trial, a population more prone to hypotensive episodes, may have played a role in the increased reliance on dialysis in the early RRT group [Citation41].
However, Gaudry et al. detected no significant change between both groups in RRT dependency after discharge from hospital. In addition, Lin et al. and Yang et al. found in their systemic reviews that timing of RRT did not significantly affect neither RRF nor RRT dependency for survived subjects [Citation12,Citation15,Citation42]. In our study, the use of furosemide stress test might be the reason why we detected a benefit from early RRT on renal function recovery as this test excluded subjects who may have unnecessarily had done hemodialysis.
From a theoretical point of view, early RRT would be associated with unnecessary risk of dialysis-related complications. However, we did not find any significant difference in the incidence of hemodialysis complications between the two groups (). Previous systematic reviews did not find any significant relationship between timing of RRT initiation and incidence of RRT-related complications [Citation12,Citation15]. However, Castro et al. found that early RRT was associated with significantly higher incidence of both hypotension and RRT-related infection [Citation25]. In addition, Bhatt et al. have also found that incidence of hypotension and hypophosphatemia was significantly higher in the early RRT group [Citation40].
Our study exhibited several strengths, such as the use of furosemide stress test for selection of subjects to exclude those who might benefit from conservative management. We carried out our study only on mechanically ventilated subjects and assessed also the parameters of respiratory mechanics before and after hemodialysis to check whether early or late hemodialysis would have significant effect on these parameters.
We faced some limitations in our study such as the use of a single modality of RRT. Unfortunately, we did not use CRRT due to financial limitations. This would have enabled us to observe whether the mode of RRT had any impact on mortality or morbidity, especially RRT-related complications. In addition, this study was conducted at a single center and had a limited sample size. A larger sample size would have enabled us to more accurately determine the average values of the data, reducing the risk of errors associated with testing a small number of potentially atypical samples.
6. Conclusion
Even after exclusion of furosemide stress test successful responders, there was no significant difference between early or late initiation of RRT as regards ICU mortality, ICU length of stay, duration of mechanical ventilation, lung compliance, or oxygenation. However, survived subjects in the early RRT group showed better recovery of renal function and significantly less dependence on RRT after discharge.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Amr M. Elsharkawy
Amr M. Elsharkawy, MSc, EDIC Assistant lecturer of critical care medicine, Alexandria Faculty of medicine, Egypt. Literature search, Data collection, Study design, Manuscript preparation.
Yasmine S. Naga
Yasmine S. Naga, MSc, PHD Assistant Professor of internal medicine and nephrology, Alexandria Faculty of medicine, Egypt Study design, Analysis of data, Manuscript preparation.
Mohamed M. Megahed
Mohamed M. Megahed, MSc, PHD Professor of critical care medicine, Alexandria Faculty of medicine, Egypt. Review of manuscript.
Amr A. El-Sayed
Amr A. El-sayed, MSc, PHD Professor of critical care medicine, Alexandria Faculty of medicine, Egypt.
References
- Bellomo R. The epidemiology of acute renal failure: 1975 versus 2005. Curr Opin Crit Care. 2006;12(6):557–560. doi: 10.1097/01.ccx.0000247443.86628.68
- Susantitaphong P, Cruz DN, Cerda J. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–1493. doi: 10.2215/CJN.00710113
- Uchino S. The epidemiology of acute renal failure in the world. Curr Opin Crit Care. 2006;12(6):538–543. doi: 10.1097/01.ccx.0000247448.94252.5a
- Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282
- Xue JL, Daniels F, Star RA. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–1142. doi: 10.1681/ASN.2005060668
- Waikar SS, Curhan GC, Wald R. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17(4):1143–1150. doi: 10.1681/ASN.2005091017
- Hoste EA, Bagshaw SM, Bellomo R. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7
- Bonventre JV. Dialysis in acute kidney injury–more is not better. N Engl J Med. 2008;359(1):82–84. doi: 10.1056/NEJMe0803765
- Bonventre JV, Basile D, Liu KD. AKI: a path forward. Clin J Am Soc Nephrol. 2013;8(9):1606–1608. doi: 10.2215/CJN.06040613
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394(10212):1949–1964. doi: 10.1016/S0140-6736(19)32563-2
- Shingarev R, Wille K, Tolwani A. Management of complications in renal replacement therapy. Semin Dial. 2011;24(2):164–168. doi: 10.1111/j.1525-139X.2011.00828.x
- Gaudry S, Hajage D, Benichou N. Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: a systematic review and individual patient data meta-analysis of randomised clinical trials. Lancet. 2020;395(10235):1506–1515. doi: 10.1016/S0140-6736(20)30531-6
- Wald R, Bagshaw SM. The timing of renal replacement therapy initiation in acute kidney injury: is earlier truly better?*. Crit Care Med. 2014;42(8):1933–1934. doi: 10.1097/CCM.0000000000000432
- Besen B, Romano TG, Mendes PV. Early versus late initiation of renal replacement therapy in critically Ill patients: systematic review and meta-analysis. J Intensive Care Med. 2019;34(9):714–722. doi: 10.1177/0885066617710914
- Yang XM, Tu GW, Zheng JL. A comparison of early versus late initiation of renal replacement therapy for acute kidney injury in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. BMC Nephrol. 2017;18(1):264. doi: 10.1186/s12882-017-0667-6
- McMahon BA, Chawla LS. The furosemide stress test: current use and future potential. Ren Fail. 2021;43(1):830–839. doi: 10.1080/0886022X.2021.1906701
- Wald R, Adhikari NK, Smith OM. Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88(4):897–904. doi: 10.1038/ki.2015.184
- NCSS Statistical software. Pass 12. Kaysville (UT), USA: NCSS, LLC; 2013.
- Kellum JA, Lameire N, Aspelin P. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. KI Supplements. 2012;2(1):1–138.
- Stawicki SP, Braslow BM, Panebianco NL. Intensivist use of hand-carried ultrasonography to measure IVC collapsibility in estimating intravascular volume status: correlations with CVP. J Am Coll Surg. 2009;209(1):55–61. doi: 10.1016/j.jamcollsurg.2009.02.062
- Cheung AK, Chang TI, Cushman WC. KDIGO 2021 Clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3):S1–s87. doi: 10.1016/j.kint.2020.11.003
- Forni LG, Darmon M, Ostermann M. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855–866. doi: 10.1007/s00134-017-4809-x
- Macedo E, Bouchard J, Mehta RL. Renal recovery following acute kidney injury. Curr Opin Crit Care. 2008;14(6):660–665. doi: 10.1097/MCC.0b013e328317ee6e
- Kirkpatrick LA. A simple guide to IBM SPSS statistics : for version 20.0. Belmont, Calif: Wadsworth Cengage Learning; 2013.
- Castro I, Relvas M, Gameiro J. The impact of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury on mortality and clinical outcomes: a meta-analysis. Clin Kidney J. 2022;15(10):1932–1945. doi: 10.1093/ckj/sfac139
- Zarbock A, Kellum JA, Schmidt C. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically Ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315(20):2190–2199. doi: 10.1001/jama.2016.5828
- Zarbock A, J G, Van Aken H. Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury (The ELAIN-Trial): study protocol for a randomized controlled trial. Trials. 2016;17(1):148. doi: 10.1186/s13063-016-1249-9
- Baek SD, Yu H, Shin S. Early continuous renal replacement therapy in septic acute kidney injury could be defined by its initiation within 24 hours of vasopressor infusion. J Crit Care. 2017;39:108–114. doi: 10.1016/j.jcrc.2016.12.014
- Lumlertgul N, Peerapornratana S, Trakarnvanich T. Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit Care. 2018;22(1):101. doi: 10.1186/s13054-018-2021-1
- Barbar SD, Clere-Jehl R, Bourredjem A. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379(15):1431–1442. doi: 10.1056/NEJMoa1803213
- Chon GR, Chang JW, Huh JW. A comparison of the time from sepsis to inception of continuous renal replacement therapy versus RIFLE criteria in patients with septic acute kidney injury. Shock. 2012;38(1):30–36. doi: 10.1097/SHK.0b013e31825adcda
- Shum HP, Chan KC, Kwan MC. Timing for initiation of continuous renal replacement therapy in patients with septic shock and acute kidney injury. Ther Apher Dial. 2013;17(3):305–310. doi: 10.1111/j.1744-9987.2012.01147.x
- Wang Q, Liu F, Tao W. Timing of renal replacement therapy in patients with sepsis-associated acute kidney injury: A systematic review and meta-analysis. Aust Crit Care. 2023;27(1). doi: 10.1186/s13054-023-04555-x
- Gruenberg DA, Shelton W, Rose SL. Factors influencing length of stay in the intensive care unit. Am J Crit Care. 2006;15(5):502–509. doi: 10.4037/ajcc2006.15.5.502
- Higgins TL, Wt M, Steingrub JS. Early indicators of prolonged intensive care unit stay: impact of illness severity, physician staffing, and pre-intensive care unit length of stay. Crit Care Med. 2003;31(1):45–51. doi: 10.1097/00003246-200301000-00007
- Zimmerman JE, Kramer AA, Ds M. Intensive care unit length of stay: benchmarking based on Acute Physiology and Chronic Health Evaluation (APACHE) IV. Crit Care Med. 2006;34(10):2517–2529. doi: 10.1097/01.CCM.0000240233.01711.D9
- Upadya A, Tilluckdharry L, Muralidharan V. Fluid balance and weaning outcomes. Intensive Care Med. 2005;31(12):1643–1647. doi: 10.1007/s00134-005-2801-3
- Seneff MG, Zimmerman JE, Knaus WA. Predicting the duration of mechanical ventilation. The importance of disease and patient characteristics. Chest. 1996;110(2):469–479. doi: 10.1378/chest.110.2.469
- de Almeida CP, Ponce D, Balbi AL. Effect of hemodialysis on respiratory mechanics in acute kidney injury patients. Hemodial Int. 2019;23(1):101–105. doi: 10.1111/hdi.12684
- Bhatt GC, Das RR, Satapathy A. Early versus lAte initiation of renal replacement therapy: Have we reached the consensus? an updated meta-analysis. Nephron. 2021;145(4):371–385. doi: 10.1159/000515129
- Bagshaw SM, Wald R, Adhikari NKJ. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. 2020;383(3):240–251.
- Lin WT, Lai CC, Chang SP. Effects of early dialysis on the outcomes of critically ill patients with acute kidney injury: a systematic review and meta-analysis of randomized controlled trials. Sci Rep. 2019;9(1):18283. doi: 10.1038/s41598-019-54777-9