ABSTRACT
Objective
To determine the effects of Dextrose 5% for maintenance and creep fluids on cumulative fluid balance (CFB) for septic shock concerning adult people.
Design
An open-label randomized clinical trial with a single center and parallel groups.
Setting
ICUs of a tertiary care academic medical center.
Patients
Adult individuals who have developed septic shock as described by the Sepsis 3 criteria.
Interventions
Dextrose 5% (n = 50) versus Saline 0.9% (n = 50) for maintenance and creep fluid for 72 hours.
Main variables of interest
The primary outcome measured was the CFB at 72 hours. Secondary outcomes assessed included mortality within 28 days, the number of days patients were free from (coma and delirium, ICU, mechanical ventilation, vasopressors), the occurrence of acute kidney injury, and disturbances in electrolyte levels.
Results
The CFB was significantly lower in Dextrose group vs Saline group (4.46 versus 6.75 L, median difference; −2.06 L; 95% CI, −2.63 to −1.35; p = P < 0.001). No significant difference mortality within 28 days (32% in the Dextrose vs 42% in the saline group; hazard ratio, 0.7; 95% CI, 0.36 to 1.34, p = 0.28). The proportions of patients with hyponatremia were higher in the Dextrose than the Saline group (31% versus 0%, p < 0.001).
Conclusions
Particularly in cases of septic shock, maintenance and creep fluids with Dextrose 5% as compared to Saline 0.9% resulted in a significantly lower CFB at 72 hours.
1. Introduction
The most common causes of death worldwide are sepsis and septic shock, accounting for more than 48 million episodes and 11 million deaths per year with Low- and middle-income countries (LMICs) account for over 85% of sepsis cases and deaths caused by sepsis [Citation1].
Intravenous (IV) fluid resuscitation is the first-line therapy for the counteraction of sepsis-induced tissue hypoperfusion [Citation2]. On the one hand, unfavorable patient outcomes in sepsis and other critical illnesses have been linked to fluid overload and positive cumulative fluid balance (CFB) with a positive correlation between the amount of CFB and mortality [Citation3] Because of this, people are looking for IV fluid strategies that are less risky. Regrettably, randomized, controlled studies assessing the efficacy of hydration restriction in people presenting with sepsis showed no improvement in patient outcomes [Citation4,Citation5]. Most studies and clinical guidelines on fluid volume management have focused at resuscitation fluids [Citation2,Citation4,Citation5] in contrast, a wide range of non-resuscitation fluids are encountered by critically ill patients, including replacement fluids, (to replace enteral and drain losses), nutrition fluids (to cover daily caloric requirements), maintenance fluids (to fulfill the daily requirements for hydration and electrolytes) as well as fluids as vehicle for medications and to keep lines open (fluid creep) [Citation6].
Maintenance and creep fluids constitute a significant portion of the overall volume of fluids consumed by critical ill patients, amounting to two-thirds or more and impose a considerable burden in terms of fluid and sodium [Citation7–10]. The selection of fluid type is particularly noteworthy. A prospective before-and-after study demonstrated that adopting Dextrose 5% as the standard maintenance fluid and as a carrier for drug infusion and boluses led to a decrease in the amount of salt supplied to critically ill individuals. This change was accompanied by enhanced urine output and a reduction in positive fluid balance [Citation11].
We conducted the Dextrose as a Maintenance fluid in Septic shock (DEMANDS) trial for assessing the efficacy of dextrose 5% for maintenance and creep fluids on cumulative fluid balance and other medical findings concerning adults diagnosed with septic shock in the ICU.
2. Methods
2.1. Experimental design and study setting
The DEMANDS study was a unicentric, open label, parallel group, randomized controlled trial. The research was conducted at a tertiary academic healthcare institution, namely the Alexandria Main University Hospital, situated in Egypt. Prior to commencement, the study was prospectively registered with the Pan African Clinical Trials Registry (PACTR202207871325490, July 11, 2022). Ethical clearance for the study protocol was granted by the Alexandria Faculty of Medicine Ethics Committee (IRB NO; 00012098) on January 21 2021 (serial number 0201447), and all participants or their legal representatives provided written informed consent.
3. Patients
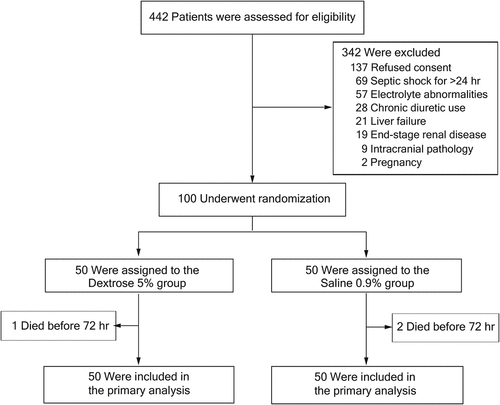
The study enrolled adult individuals (18 years or more) who were admitted to the ICU and met the diagnostic criteria for septic shock, characterized by suspected or confirmed infectious etiology, a plasma lactate level of ≥2 mmol/L, and the necessity for continuous vasopressor infusion therapy [Citation12]. Eligibility for participation was contingent upon the onset of shock occurring within the 24-hour period preceding the screening process.
The exclusion criteria were; chronic diuretic use, end-stage renal failure, liver failure, pregnancy, severe heart failure, patients with intracranial pathology (eg; traumatic brain injury, stroke), and electrolyte disorders (Individuals exhibiting a serum sodium concentration deviating from the range of 130 to 150 mmol/L or a serum potassium level falling outside the boundaries of 3 to 5 mmol/L).
3.1. Randomization
Eligible participants were allocated randomly in equal proportions to one of two parallel treatment arms (1:1 ratio) through a computerized random number generation process. The randomization sequence was concealed in consecutively numbered, opaque, sealed envelopes that were opened following the recruitment of each patient. Participants were assigned to receive either a 5% dextrose solution or 0.9% sodium chloride solution. This was an open-label study design, with no masking of treatment allocation for patients, clinicians, or investigators involved.
Initial resuscitation in ER was according to surviving sepsis campaign then patients were randomized one of two arms.
Half of the infusion fluids given in the ICU were given in the form of water; D5%. The other half of the infusion fluids were given in the form of NS. Once resuscitation targeted parameter are achieved (MAP >70 mmHg for at least three hours & lactate clearance), all fluids given e.g. vehicle of drug infusion, maintenance or even boluses were given in the form of water; D5% for first 72 hours in the ICU. The treating clinicians determined the volume and infusion rate of each individual patient.
Received an intravenous infusion of 0.9% NaCl as the routine maintenance fluid and as a vehicle of drug infusion and boluses during the first three days in ICU. The treating clinician determined the volume and infusion rate of each fluid.
Other fluids (resuscitation, nutrition, and blood products) are prescribed at the discretion of the healthcare provider.
3.2. Data collected
Baseline patient characteristics collected consisted of age, sex, Acute Physiology and Chronic Health Evaluation (APACHE) II score [Citation13], Sepsis-related Organ Failure Assessment (SOFA) score [Citation14], Charlson comorbidity index [Citation15], and source of sepsis. The data for the type of fluids, total input, and output were collected in the enrolled patients over 72 hours after randomization. Samples for serum electrolytes, creatinine, blood gases, and lactate were collected at following time points: 24, 48, and 72 hours, as well as at any time between these time points at the discretion of the treating team. Treatment with vasopressors and mechanical ventilation was also recorded daily until day 28. Delirium was evaluated using the Confusion Assessment Method for the ICU (CAM-ICU) [Citation16] twice during each 12 h shift.
3.3. Outcome measures
The cumulative fluid balance at 72 hours post-randomization was the primaryendpoint of this investigation, found by subtracting the total fluid output (urinary output, drain outputs, and ultrafiltration) from the entire fluid input (intravenous and enteral). Insensible losses were not accounted for in this calculation. Secondary clinical outcomes assessed included 28-day mortality, change in Sequential Organ Failure Assessment (SOFA) score from baseline to 72 hours (delta-SOFA), ventilator-free days, vasopressor-free days, coma-free and delirium-free days, occurrence of acute kidney injury defined as Acute Kidney Injury Network (AKIN) score [Citation17] of 1 or higher, requirement for renal replacement therapy, intensive care unit length of stay, hyponatremia (<135 mmol/L), hypernatremia (>145 mmol/L), hyperchloremia (>109 mmol/L), and hypochloremia (<98 mmol/L).
3.4. Statistical analysis
Assuming a cumulative fluid balance at 72 hours of 5 ± 2.5 L [Citation18]. A power calculation indicated that 90 patients (45 per group) would provide 80% power to detect a 1.5-liter difference in the primary outcome, with a two-sided alpha of 0.05. To account for a 10% dropout rate, the target was set at 100 participants.
Categorical variables were summarized as frequencies and proportions, with comparisons made using Pearson’s chi-square or Fisher’s exact test. Effect sizes for categorical outcomes were reported as absolute differences with 95% confidence intervals (CI). Continuous data were presented as medians with interquartile ranges (IQR) and analyzed using the Mann-Whitney U test. Effect sizes were quantified by median differences and 95% CIs [Citation19]. Survival analyses used Kaplan-Meier and log-rank tests, adhering to the intention-to-treat principle, with statistical significance set at p < 0.05. Analyses were conducted with MedCalc® software (version 20.019, Ostend, Belgium).
4. Results
4.1. Trial population
Between August 20 2022, and October 7 2023, we recruited 100 patients, with 50 assigned to the dextrose group and 50 to the saline group (). All randomized participants were monitored until they were discharged from the hospital. Baseline characteristics of the patients were largely similar across the two groups ().
Table 1. Characteristics of the patients at baseline.
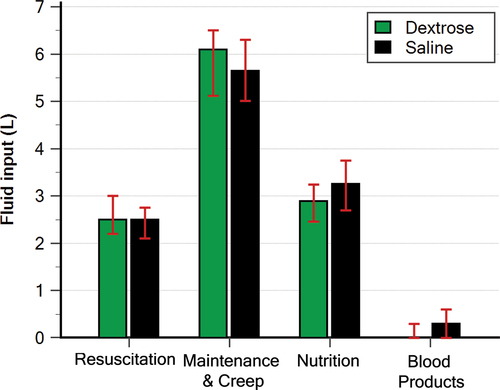
Patients in both groups received the same amount of resuscitation fluid, maintenance and creep fluid, and blood products. Whereas nutrition fluid was less in the Dextrose group than the saline group (2.89 L versus 3.25 L; p = 0.012; median difference; −0.35 L; 95% CI, −0.61 to −0.08; ). None (0%) of the patients in this trial received de-resuscitation treatment (diuretics or ultrafiltration) during the intervention period.
4.2. Primary outcome
The CFB was significantly lower in Dextrose group vs Saline group (4.46 versus 6.75 L, median difference; –2.06 L; 95% CI, −2.63 to −1.35; p < 0.001) (, and ). The median cumulative volume of fluid received comparable between the two groups (11.7 L; IQR, 10.8–12.1 L in the Dextrose group and 11.9 L; IQR, 11–12.5 L in the Saline group; p = 0.16; ). The difference in balance between the two groups was derived by a higher output in the Dextrose group ().
Figure 3. Cumulative fluid input, output, and balance. Patients in both groups received similar amount of fluid input (A) the higher fluid output in the dextrose group(B) derived the difference in fluid balance between the two groups(C).
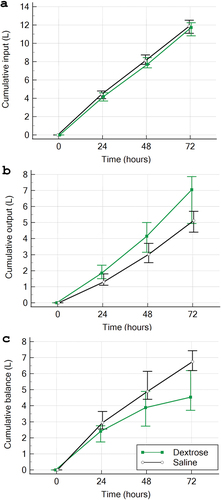
Table 2. Outcomes.
4.3. Secondary outcomes
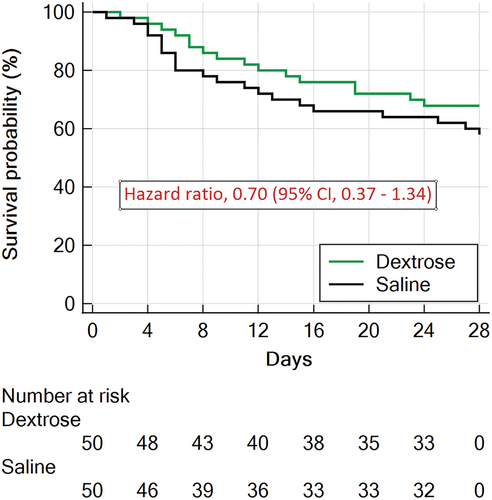
By the 28-day point, 16 (32%) patients in the dextrose group and 21 (42%) patients in the saline group had experienced mortality (hazard ratio, 0.7; 95% CI, 0.36 to 1.34, p = 0.28) (, ). In the overall cohort, no statistically significant differences were observed between the treatment groups in terms of the change in Sequential Organ Failure Assessment (SOFA) score from baseline to 72 hours (delta-SOFA score), ventilator-free days, vasopressor-free days, coma-free and delirium-free days, incidence of acute kidney injury (defined as an Acute Kidney Injury Network [AKIN] score ≥ 1), requirement for renal replacement therapy, or intensive care unit length of stay ().
Figure 4. Survival curves censored at day 28 for the two groups in the intention-to-treat population.
Participants allocated to the dextrose solution arm exhibited a significantly higher prevalence of hyponatremia (31% versus 0%; difference, 31%; 95% CI 17–45%; p < 0.001) and hypochloremia (22% versus 0%; difference, 20%; 95% CI 7–34%; p = 0.002) compared to those in the saline solution group. Conversely, the saline solution group demonstrated a significantly higher incidence of hypernatremia (17% versus 0%; difference, 8%; 95% CI 1–19%; p = 0.003) and hyperchloremia (75% versus 1%; difference, 74%; 95% CI 58–84%; p < 0.001) relative to the dextrose solution group ().
5. Discussion
Adults hospitalized (ICU) with septic shock were the subjects of this randomized, open-label clinical study, using dextrose 5% for maintenance and creep fluids reduced cumulative fluid balance at 72 hours compared to saline 0.9%with more frequent hyponatremia which is mostly mild.
Excessive fluid accumulation has long been recognized as the primary culprit behind iatrogenic fluid overload, garnering significant attention in perioperative and critical care medicine research. Nonetheless, an equally crucial yet often overlooked factor is the quantity of sodium administered [Citation20]. Maintenance When patients are unable to take in fluids or meals orally, their daily hydration and electrolyte needs are usually met using intravenous fluid solutions [Citation21]. Among these, It is usual practice to use 0.9% saline as the fluid, serving not only as a maintenance fluid but also as a diluent for medications [Citation8]. The preference for isotonic fluids like 0.9% saline in maintenance therapy stems from the apprehension of hyponatremia, particularly in pediatric patients. This apprehension has led to the widespread adoption of isotonic fluids in adult care as well [Citation21]. Serum sodium levels differed significantly between the two therapies, as shown by our study’s secondary outcomes; more patients in the first group had levels <135 mmol/L. Regardless, doctors didn’t stop the trial because patients were showing signs of hyponatremia, which raises the intriguing possibility that adults don’t experience the same kinds of problems as children. In the single randomized controlled trial comparing isotonic and hypotonic maintenance fluids in adults, no disparity was found in the incidence of significant hyponatremia between the groups. However, Clinical fluid overload was more common in the group given isotonic fluid [Citation8].When deciding between isotonic sodium-rich fluid and hypotonic fluid, it is important to consider the possibility of hyponatremia, which is usually mild and asymptomatic, as well as the risk of volume overload.
Patients in the Dextrose group showed a numerically lower mortality and a trend toward better organ dysfunction measures delta SOFA, days free of (ventilator, vasopressor, coma, delirium) that did not meet statistical significance, but the clinical significance warrants further investigation.
Our study has several strengths. First, the complete data collection, intention to treat analysis, and zero loss of follow-up. Second, to our knowledge, this trail is the first RCT to address the issue of maintenance and creep fluids concerning septic shock critically ill patients. Third, the decision-making about patients’ overall treatment, including fluid amount, was delegated to the physicians, and the protocol was designed to be practical in order to mirror standard care. Forth, the trial was conducted in low- middle-income setting, where sepsis burden is considerably higher and for which data were under-represented in the literature [Citation1].
This investigation is limited by; Firstly, the unicentric nature of the study design raises concerns regarding the extrapolation and broader applicability of the findings. Secondly, the open-label approach adopted in this trial, wherein patients and study personnel were cognizant of the treatment group allocations, introduces the potential for performance bias to influence the observed outcomes. Third, we chose an intervention period of 72 hours for fluid administration and balance assessment. Administration for a longer time might have more pronounced effects on balance and electrolytes. However, the timepoint at which There is a substantial correlation between fluid balance and death is 72 hours. [Citation3] Lastly, there was insufficient power to detect statistically significant variations in mortality and other clinical consequences. While, Dextrose appeared to reduce cumulative fluid balance, the clinical relevance requires investigations in subsequent studies.
6. Conclusions
In a randomized unicentric controlled trial, individuals with septic shock who received maintenance and creep fluids with dextrose 5% had lower cumulative fluid balance at 72 hours as compared with those who received saline 0.9%, were more likely to develop hyponatremia and less likely to develop hyperchloremia. The impact of these findings on mortality and other patient-centered outcomes require further evaluation in a subsequent larger clinical trial.
Authors’ contributions
Akram Muhammad Fayed (Conceptualization; Project administration; Supervision; Writing – review & editing).
Mohamed Osman Elsayed Osman (Conceptualization; Data curation; Investigation; Resources; Writing – review & editing).
Mohammed Mostafa Megahed (Supervision; Writing – review & editing)
Mohamed Abdelalim Abdelhady (Supervision; Writing – review & editing)
Ahmad Sabry Saleh (Conceptualization; Formal analysis; Methodology; Visualization; Writing – original draft).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Akram Muhammad Fayed
Akram Muhammad Fayed, MBBCh, PhD, ABIM, Professor of Critical Care Medicine, Faculty of Medicine, Alexandria University, Egypt.
Mohamed Osman Elsayed Osman
Mohamed Osman Elsayed Osman, MBBCh, MSc, Assistant Lecturer, Critical Care Medicine, Faculty of Medicine, Alexandria University, Egypt.
Mohammed Mostafa Megahed
Mohammed Mostafa Megahed, MBBCh, PhD, Professor of Critical Care Medicine, Faculty of Medicine, Alexandria University, Egypt.
Mohamed Abdelalim Abdelhady
Mohamed Abdelalim Abdelhady, MBBCh, PhD, Assistant Professor of Critical Care Medicine, Faculty of Medicine, Alexandria University, Egypt.
Ahmad Sabry Saleh
Ahmad Sabry Saleh, MBBCh, MSc, Physician and Biostatistician, Alhayat Clinic, Edku, el-Beheira, Egypt.
References
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7
- Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi: 10.1097/CCM.0000000000005337
- Messmer AS, Zingg C, Muller M, et al. Fluid overload and mortality in adult critical care patients-a systematic review and meta-analysis of observational studies. Crit Care Med. 2020;48(12):1862–1870. doi: 10.1097/CCM.0000000000004617
- Meyhoff TS, Hjortrup PB, Wetterslev J, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med. 2022;386(26):2459–2470. doi: 10.1056/NEJMoa2202707
- Shapiro NI, Douglas IS, Brower RG, et al. Early restrictive or liberal fluid management for sepsis-induced hypotension. N Engl J Med. 2023;388(6):499–510. doi: 10.1056/NEJMoa2212663
- Perez Nieto OR, Wong A, Lopez Fermin J, et al. Aiming for zero fluid accumulation: first, do no harm. Anaesthesiol Intensive Ther. 2021;53(2):162–178. doi: 10.5114/ait.2021.105252
- Van Regenmortel N, Verbrugghe W, Roelant E, et al. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44(4):409–417. doi: 10.1007/s00134-018-5147-3
- Bihari S, Watts NR, Seppelt I, et al. Maintenance fluid practices in intensive care units in Australia and New Zealand. Crit Care Resusc. 2016;18(2):89–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27242106
- Magee CA, Bastin MLT, Laine ME, et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study. Crit Care Med. 2018;46(8):1217–1223. doi: 10.1097/CCM.0000000000003191
- Nihlen S, Kawati R, Rasmusson J, et al. Hidden sources of fluids, sodium and potassium in stabilised Swedish ICU patients: a multicentre retrospective observational study. Eur J Anaesthesiol. 2021;38(6):625–633. doi: 10.1097/EJA.0000000000001354
- Bihari S, Prakash S, Potts S, et al. Addressing the inadvertent sodium and chloride burden in critically ill patients: a prospective before-and-after study in a tertiary mixed intensive care unit population. Crit Care Resusc. 2018;20(4):285–293. doi: 10.1016/S1441-2772(23)00968-7
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287
- Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22(7):707–710. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8844239
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3558716
- Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012
- Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713
- Koonrangsesomboon W, Khwannimit B. Impact of positive fluid balance on mortality and length of stay in septic shock patients. Indian J Crit Care Med. 2015;19(12):708–713. doi: 10.4103/0972-5229.171356
- Staffa SJ, Zurakowski D. Calculation of confidence intervals for differences in medians between groups and comparison of methods. Anesth Analg. 2020;130(2):542–546. doi: 10.1213/ANE.0000000000004535
- Van Regenmortel N, Moers L, Langer T, et al. Fluid-induced harm in the hospital: look beyond volume and start considering sodium. From physiology towards recommendations for daily practice in hospitalized adults. Ann Intensive Care. 2021;11(1):79. doi: 10.1186/s13613-021-00851-3
- Moritz ML, Ayus JC, Ingelfinger JR. Maintenance intravenous fluids in acutely Ill patients. N Engl J Med. 2015;373(14):1350–1360. doi: 10.1056/NEJMra1412877