ABSTRACT
Background
Caudal epidural prolotherapy injection has emerged as a successful treatment for managing chronic pain. This study is a prospective randomized, double-blinded clinical trial. It assesses and compares the effectiveness of prolotherapy versus steroid injection for pain relief in cases of failed back surgery syndrome (FBSS). It involved randomly assigning 90 patients with FBSS to one of two equal groups: steroids or prolotherapy. The VAS was used to measure pain as the primary outcome, while disability was measured by the Oswestry disability index (ODI). McGill Pain Questionnaire (MPQ) and complications were recorded as secondary outcomes.
Results
The VAS improved significantly from the baseline in both groups at all follow-up periods (p < 0.001). Nonetheless, after 2, 4, and 6 weeks post-injection, there was no statistically significant difference between the two groups, while at 8 weeks, 3, and 6 months, the VAS was statistically significantly lower in the steroids group than in the prolotherapy group (p < 0.001). The ODI and MPQ improved significantly from the baseline in both groups at all follow-up periods (p < 0.001). However, after 2 and 4 weeks post-injection, there was no statistically significant difference between the two groups; but, at 6 and 8 weeks, 3, and 6 months, the ODI and MPQ were statistically significantly lower in the steroid group compared to the prolotherapy group (p < 0.001). Only one patient in the steroid group suffered from pain and swelling at the injection site without a statistically significant difference between the two groups.
Conclusion
Caudal epidural prolotherapy injection has good efficacy in managing pain that can be similar to steroid injection but shorter in duration in cases of FBSS with comparable safety profile.
1. Introduction
“Lumbar spinal pain of unknown origin either persisting despite the surgical intervention or appearing after surgical intervention for spinal pain originally in the same topographical location “is the definition of failed back surgery syndrome (FBSS) [Citation1]. Medication, physical therapy, and other conservative therapies, including nerve blocks, are among the conventional treatments for FBSS [Citation2]. The use of repeated spinal surgery as a way of treatment does not guarantee a favorable result. Thus, when possible, non-invasive methods have been preferred for the treatment of FBSS [Citation3]. Caudal epidural steroid injection (CESI) is commonly used to alleviate radicular pain, and has been utilized extensively to treat lower back pain [Citation4] and frequently performed to manage radicular symptoms [Citation5]. Interest in regenerative medicine is rising. Numerous randomized controlled studies have demonstrated the beneficial effects of prolotherapy, one of the most popular injections that has recently been introduced. Prolotherapy can decrease pain, especially in cases of chronic low back pain (CLBP), and a 5% glucose subcutaneous injection has been shown to help treat neurogenic pain and reduce allodynia and hyperalgesia [Citation6]. CESIs were carried out either blindly or with fluoroscopy guidance for several decades. Ultrasound (US) guidance has been more commonly used in CESIs recently [Citation7].
This study aims to compare the efficacy of combined US fluoroscopy-guided caudal epidural prolotherapy versus steroid injection for chronic pain management in cases of FBSS where a VAS score reduction by 50% or more at 2 weeks post-injection is considered successful.
2. Patients and methods
This clinical study is prospective, randomized, controlled, double-blinded, and was conducted in the period between September 2022 and January 2024. In terms of reporting randomized clinical trials, it adhered to CONSORT and supported the Helsinki Declaration. The approved sample size was 70 patients. Ninety patients were included in the study, aged 20–70 years who had been diagnosed with FBSS and had been experiencing CLBP for longer than three months as determined by a thorough evaluation that included medical history, and physical examination. Additionally, positive results from an MRI showed that the spinal nerve roots were being compressed or irritated following surgery for a variety of conditions, including lumbar disc herniation, spinal canal stenosis, osteophytes, spondylolisthesis, and foramen stenosis. The included patients did not require any additional recurrent surgery.
Exclusion criteria included the refusal of the patient, pregnant women, patients having systemic diseases, or infection at the injection site; history of allergy to the contrast medium, individuals on anticoagulants; immunocompromised patients, renal impairment or dialysis, neurological deficits, cauda equina syndrome, motor weakness, acute disc rupture, use of opioids or substantial concomitant depression or inflammatory joint disease. Also, the injection of steroids is contraindicated in cases with osteoporosis or uncontrolled diabetes mellitus, so they were also excluded.
Patients were randomly categorized into two subgroups using a closed envelope method by a simple randomization sheet:
Prolotherapy group: 45 patients received US and fluoroscopy-guided caudal epidural injection of 5 ml bupivacaine 0.5%+ 4 ml omnipaque contrast (350 mg/ml) + 10 ml dextrose 25% + 6 ml distilled water.
Steroid group (Group S): 45 patients received US and fluoroscopy-guided caudal epidural injection of 1 ml methylprednisolone (40 mg/ml) + 4 ml omnipaque contrast (350 mg/ml) + 5 ml bupivacaine 0.5% + 15 ml distilled water [Citation1].
Preparation of the drugs was performed by an investigator not participating in the study. Both the investigator and the patient were blinded to the study drugs.
Full history and clinical examination were performed. All patients were evaluated using MRI for the lumbar region, and radiological signs of FBSS were detected as recurrent disc herniation, epidural scar tissue, and implant mal-alignment. Routine and other lab investigations were conducted. Standard fasting guidelines were followed before the procedure. Patients were injected in the operating room under complete aseptic conditions. A full monitor was attached to all patients undergoing the procedure, and a 20-gauge cannula was inserted. The injection was done using a Sono site US (M-turbo, Bothell, WA, USA) with a high-frequency transducer (10 HZ).
Patients were placed in a prone position; US probe sterilization was done then the needle entry point was anesthetized by 3 cm of lidocaine 2% subcutaneously. The ultrasound probe was first placed transversely, then it was turned 90 degrees to settle between the two sacral cornua and to get a longitudinal view of the sacral hiatus. Finally, using US guidance, the epidural needle was inserted in the caudal epidural space [Citation2]. Using fluoroscopy, confirmation of the needle site was made, and then patients in both groups were injected, each according to the assigned injectate. Patients were followed up at regular intervals of 2, 4, 6, 8 weeks, 3, and 6 months post-injection. After two weeks, a 50% reduction in the VAS from the pre-injection level or above is deemed effective. One gram paracetamol tablets were prescribed for all patients twice daily for 3 days post-injection to relieve any pain or tenderness at the injection site caused by the procedure.
This study included a single injection of either prolotherapy or steroids for patients in both groups. Patients with VAS more than 50% at any time during the follow-up periods were re-injected with steroids, excluded from the current study, and followed up separately.
The statistical package for social science (SPSS) application (ver 25) was used for data collection and analysis [Citation3]. As appropriate, data were recorded as either numerical or categorical. After the results of the Kolmogorov–Smirnov test for the normality of the variable distribution showed significance, non-parametric statistics were used [Citation4]. Minimum, maximum, median, 95% Confidence Interval of the median, and 25th to 75th percentiles were used to characterize the data. Two independent, non-normally distributed research subgroups were compared using the Mann-Whitney U test [Citation5]. Using Friedman’s test, comparisons between related samples were made [Citation6]. p-value was adjusted using the Bonferroni correction method [Citation7].
With an 80% power of the study, a beta error was allowed up to 20% when calculating the sample size. A 95% significant level was used along with a 5% alpha level. At p-value < 0.05, statistical significance was assessed [Citation8].
The pain was assessed by VAS as the primary outcome, disability by the Oswestry Disability Index (ODI), as well as McGill pain questionnaire, and any complications were recorded as secondary outcomes.
3. Results
One hundred and fifty patients with FBSS and indicated for caudal epidural injection were assessed for eligibility, then 60 patients were excluded either due to not meeting the inclusion criteria or refusal to perform the procedure. The remaining 90 patients were randomized into 2 groups either steroid or prolotherapy, 45 each. Patients were followed up for up to 6 months. Seven patients were excluded throughout the follow-up periods because they were re-injected, so the final number of patients at 6 months was 40 patients in the prolotherapy group and 43 patients in the steroid group ()
Figure 1. Consort flow diagram illustrating the overall number of patients as well as the number of patients in each group.
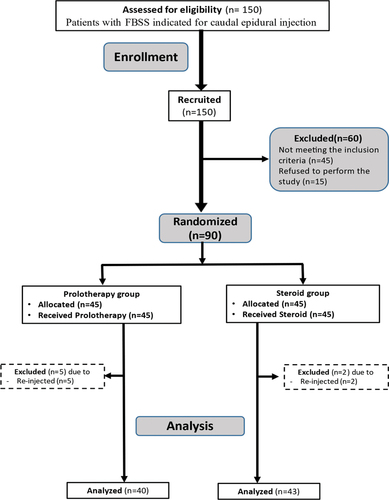
Consort flow diagram illustrating the overall number of patients as well as the number of patients in each group ().
Regarding age and sex, no statistically significant differences were recognized between the two groups (p = 0.903, 0.833 respectively) (). The VAS improved significantly from the baseline score in both groups at all follow-up periods (p < 0.001). Nonetheless, at 2-, 4-, and 6-weeks post-injection, there was no statistically significant difference between the two groups (p = 0.795, p = 0.232, p = 0.511 respectively) while at 8 weeks, 3-, and 6-months post-injection the VAS was statistically significantly lower in the steroid group than in the prolotherapy group (p < 0.001) (). The ODI improved significantly from the baseline score in both groups at all follow-up periods (p < 0.001); however, at 2 and 4 weeks post-injection, no statistically significant difference was recognized between the two groups (p = 0.886, p = 0.993 respectively), while at 6-, 8-weeks, 3- and 6-months post-injection the ODI was statistically significantly lower in the steroid group than in the prolotherapy group (p < 0.001) (). The MPQ score improved significantly from the baseline in both groups at all follow-up periods (p < 0.001) but at 2 and 4 weeks post-injection, no statistically significant difference was noted between the two groups (p = 1.00). While at 8 weeks, 3, and 6 months post-injection the MPQ was statistically significantly lower in the steroid group than in the prolotherapy group (p < 0.001) (). Pain and swelling at the injection site were the only side effects encountered in the steroid group, with no complications in the prolotherapy group, and no statistically significant difference between them was noted (p = 0.3125).
Figure 2. VAS in the studied groups as shown by the box and whisker graph, the thick line in the middle of the box indicates the median, and the box itself represents the inter-quartile range. After the exclusion of the outliers (circles), and the extremes (asterisks), the whiskers indicate the minimum and maximum.
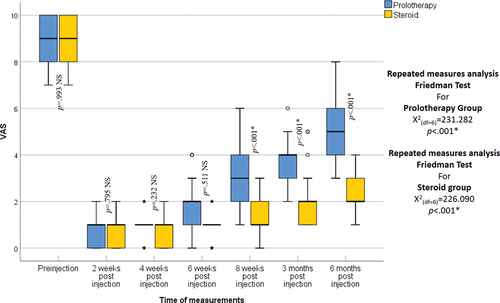
Figure 3. Oswestry disability index in the studied groups as shown by the box and whisker graph, the thick line in the middle of the box indicates the median and the box itself represents the inter-quartile range. After the exclusion of the outliers (circles), and the extremes (asterisks), the whiskers indicate the minimum and maximum.
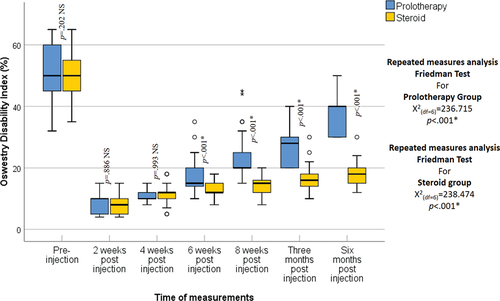
Figure 4. McGill pain questionnaire in the studied groups as shown by the box and whisker graph. The thick line in the middle of the box indicates the median and the box itself represents the inter-quartile range. After the exclusion of the outliers (circles), and the extremes (asterisks), the whiskers indicate the minimum and maximum.
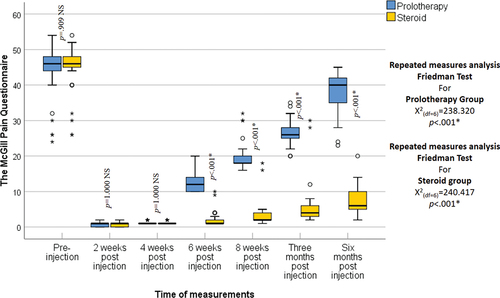
Table 1. Baseline comparison of age (years) and sex between the two groups under study, 45 patients in each group.
4. Discussion
In cases of FBSS, the current study evaluates the effectiveness of injecting caudal epidural prolotherapy against steroids for treating CLBP.
Caudal epidural injections of prolotherapy and steroids helped relieve pain after FBSS in terms of VAS score, disability score (ODI), and MPQ. However, CESI had a longer lasting effect.
In consistence with our study, a study carried out by Celenlioglu AE et al. [Citation9] compared caudal and transforaminal epidural steroid injections in patients who had undergone single-level discectomy and complained of FBSS, 56 patients with low back and radicular pain related to epidural fibrosis were included. Numerical Rating Scale (NRS-11) was used to evaluate patients before the procedure, one hour, three weeks, and three months post-injection. They concluded that NRS-11 demonstrated a substantial drop in both groups at all follow-up periods (p < 0.001).
Maniquis-Smigel L, et al. [Citation10] studied the analgesic impact of caudal epidural D5W and its cumulative effects in individuals with persistent low back and buttock/leg pain. D5W with a volume of 10 ml without anesthetic were injected in the caudal epidural space every two weeks for four treatments and then as needed for a year. The authors measured the NRS and the ODI and concluded that after receiving an epidural D5W injection without anesthesia, participants in a long-term follow-up reported improvements in their ODI and NRS scores when compared to their baseline status, and the majority of these improvements were attained within three months of the injection and remained stable for a full year. This analgesia suggested a potential sensorineural effect that dextrose has on neurogenic pain and it can be attributed to the multiple injections given (4 injections) denoting that prolotherapy requires multiple injections for better results and a longer duration of action. However, improvement in the current study occurred in the early follow-up periods as only one injection of prolotherapy was done.
In contrast to the current study, Rabago D et al. [Citation11] carried out a study that was prospective, randomized, and double-blinded on 35 patients to assess the short-term analgesic efficacy of D5W epidural injection in comparison to normal saline for non-surgical cases of CLBP. A caudal approach was used to administer a single fluoroscopy-guided epidural injection of 10 mL of either 0.9% saline or 5% dextrose. The numerical rating scale (NRS, 0–10 points) was used to assess the pain score at baseline, as well as at 15 min, 2, 4, and 48 h as well as two weeks after the injection. Dextrose participants reported greater NRS change at 15 min, 2 h, 4 h, and 48 h, but not at 2 weeks. This may be explained by the lower concentration of dextrose that was injected (5%) compared to the current study’s injection concentration (10%), as well as the smaller injectate volume (10 ml) compared to the present study’s total injection volume (25 ml). Additionally, the study’s sample size is small (35 cases).
Furthermore, a study by Jindal et al. [Citation12] examined how psychological distress screening functions to predict the results of caudal epidural steroid injection in CLBP. Ninety-six patients suffering from CLBP were enrolled in this prospective cohort study. A 30 ml total volume caudal epidural steroid injection guided by fluoroscopy was administered. The injection consisted of 80 mg methylprednisolone (2 ml in volume), 8 mg lignocaine combined with normal saline, and 1 ml of contrast (iopamidol). MPQ was assessed for pre-injection at 6, 12, and 26 weeks post-injection. MPQ declined significantly at 6 and 26 weeks post-injection. This can be due to a higher dose of steroids (80 mg) and a higher volume (30 ml) when compared to that done in the present study (40 mg, 25 ml in volume).
The current study demonstrated no major complications. In contrast, in a study conducted by Akkaya T et al. [Citation13] comparing fluoroscopy and US-guided steroid injection in the caudal epidural space in cases of post-laminectomy, 30 patients were enrolled; facial flushing was reported by 2 patients, and only one patient had a vasovagal reaction in one group, while in the other group, facial flushing and headache occurred in 1 one patient each. There was no evidence of hematoma, infection, or bleeding. The complications did not show any statistically significant difference between both groups.
Limitations: The follow-up period was short (six months), which might not be enough to record the injected medications’ long-term effects; the sample size may not be sufficient to detect the actual incidence of complications; moreover, multiple serial injections of prolotherapy should have been studied to assess its long-term effectiveness.
5. Conclusion
Caudal epidural prolotherapy injection is effective in managing pain that can be similar to steroid injection in cases of failed back surgery syndrome with a comparable safety profile. However, the duration of action may be shorter.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Tagowski M, Lewandowski Z, Hodler J, et al. Pain reduction after lumbar epidural injections using particulate versus non-particulate steroids: intensity of the baseline pain matters. Eur Radiol. 2019;29(7):3379–3389. doi: 10.1007/s00330-019-06108-9
- Bubic I, Oswald J. Ultrasound-guided caudal epidural steroid injection for back pain: a case report of successful emergency department management of radicular low back pain symptoms. J Emergency Med. 2021;61(3):293–297. doi: 10.1016/j.jemermed.2021.04.015
- Spss Ibm. Ibm Corp. Released, statistics for windows, version 25.0. Armonk, Ny: Ibm Corp; 2017.
- Field A. Discovering statistics using ibm spss statistics. 4th ed. London (CA), New Delhi: Sage Publications Ltd; 2013.
- Mann H, Whitney D. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Statist. 1947;18(1):50–60. doi: 10.1214/aoms/1177730491
- Friedman M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J Am Stat Assoc. 1937;32(200):675–701. doi: 10.1080/01621459.1937.10503522
- Schober P, Vetter T. Adjustments for multiple testing in medical research. Anesthes Analges. 2020;130(1):99. doi: 10.1213/ANE.0000000000004545
- Curran-Everett D. Evolution in statistics: p values, statistical significance, kayaks, and walking trees. Bethesda (MD): American Physiological Society; 2020. p. 221–224.
- Celenlioglu AE, Sencan S, Bilim S, et al. Comparison of caudal versus transforaminal epidural steroid injection in post lumbar surgery syndrome after single-level discectomy: a prospective, randomized trial. Pain Physic. 2022;25(2):161–169.
- Maniquis-Smigel L, Reeves KD, Rosen HJ, et al. Analgesic effect and potential cumulative benefit from caudal epidural D5W in consecutive participants with chronic low-back and buttock/leg pain. J Altern Complement Med. 2018;24(12):1189–1196. doi: 10.1089/acm.2018.0085
- Maniquis-Smigel L, Dean Reeves K, Rosen HJ, et al. Short term analgesic effects of 5% dextrose epidural injections for chronic low back pain: a randomized controlled trial. Anesth Pain Med. 2016;7(1). doi: 10.5812/aapm.42550
- Jindal R, Rudol G, Okafor B, et al. Role of psychological distress screening in predicting the outcomes of epidural steroid injection in chronic low back pain. J Clin Orthop Trauma. 2021;19:26–33. doi: 10.1016/j.jcot.2021.04.027
- Akkaya T, Ozkan D, Kertmen H, et al. Caudal epidural steroid injections in post laminectomy patients: comparison of ultrasonography and fluoroscopy. Turk Neurosurg. 2017;27(3):420–425. doi: 10.5137/1019-5149.JTN.16171-15.1
