ABSTRACT
Background
This study was designed to compare the effect of nalbuphine versus dexmedetomidine on the incidence of post-operative emergence agitation (EA) in pediatric patients undergoing elective lower abdominal surgical procedures during anesthesia with sevoflurane.
Methods
A prospective, controlled trial of 135 children aged 3–6 years scheduled for elective lower abdominal surgery was randomly assigned to one of the three equal groups receiving either nalbuphine 0.1 mg/kg IV (Group N), dexmedetomidine 0.5 mic/kg IV in (Group D) or a saline solution (Group C) at 10 min before the end of surgery in a double-blind manner. Post-operative EA was assessed with pediatric anesthesia emergence delirium (PAED) scale.
Results
The incidence of EA was significantly low in children in Group N and Group D compared to placebo group (p = 0.011) with no significant difference between Group N and Group D. The number of patients receiving nalbuphine and post-operative nalbuphine consumption during PACU stay were significantly higher in Group C than in Groups N and D (p = 0.024 and 0.001 respectively) with no significant differences between Group N and Group D. There was no significant difference regarding pain between the three groups at 5, 10, 20 and 30 min postoperatively. No significant difference among groups regarding the time to Aldrete score. The overall incidence of vomiting in our study was similar in the three groups.
Conclusion
Nalbuphine 0.1 mg/kg was comparable to dexmedetomidine 0.5 mic/kg for decreasing the incidence and severity of EA in the PACU in sevoflurane-anesthetized children when administered intravenously 10 min before the end of surgery.
Trial registration
This study was registered at ClinicalTrials.gov NCT05273671 (10/03/2022).
1. Introduction
Sevoflurane is a commonly used inhalational anesthetic for induction and maintenance of anesthesia in pediatric patients [Citation1]. However, receiving sevoflurane anesthesia may be frequently associated with higher incidence of emergence agitation (EA) ranging up to 80% [Citation2–5].
EA is a complex troublesome phenomenon that includes nervousness, excitement, confusion, aimlessness, and incoherence in the early stages of awakening from general anesthesia [Citation1,Citation2,Citation6]. Although, EA often has a brief duration and ends on its own over time without pharmacologic intervention; it can lead to unintended extubation, self-injury, surgical dehiscence, falling out of bed, dissatisfaction for parents and healthcare providers, disconnected cables, prolonged stay in recovery room, and extra nursing care, hence, it may increase medical care costs [Citation6].
Prescriptions of various adjuvants, e.g. propofol, fentanyl, α 2-adrenergic receptor agonist and ketamine have been utilized to reduce the occurrence and severity of EA in pediatric patients, with variable success [Citation4–12]. Dexmedetomidine (DEX) is a highly selective α2 adrenergic agonist with a higher α 2/α 1 activity ratio of (1600:1) when compared to clonidine (200:1) [Citation8]. It has been reported that using DEX is an effective adjuvant for prevention of EA because of dose-dependent analgesic, sedative, and anxiolytic properties [Citation8–10].
Nalbuphine hydrochloride (NAL) is a semi-synthetic opioid agonist antagonist with high affinity for kappa and mu opioid receptor with analgesic and sedative properties. A big margin of safety is one of the pros that NAL displays, hence it is usually used for children. It has very few adverse effects, such as nausea, vomiting, pruritis, constipation, and respiratory depression [Citation13–16]. Earlier research has indicated that nalbuphine may reduce the risk of EA [Citation13,Citation14].
We postulated that nalbuphine might be a suitable medication to prevent agitation following sevoflurane-based anesthesia in children. The aim of this clinical trial was to compare the effect of Nalbuphine versus Dexmedetomidine on the incidence of post-operative emergence agitation (as a primary endpoint), time to LMA removal, time to emergence, postoperative pain scale, Ramsay sedation score, post-anesthesia care unit (PACU) stay time, and possible postoperative adverse effects (as a secondary endpoint) in pediatric patients undergoing elective lower abdominal surgeries (open inguinal herniotomy and hypospadias) during anesthesia with sevoflurane.
2. Methods
This prospective, double-blinded, randomized, placebo-controlled comparative clinical trial was conducted from April 2022 to October 2022. The World Medical Association Declaration of Helsinki ethical principles for pediatric medical research has guided the trial’s conduct. The Institutional Review Board granted approval for this study (approval number 00012098), and it was registered at ClinicalTrials.govNCT05273671 (10/03/2022).
A written informed consent for each patient was obtained from at least one of the parents or guardians. Children of ASA physical status I or II, aged 3–6 years of both sexes, and within the normal range of weight were included in this study. They were scheduled for elective lower abdominal surgery (open inguinal herniotomy and hypospadias) with the expected duration of 60–180 min under sevoflurane based general anesthesia. A child who was severely agitated at the induction of anesthesia (very much or extremely inconsolable), had a history of allergy to the studied drug, was already contraindicated from caudal block due to a local or systemic infection, coagulopathy, or failure of the caudal block were among the exclusion criteria, as was the refusal of the legal guardian, emotional and psychological disorders, cognitive or developmental delays, and any neurological condition associated with difficulty communicating with nurses.
The day before surgery, according to the hospital protocol, every patient was submitted to the standard pre-operative evaluation. All the children fasted 6 h for solids preoperatively and received clear liquids until 2 h before induction of anesthesia. EMLA cream was applied to the dorsum of both hands 1 h before surgery. In the holding area, all children were pre-medicated with 0.3 mg/kg oral midazolam (maximum dose of 12 mg) approximately 30 min before separation from the parents. Children who refused intake of oral premedication were excluded.
In the operating room, children were monitored with pulse oximetry, noninvasive blood pressure, electrocardiogram, capnography, temperature probe, peripheral nerve stimulator, inspiratory and expiratory gas concentrations throughout the surgery. All children received a standardized anesthetic technique. With children under 100% oxygen (6 L/min), general anesthesia was induced by sevoflurane via face mask with gradual increases every few breaths until reaching an eight-volume percent. Once consciousness was lost, a peripheral venous access by a 22-gauge was established and 10 ml/kg of Ringer’s Lactate infusion was provided over 20 min followed by standard fluid maintenance therapy according to the weight of the patient. Rocuronium 0.6 mg/kg was injected and when adequate depth of anesthesia was achieved, a laryngeal mask airway (LMA Classic TM) of appropriate size for the age and weight of the child was lubricated with water-soluble jelly and placed according to the instructions of manufacturer. Immediately following the induction of anesthesia, Paracetamol 15 mg/kg (Perfalgan® 100 ml vial UPSA France) and Dexamethasone 0.3 mg/kg were given intravenously to each child. End-tidal concentration of 23% sevoflurane in 40% O2 with air was used for the maintenance of anesthesia. A semi-closed circular system was used throughout anesthesia, and lung ventilation was controlled to maintain the end tidal carbon dioxide tension of 32–38 mmHg. In lateral position, bupivacaine 1.0 ml/kg 0.25% was administered into the caudal space by using a 22-gauge needle in all children; then, the patients were returned to the supine position. Failure of the caudal block was recognized as an increase in mean arterial blood pressure (MAP) and heart rate >10% than pre-incisional baseline at the start of surgery and excluded from the study. No propofol or narcotics was given during the procedure.
A parallel trial design with equal allocation was adopted where the allocated children were assigned into three groups at random using computer-generated random numbers that were sealed in opaque envelopes: Group N: received 0.1 mg/kg Nalbuphine (NAL) diluted in 10 ml I.V 10 min before the end of surgery n = 45). Group D: received 0.5 mic/kg dexmedetomidine diluted in 10 ml I.V 10 min before the end of surgery, n = 45). Group C: received a saline solution 10 min before the end of surgery, n = 45).
The study medications were prepared and the master codes were kept by an independent physician who does not supervise or administer anesthesia, and each drug was administered slowly over 10 min by an observer who was completely blinded to the study and to the allocated group. So the attending anesthesiologist, the parents, data collectors, and the children remained all unaware of the group to which the patient was assigned. At the end of the surgical procedure, O2 100% >5 L/min were continued after the discontinuation of sevoflurane. Any residual muscle relaxation, as confirmed by train-of-four (TOF) monitoring, was reversed with 50 μg/kg neostigmine and 20 μg/kg atropine intravenously. Oropharyngeal suction was performed, and once the patient showed a regular respiratory pattern, the LMA was removed while the patient was unconscious. Afterward, children were moved to the post-anesthesia care unit (PACU) into which their vital signs would be monitored, where one of the parents was waiting and stayed with them until discharge to the ward. The following time intervals were recorded: duration of anesthesia (time from the start of induction till discontinuation of sevoflurane in minutes), duration of surgery (from skin incision to final skin sutures in minutes), the time to LMA removal (defined as time from the end of surgery to LMA removal) and emergence time (defined as the time from discontinuation of sevoflurane till the child opened his or her eyes spontaneously).
Upon admission to the PACU, an independent, well-trained PACU nurse who was blinded to the study protocol was responsible for observing and recording the measurements. The incidence and severity of EA was evaluated using pediatric anesthesia emergence delirium (PAED) scale which comprises five psychometric items which describe the child’s emergence behavior in the recovery room, scores ranging from 0 to 20 at 5 min (except for a few children who were not yet awake), 10, 20 and 30 min post-operatively () [Citation17]. Patients were classified with ED if their PAED score was ≥10, and severe ED was defined as PAED score of ≥15. In PACU, the Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) was used to measure postoperative pain based on the following characteristics of behavior: crying, facial expressions, verbal behavior, body position, touching the wound, and leg movement [Citation18]. It was measured at 5, 10, 20 and 30 min post-operatively. NAL 0.1 mg/kg was given intravenously to treat EA (score ≥10) lasting more than 3 min or to treat pain whenever the CHEOPS is greater than 6. If EA continued for more than 1 min after drug administration or further pain control was required, 0.05 mg/kg of NAL was given IV as needed with at least a 10 min time interval between each dose for a maximum dose of 0.2 mg/kg. Ramsay sedation score was used to assess sedation at 10 min after PACU admission [Citation19]. Children who were completely awake, had stable vital signs for 30 min, with no nausea, vomiting, or pain, and with modified Aldrete post-anesthesia score equal or greater than 9 were deemed ready to be released from the PACU [Citation20]. The time to reach a score of ≥9 was recorded. Patients were transferred to the ward when fulfilling the discharge criteria and PACU stay time (from arrival to the PACU until discharge) was recorded. Incidence of immediate postoperative adverse events in the PACU, such as vomiting, oxygen desaturation (defined as SpO2, <90%), hypotension (SBP < 70 mmHg) and bradycardia (HR < 60 b·min−1) were also noted. Vomiting was treated with ondansetron 0.15 mg/kg intravenously if needed.
Table 1. Pediatric anesthesia emergence delirium scale (PAED)[Citation17].
2.1. Statistical analysis
We chose the incidence rate of post-operative EA in the PACU as the primary endpoint to calculate the required sample size, according to a previous study [Citation21]. A sample size of 42 patients per study group was estimated to achieve an 80% power to detect a statistically significant difference of 5% between the EA using a two-sided chi-squared test with a confidence of 95%. Forty-five patients were enrolled in each group to account for possible dropouts. Statistical analysis was performed using the Statistical Package for Social Sciences software (SPSS 12.0 for windows; SPSS Inc, Chicago IL, USA). The data was expressed as means ± SDs, number of patients (n) or percentage (%). Between-group comparisons for the numerical data were analyzed using Student’s t-test to compare between two groups, while ANOVA test was used when comparing between more than two groups. Categorical data were analyzed using the χ2 test and Fisher’s exact test (when indicated), p values <0.05 were regarded as statistically significant.
3. Results
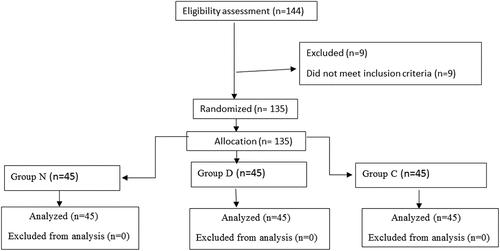
One hundred forty-four candidates on the list of patients scheduled for surgery were identified as possible participants. Of these, nine were declined to participate in the study due to exclusion criteria. Finally, the remaining 135 patients were eligible to participate in the study and were allocated into three equal groups (45 each) to participate in the study and none of them dropped out (). There were no significant differences among the three groups with respect to age, sex, weight, ASA physical status, duration of surgery, duration of anesthesia and type of surgery (p > 0.05, ).
Table 2. Demographic data.
The incidence of EA was significantly less in children who received either nalbuphine or dexmedetomidine (15.6%, and 13.3%, respectively, ) compared to that in the placebo group (40%) (p = 0.011, ), while there was no significant difference between nalbuphine and dexmedetomidine groups. The mean PAED scale was significantly higher in Group C than in Groups N and D at 5, and 10 min postoperatively (p = 002, and 0.016, respectively, ) () with no significant differences between Group N and Group D, while no significant difference among groups at 20, and 30 min postoperatively (p = 063, and 0.230, respectively, ) ().
Figure 2. Pediatric anesthesia emergence delirium (PAED) scale. Values are presented as mean ± SD.
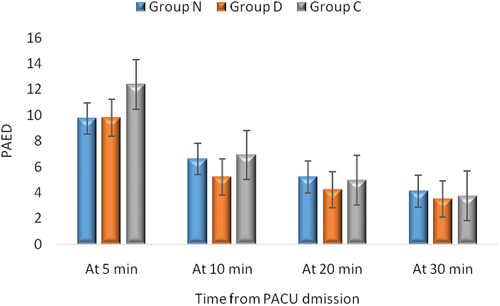
Table 3. Incidence of emergence agitation.
Table 4. Comparison between the mean PAED in different studied groups.
There was no significant difference between the three groups regarding time to LMA removal (min) (p = 0.735, ). Emergence time was significantly lower in Group C than in Groups N and D (p = 0.001, ) with no significant differences between Group N and Group D. There was no significant difference among groups regarding time to Aldrete score, Ramsay sedation score and PACU stay (p = 0.133, 0.115 and 0.060, respectively, ). The number of patients receiving nalbuphine and postoperative nalbuphine consumption during PACU stay was significantly higher in Group C than in Groups N and D (p = 0.024 and 0.001, respectively, ) with no significant differences between Group N and Group D. There was insignificant difference between the three groups regarding occurrence of vomiting and hypotension (p = 0.108, and 0.297 respectively), ). No incidence of desaturation or bradycardia in PACU was observed in any patient. There is no significant difference between the three groups regarding Children’s Hospital of Eastern Ontario Pain Scale, at 5, 10, 20 and 30 min postoperatively (p > 0.05) ().
Figure 3. Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) of the studied groups. Values are presented as mean ± SD. Non-significant difference between the three groups (p > 0.05).
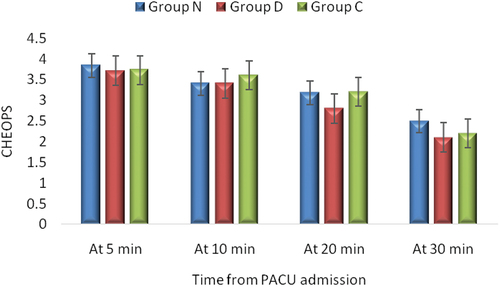
Table 5. Post-operative profiles.
4. Discussion
In this prospective, comparative, double-blinded, randomized, controlled clinical trial, we noted that the administration of either a single dose of intravenous nalbuphine 0.1 mg/kg or dexmedetomidine 0.5 mic/kg 10 min before the end of sevoflurane based anesthesia was comparable in reducing the incidence of EA (15.6% and 13.3%, respectively) if compared with placebo (40%) with significantly lower need for postoperative rescue medication for pain and agitation during PACU stay in both groups compared to placebo group. In accordance with these findings, previous reports showed that nalbuphine at a dose of 0.1 mg/kg administered at the end of sevoflurane anesthesia to children aged 6 months to 8 years decrease the incidence of EA for pediatric cerebral magnetic resonance imaging more effectively than ketamine 0.25 mg/kg without prolonging the awakening time [Citation13]. In another study performed by, Goyal et al., they showed that nalbuphine 0.1 mg/kg was significantly more effective than propofol 1 mg/kg in decreasing the incidence and severity of EA when administered intravenously 5 min before the end of cochlear implant surgery in children after sevoflurane anesthesia. This may be attributed to its sedative and potent analgesic effects, without affecting the length of stay in PACU [Citation22]. Furthermore, kim and colleagues, compared nalbuphine 0.1 mg/kg versus propofol 1 mg/kg administered at the end of strabismus surgery under sevoflurane anesthesia and reported that nalbuphine decreased the incidence of EA in pediatric patients compared to placebo group without delaying recovery, but propofol did not reduce EA [Citation23]. Likewise, Nan et al. demonstrated that, nalbuphine 0.1 mg/kg administered intravenously 30 min before the end of surgery significantly decreased the incidence and severity of EA during PACU stay in children aged 3 to 6 years undergoing dental surgery under sevoflurane anesthesia compared to placebo but did not delay discharge from PACU, also, no significant differences in the incidence of PONV or other adverse events [Citation24]. The findings of the current study are consistent with two meta-analyses conducted by Gyanesh and Sun [Citation8,Citation9]. A study by Sharma et al. tried intravenous infusion of dexmedetomidine (1 µg/kg) before induction of anesthesia in children undergoing adenotonsillectomy over 10 min and reported that the incidence of EA became significantly lower without excessive sedation, desaturation, or any other drug-related side effects [Citation25]. In another investigation, Shi et al., demonstrated that dexmedetomidine 0.5 µg/kg significantly reduced the incidence of EA compared to saline control (31.1% vs 53.3%) when administered intravenously over 10 minafter induction of anesthesia in children undergone tonsillectomy with sevoflurane anesthesia [Citation11]. A recent systematic review revealed that dexmedetomidine significantly lowered the incidence of post-anesthesia emeregence delirium or agitation in pediatric patients compared with placebo [Citation12]. In the present study, in spite that all children received caudal block, the incidence of EA in placebo group was still relatively high (40%) suggesting the fact that painless treatment is not solely enough for the prevention of EA in pediatric patients after sevoflurane anesthesia [Citation26]. In the current study, the beneficial effect of nalbuphine and dexmedetomidine may be attributed to the role of their sedative and tranquilizing effect [Citation12,Citation27]. Both Nalbuphine and Dexmedetomidine showed prolonged emergence times compared to placebo group, may be related to their sedative effect, however, the use of these drugs would not delay time to Aldrete score. Ashraf et al., evaluated IV infusion of 0.5 µg/kg dexmedetomidine versus IV infusion of 0.1 mg/kg nalbuphine over 10 min after induction of anesthesia in un-pre-medicated children aged 2–5 years for prevention of EA following adenotonsillectomy under sevoflurane anesthesia but differently, they reported conflicting results that dexmedetomidine was superior to nalbuphine in terms of reducing the incidence of postoperative EA, with more postoperative sedation but similar length of stay in PACU [Citation27]. Even though their study applied the pediatric anesthesia emergence delirium (PAED) scale to measure EA, our study considerably differs from theirs due to differences in study design, patient characteristics, the need for premedication, different anesthetic techniques, different types of surgical procedures, and, finally, the timing of medication administration. We observed the patients for 30 min after emergence in PACU as the greatest incidence of agitation is usually observed during that time, and its duration is generally limited [Citation26]. Fortunately, the overall incidence of vomiting in our study was similar in the three groups, this finding may be attributed to antiemetic properties of dexamethasone administered in the three groups [Citation28]. Shuangshuang et al. reported that, in children undergoing strabismus surgery, dexmedetomidine (0.5 μg/kg) decreased the incidence of PONV without increasing the duration of extubation or recovery [Citation29]. Moreover, nalbuphine has antagonist activity at µ receptors so it is less likely to cause nausea and vomiting when compared with morphine [Citation14]. There was no evidence of oxygen desaturation which indicates that the doses used were safe and validates the results of other study. Our study has several limitations. First, we did not assess pediatric patients’ anxiety levels preoperatively. Second, a parent/legal guardian presence during anesthetic induction in the operating room was missing. Third, we could not assess the incidence of delayed PONV after PACU discharge. Finally, we did not assess the parental anxiety profile.
5. Conclusion
In conclusion, the use of nalbuphine 0.1 mg/kg IV was comparable to dexmedetomidine 0.5 mic/kg iv for reducing the incidence and severity of EA in sevoflurane-anesthetized children undergoing lower abdominal surgery when administered intravenously 10 min before the end of surgery without delay in PACU stay.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20905068.2024.2378237
Additional information
Notes on contributors
Ayman Mohamed Maaly
Dr. Ayman Mohamed Maaly Lecturer of Anesthesia & Surgical intensive care, Faculty of Medicine, Alexandria University, Egypt.
Adel Mahgoub
Dr. Adel Mahgoub Associate professor of Anesthesia & Surgical intensive care, Faculty of Medicine, Alexandria University, Egypt.
Yasser Osman
Dr. Yasser Osman Professor of Anesthesia & Surgical intensive care, Faculty of Medicine, Alexandria University, Egypt.
Ashraf Arafat Abdelhalim
Dr. Ashraf Arafat Abdelhalim Professor of Anesthesia & Surgical intensive care, Faculty of Medicine, Alexandria University, Egypt.
Mohammad Gomaa
Dr. Mohammad Gomaa Lecturer of Anesthesia & Surgical intensive care, Faculty of Medicine, Alexandria University, Egypt.
References
- Moore AD, Anghelescu DL. Emergence delirium in pediatric anesthesia. Pediatr Drugs. 2017;19(1):11–20. doi: 10.1007/s40272-016-0201-5
- Kanaya A. Emergence agitation in children: risk factors, prevention, and treatment. J Anesth. 2016;30(2):261–267. doi: 10.1007/s00540-015-2098-5
- Kuratani N, Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trials. Anesthesiology. 2008;109(2):225–232. doi: 10.1097/ALN.0b013e31817f5c18
- Aouad MT, Yazbeck-Karam VG, Nasr VG, et al. A single dose of propofol at the end of surgery for the prevention of emergence agitation in children undergoing strabismus surgery during sevoflurane anesthesia. Anesthesiology. 2007;107(5):733–738. doi: 10.1097/01.anes.0000287009.46896.a7
- Abdelhalim AA, Alarfaj AM. The effect of ketamine versus fentanyl on the incidence of emergence agitation after sevoflurane anesthesia in pediatric patients undergoing tonsillectomy with or without adenoidectomy. Saudi J Anaesth. 2013 Oct;7(4):392–398. doi: 10.4103/1658-354X.121047
- Lee S-J, Sung T-Y. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. 2020 Dec;73(6):471–485. doi: 10.4097/kja.20097
- Ramachandran A, Palanisamy N, VidyaM V, et al. Comparison of dexmedetomidine in two different doses on emergence agitation in children under sevofluraneanaesthesia: a double-blind randomised controlled trial. Indian J Anaesth. 2021 Jul;65(7):519–524. doi: 10.4103/ija.IJA_168_21
- Zhu M, Wang H, Zhu A, et al. Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: different administration and different dosage. PLOS ONE. 2015 Apr 13;10(4):e0123728. doi: 10.1371/journal.pone.0123728
- Sun L, Guo R. Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: a meta-analysis of randomized controlled trials. ActaAnaesthesiolscand. 2014;58(6):642–650. doi: 10.1111/aas.12292
- Ahmed M, Hany M. Effect of intranasal dexmedetomidine on emergence agitation after sevoflurane anesthesia in children undergoing tonsillectomy and/or adenoidectomy. Saudi J Anaesth. 2017 Apr-Jun;11(2):137–143. doi: 10.4103/1658-354X.203020
- Shi M, Miao S, Gu T, et al. Dexmedetomidine for the prevention of emergence delirium and postoperative behavioral changes in pediatric patients with sevoflurane anesthesia: a double-blind, randomized trial. Drug Des Devel Ther. 2019;13:897‐905. doi: 10.2147/DDDT.S196075
- Rao Y, Zeng R, Jiang X, et al. The effects of dexmedetomidine on emergence agitation or delirium in children after anesthesia-a systematic review and meta-analysis of clinical studies. Front Pediatr. 2020;8:329. doi: 10.3389/fped.2020.00329
- Dalens BJ, Pinard AM, Letourneau DR, et al. Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. AnesthAnalg. 2006;102(4):1056–1061. doi: 10.1213/01.ane.0000200282.38041.1f
- Kubica-Cielińska A, Zielińska M. The use of nalbuphine in paediatricanaesthesia. Anaesthesiol Intensive Ther. 2015;47(3):252–256. doi: 10.5603/AIT.2015.0036
- Bressolle F, Khier S, Rochette A, et al. Population pharmacokinetics of nalbuphine after surgery in children. Br J Anaesth. 2011;106(4):558–565. doi: 10.1093/bja/aer001
- Schnabel A, Reichl SU, Zahn PK, et al. Nalbuphine for postoperative pain treatment in children. Cochrane Database Syst Rev. 2014;2019(7): CD009583. doi: 10.1002/14651858.CD009574.pub2
- Ringblom J, Wåhlin I, Proczkowska M. A psychometric evaluation of the pediatric anesthesia emergence delirium scale. PediatrAnaesth. 2018;28(4):332–337. doi: 10.1111/pan.13348
- McGrath PJ, Johnson G, Goodman JT, et al. CHEOPS: a behavioral scale for rating postoperative pain in children. In: Field H, editor. Advances in pain research and therapy. 9th ed. (NY): Raven Press; 1985. p. 395–402.
- Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2(5920):656–659. doi: 10.1136/bmj.2.5920.656
- Aldrete JA, Kroulik D. A postanesthetic recovery score. J Am CollSurg. 2007;205(5):e3–e4. doi: 10.1016/j.jamcollsurg.2007.07.034
- Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi J Anaesth. 2013;7(3):296–300. doi: 10.4103/1658-354X.115363
- Goyal S, Audichya PC, Soni K, et al. Prevention of sevoflurane related emergence agitation in children undergoing cochlear implant: a comparison of nalbuphine and propofol without prolonging the awakening time. Eur J Pharm Med Res. 2006;3:300–305.
- Kim HJ, Kim HS, Kim SD. Effects of propofol and nalbuphine on emergence agitation after sevoflurane anesthesia in children for strabismus surgery. Korean J Anesthesiol. 2008;55(5):575–578. doi: 10.4097/kjae.2008.55.5.575
- Zhao N, Wu Y, Yu C. Effect of intravenous nalbuphine on emergence agitation in children undergoing dental surgery under sevoflurane anesthesia. Int J ClinExp Med. 2018;11(9):10215–10222.
- Sharma K, Kumar M, Gandhi R. Effect of single-dose dexmedetomidine on intraoperative hemodynamics and postoperative recovery during pediatric adenotonsillectomy. Anesth Essays Res. 2019;13(1):63–67. doi: 10.4103/aer.AER_178_18
- Somaini M, Engelhardt T, Fumagalli R, et al. Emergence delirium or pain after anaesthesia–how to distinguish between the two in young children: a retrospective analysis of observational studies. Br J Anaesth. 2016 Mar;116(3):377–383. doi: 10.1093/bja/aev552
- Ashraf EE, Mostafa GM, Amr ZM. Dexmedetomidine versus nalbuphine in prevention of emergence agitation following adenotonsillectomy in pediatrics. Egypt J Anaesth. 2020;36(1):24–29. doi: 10.1080/11101849.2020.1728865
- Wakamiya R, Seki H, Ideno S, et al. Effects of prophylactic dexamethasone on postoperative nausea and vomiting in scoliosis correction surgery: a double-blind, randomized, placebo-controlled clinical trial. Sci Rep. 2019;9(1):2119. doi: 10.1038/s41598-019-38764-8
- Li S, Liu T, Xia J, et al. Effect of dexmedetomidine on prevention of postoperative nausea and vomiting in pediatric strabismus surgery: a randomized controlled study. BMC Ophthalmol. 2020 Mar 5;20(1):86. doi: 10.1186/s12886-020-01359-3