ABSTRACT
Objective: To determine if the interpretation of urodynamic studies (UDS) in children without a rectal catheter may be similar to multi-channel studies, as UDS in children are challenging and can sometimes be difficult to interpret.
Patients and methods: In this retrospective pilot study, 115 paediatric pressure–flow studies were included. A blinded investigator was given two sets of UDS traces. The first set had the vesical trace of all children and the second set had the multi-channel trace. The agreement between the interpretations of both the sets was tested by Cohen’s κ, and sensitivity, specificity, and predictive values were expressed with 95% confidence intervals (CIs). The voiding pattern was compared and Pearson’s correlation coefficient was used to analyse the pressure at maximum urinary flow (Qmax).
Results: The most common indications for UDS were neurogenic bladder and posterior urethral valves. The interpretation of compliance and detrusor overactivity by single-channel analysis had a positive predictive value of 92.1% (95% CI 84.7–96.1%) and 89.4% (95% CI 78.3–95.6%), respectively, and a negative predictive value of 100% and 97.1% (95% CI 89.5–99.2%) respectively, in comparison to multi-channel analysis. Children with underactive detrusor were identified reliably by analysing the straining pressure pattern and flow curve. Amongst children who voided, the pressure at Qmax showed a moderate correlation (Pearson’s coefficient = 0.53) between the two groups.
Conclusion: Rectal catheters may be avoided in a carefully selected group of children undergoing UDS who only need filling phase assessment.
Abbreviations: DO: detrusor overactivity; EBC: expected bladder capacity; Pabd: abdominal pressure; Pdet: detrusor pressure; PUV: posterior urethral valve; (N)(P)PV: (negative) (positive) predictive value; Pves: vesical pressure; Qmax: maximum urinary flow rate; UDS: urodynamic studies; UI: urinary incontinence
Introduction
Urodynamic studies (UDS) in children have a well-established role in the management of complex urological and neuro-vesical pathologies [Citation1,Citation2]. Multi-channel cystometry is currently the ‘gold standard’ for urodynamic evaluation [Citation3]. Although the placement of a rectal catheter in addition to a vesical catheter enables subtraction of abdominal pressure (Pabd) from vesical pressure (Pves), it is sometimes counterproductive in children. Distress caused during the procedure often makes them uncooperative. The traces thus obtained are hard to interpret due to excessive artefacts. In addition, the incremental benefit of measuring the Pabd may be of questionable value in certain groups of patients where the only indication for this procedure is to assess bladder compliance and detrusor overactivity (DO) to decide on fluid intake, the frequency of clean-intermittent catheterisation, and anticholinergic medications.
Based on these observations in routine practice, the present study aimed to determine if pressure–flow studies in children can be interpreted satisfactorily with single-channel cystometry to avoid the discomfort of a rectal catheter. There are few studies that have evaluated the role of one-channel cystometry [Citation4,Citation5] in identifying bladder overactivity and stress urinary incontinence (UI) in adults. Ricci et al. [Citation6] found single-channel cystometry to be a useful adjunct to clinical examination in women with UI. Single-channel cystometry interpreted carefully in relation to clinical findings is considered a reasonably accurate, safe and cheap method for diagnosing neurogenic bladder dysfunction, especially in spinal cord injuries [Citation7]. In another study comparing single- and multi-channel cystometry as a screening tool for detrusor instability in women, single-channel studies had acceptable specificity but the predictive value was poor as a screening tool [Citation8]. There are no such studies in the paediatric population to our knowledge.
Patients and methods
Study population and design
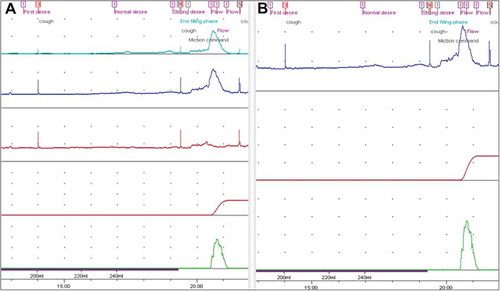
A retrospective cohort study was conducted, which included all children who underwent UDS at our centre during the period July 2014 to June 2017. In all, 118 successfully completed UDS were conducted in this period and 115 were included in this study. Three children had two UDSs during this period and only the first study was included in these cases. UDSs of children aged >1 year up to 16 years were included, which is considered the upper age-limit for paediatric patients at the authors’ centre. Incomplete studies and patients that lacked relevant clinical data were omitted. Data were acquired from the hospital’s electronic database. The single-channel traces were obtained by digital subtraction of the Pabd and Pves traces from the multi-channel trace (). Patient identifiers were removed and only relevant clinical data were provided. Traces were interpreted by a blinded paediatric urologist with >10-years’ experience working in the same unit. Single-channel traces were first assigned for interpretation and the findings were recorded. The same investigator was then assigned the multi-channel traces for interpretation. The two sets of interpretations were then compared.
UDS
All children had a detailed history taken, examination, and completed a 48-h bladder diary. Ultrasonography of the abdomen was used for upper tract assessment, uroflowmetry, and renal function tests. All children had undergone multi-channel cystometry by standard institutional protocols based on the International Children’s Continence Society guidelines [Citation9]. Studies were done in the sitting position if the child was able, and all studies were done in the presence of the parents with informed written consent. No drugs or sedatives were used during the study. Patients were asked to come for the test after emptying their bowel if possible, or the study was re-scheduled for the next day after administrating a laxative the previous night.
Filling and simultaneous measurement of Pves were done by a 6-F double-lumen transurethral catheter placed in the bladder. Lignocaine jelly (0.2%) was used to aid the introduction of the catheter. Pabd was measured simultaneously with a small rectal balloon catheter. Normal saline solution (0.9%) at room temperature [Citation10] was instilled in a retrograde manner, with physiological filling rate calculated as weight (in kg) divided by 4. At the beginning of the filling phase and at regular intervals, the child was instructed to cough to ensure that the abdominal and vesical pressure lines were balanced, when possible [Citation11].
Detrusor pressure (Pdet) was calculated by subtracting Pabd from Pves. The Pabd and Pves traces were digitally masked to obtain a trace with only the Pves for comparison. Voiding command was given at normal desire to void in older children. In non-toilet-trained children, signs of discomfort or whenever the child voided was considered as the end-filling phase.
Outcome measurement
Comparisons between the interpretation of multi-channel UDS and of the Pves trace alone were made with respect to bladder compliance, detrusor instability, and voiding pressures.
Poor compliance in a multi-channel trace was defined as bladder pressure at the expected bladder capacity (EBC) of >10 cmH2O. Similarly, an increase in Pves (∆Pves) by ≥10 cmH2O at EBC for age was the threshold to detect poor compliance in the single-channel trace [Citation12]. In children where EBC was not reached during the filling phase, pressure at end-filling was used to determine the compliance. The EBC for age was determined by the equation derived by Koff [Citation13], which is important to take into consideration, as unlike adults, compliance changes with age in children. DO was reported when there were involuntary provoked or unprovoked contractions with an increase in pressure by 15 cmH2O during the filling phase from the baseline [Citation14].
The voiding phase was analysed by:
The pattern of flow curves and
Comparing pressures at maximum urinary flow (Qmax).
In children who strained to void, the blinded investigator compared the pattern of the pressure and flow curves to identify if the child was straining to void, as absolute pressure measurements were not possible. Children who could not void, or voided <50% of the EBC [Citation15], were also excluded from analysis of pressures during voiding. In others, the Pdet at Qmax (pressure at Qmax on conventional pressure–flow studies) and ∆Pves at Qmax (the corresponding Pves, after accounting for the baseline Pves at the start of filling phase) were compared.
Statistical methods
Data entry was done using EpiData Entry Client (EpiData Association, Odense, Denmark). Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS®), version 20 (SPSS Inc., IBM Corp., Armonk, NY, USA). Categorical data were analysed using descriptive statistics and reported as frequency and percentage. Compliance and DO derived from both methods in the study were represented by 2 × 2 contingency tables and Cohen’s κ value with 95% CI was calculated to assess the agreement between the two methods [Citation16]. Sensitivity, specificity, positive predictive value (PPV) and negative PV (NPV) were calculated for single-channel cystometry considering multi-channel as the ‘gold standard’. The correlation between the voiding pressures (Pdet at Qmax and ∆Pves at Qmax) were analysed by calculating the Pearson’s coefficients.
Results
Demography
In all, 115 paediatric UDS were reviewed, which included 86 boys and 29 girls (). The median (interquartile range) age of the children was 12 (8–15) years. Neurogenic bladder was the commonest indication for UDS (56 children), followed by posterior urethral valves (PUVs; 41 children). Children with anatomical abnormalities and dysfunctional voiding were few in number, with 14 and four, respectively, in each group.
Table 1. Demographic data.
Filling phase
In , the number of children with normal and decreased compliance by both methods of interpretation is shown. Interpretation of compliance was concordant in 109 of the 115 children (94.7%), with a sensitivity of 100% (95% CI 94.9–100%) and specificity of 86.7% (95% CI 73.2–94.95%). The PPV was 92.1% (95% CI 84.7–96.1%) and NPV was 100%. The agreement between the two methods of interpretation was very good; the κ value was 0.89 (95% CI 0.80–0.98).
Table 2. Comparison of interpretation of compliance and DO between single- and multi-channel studies.
The next parameter compared was DO. shows the comparison of DO as interpreted by a single- and multi-channel trace. Results of the blinded interpretations were concordant in 94% (108/115 children), with a sensitivity of 95.5% (95% CI 84.5–99.4%) and specificity of 93% (95% CI 84.3–97.7%). The PPV of the single-channel trace was 89.4% (95% CI 78.3–95.6%) and NPV was 97.1% (95% CI 89.5–99.2%). The κ value, which represents the agreement between the two methods, was 0.87 (95% CI 0.78–0.96).
Voiding phase
Voiding pattern analysis
In all cases, the flow curve in conjunction with the pressure curves could reliably predict if the child was straining to void. In children who had a straining pattern whilst voiding (n = 55; ), although actual voiding pressures could not be compared, the blinded investigator could identify straining pattern of flow and pressure curves suggestive of an underactive detrusor. This was done reliably with single-channel cystometry in all patients.
Table 3. Breakdown of children in each category who were included for analysis of voiding pressures.
Voiding pressure analysis
The voiding phase pressures were analysed in a total of 41 children of which 35 had PUVs. Correlation between Pdet at Qmax [mean (SD) was 50.36 (20.24) cmH2O] and ∆Pves at Qmax [mean (SD) was 58.36 (21.07) cmH2O] was acquired by calculating the Pearson’s coefficient, the value of which was 0.53 (P < 0.05). In all, 19 children were excluded due to either low voided volume or low bladder capacity. Another 55 were excluded as they strained to void, which made accurate voiding phase pressure measurement impossible in these children.
Discussion
Pressure–flow studies are challenging and time-consuming in children. Trained personnel and a friendly environment are prerequisites for studying bladder function in children [Citation1,Citation17]. Although invasive urodynamic evaluation with multi-channel cystometry gives an accurate assessment of pressures to assess lower urinary tract dysfunction (LUTD), it can cause a great deal of discomfort to children resulting in artefacts. Although there is no scale to measure the degree of physical and emotional distress, it has been well documented in adults [Citation18].
It is important to be aware of the various causes of artefacts that may be present in a paediatric UDS. Most children are either anxious or restless during the study. Straining, change in position, movement of the tubes, rectal contractions, and faulty calibration can lead to artefacts [Citation19]. DO may be precipitated by irritation caused by the catheter, cold saline, supra-physiological filling rate, and coughing. Additionally, the placement of catheters in a narrow urethra may cause difficulty in voiding. Bladder sensation was not studied, as it is subjective and relevant only in older, toilet-trained children.
In the present study, the Cohen’s κ was used to compare both tests instead of McNemar’s test, due to the small number of observations in two of the cells in the contingency table () making it unsuitable for our analysis. There was very good agreement between single- and multi-channel cystometry for diagnosing poor compliance with a PPV and NPV of >92%. Likewise, there was very good agreement between single- and multi-channel traces with respect to DO. Five children were over-diagnosed with DO by the single-channel trace due to intermittent rectal contractions during the study.
The interpretation of the filling phase of the study did not change, irrespective of the availability of the Pabd trace in most of the patients. Single-channel interpretation had a high NPV of 100% and 97% for the diagnosis of poor compliance and DO, respectively. Six children were over-diagnosed as having poor compliance, while five were over-diagnosed with DO by single-channel interpretation. False positives were equally distributed in the neurogenic bladder and PUV groups. False positive DO on single-channel interpretation corresponded to an increase in Pabd. The benefit of avoiding the discomfort of a rectal catheter vs a small chance of over-diagnosis of poor compliance and DO is debatable.
Comparison of the voiding phase between the two methods of interpretation is primarily based on: (i) the flow and pressure curve pattern and (ii) voiding pressures. Children with neurogenic bladders were excluded from the analysis of voiding pressure. Children with neurogenic bladder are often on intermittent clean catheterisation and the usual indications to do a pressure–flow study in these patients are to determine upper tract safety, the need for bladder drainage and anticholinergic medication. Although accurate voiding phase pressure measurements are not possible without measuring Pabd, in children with underactive and decompensated bladders, the staccato or interrupted pattern of flow, along with careful interpretation of the Pves trace could reliably identify abdominal straining, thus enabling clinical decision-making.
In children with non-neurogenic LUTD, as mentioned earlier, the interpretation of the filling phase was concordant in most cases. However, the correlation of voiding pressures, by comparing absolute values of Pdet and ∆Pves at Qmax was only moderate. This subgroup included children with dysfunctional voiding as well as children with sequelae of PUV fulguration. As the correlation is at best, moderate, pressure–flow studies without the Pabd trace for clinical scenarios that require meticulous assessment of the voiding phase are not recommended by the authors.
There is no literature available with regard to single-channel UDS in children. In the present study, the single-channel traces were obtained from the multi-channel traces that were available for the children. This negated any other possible confounders influencing the pressure traces. Comparison of a single- and multi-channel study done at two different times is not comparable due to multiple other confounders, especially in children. At the same time, a derived single-channel trace may be a source of bias, as these traces were balanced with the help of a rectal catheter at the time of multi-channel study. This may have resulted in a falsely accurate interpretation of the derived single-channel graph.
Another important limitation of the present study is the retrospective nature of the study. Secondly, the relatively small sample size may not be sufficient to establish the non-inferiority of single-channel cystometry. Additional blinded independent reviewers would help in determining inter-observer consistency in interpretation. Most children included in the present study had neurogenic bladder or PUVs and the results may not be generalisable across all diagnoses. One may also argue that most children with a neurogenic bladder are insensate and may not be bothered by a rectal catheter. However, the authors believe that the emotional and psychological aspects cannot be ignored in children, and it is of value if the rectal catheter can be avoided in some.
Although the present study had its limitations, these results should encourage larger prospective studies to be undertaken to conclusively address this hypothesis, because often in our pursuit for accurate diagnoses the discomfort and trauma caused by these investigations are often over-looked, especially in children. The ideal study design would be to do the single- and multi-channel study consecutively in the same sitting and compare the same with a t-test.
Conclusion
This retrospective blinded analysis suggests that rectal catheters may be avoided in a carefully selected group of children requiring assessment of only the storage phase.
Ethical approval
This retrospective study was in accordance with the ethical standards of the institution and the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Acknowledgments
We acknowledge Mr Emmanuel, Urology Technician, for maintaining a database of patients undergoing UDS at our institute, and Dr Shailaja for data entry.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hjälmås K. Urodynamics in normal infants and children. Scand J Urol Nephrol Suppl. 1988;114:20–27.
- Drzewiecki BA, Bauer SB. Urodynamic testing in children: indications, technique, interpretation and significance. J Urol. 2011;186:1190–1197.
- Hjälmås K, Hoebeke PB, de Paepe H. Lower urinary tract dysfunction and urodynamics in children. Eur Urol. 2000;38:655–665.
- Wyndaele JJ, THi HV, Pham BC, et al. The use of one-channel water cystometry in patients with a spinal cord lesion: practicalities, clinical value and limitations for the diagnosis of neurogenic bladder dysfunction. Spinal Cord. 2008;47:526–530.
- Sutherst JR, Brown MC. Comparison of single and multichannel cystometry in diagnosing bladder instability. Br Med J (Clin Res Ed). 1984;288:1720–1722.
- Ricci Arriola P, Solá Dalenz V, Pardo Schanz J. Study of female urinary incontinence with single channel urodynamics: comparison of the symptoms on admission. Analysis of 590 females [Article in Spanish]. Arch Esp Urol. 2009;62:115–123.
- Zamli AH, Ratnalingam K, Yusmido YA, et al. Diagnostic accuracy of single channel cystometry for neurogenic bladder diagnosis following spinal cord injury: a pilot study. Spinal Cord Ser Cases. 2017;3:16044.
- Sand PK, Hill RC, Ostergard DR. Supine urethroscopic and standing cystometry as screening methods for the detection of detrusor instability. Obstet Gynecol. 1987;70:57–60.
- Bauer SB, Nijman RJM, Drzewiecki BA, et al. International children’s continence society standardization report on urodynamic studies of the lower urinary tract in children. Neurourol Urodyn. 2015;34:640–647.
- Bael A, Lax H, de Jong TP, et al. The Relevance of urodynamic studies for urge syndrome and dysfunctional voiding: a multicenter controlled trial in children. J Urol. 2008;180:1486–1495.
- Lu T, Liao L. Typical value ranges and typical signal patterns in the initial cough in patients with neurogenic bladder: quality control in urodynamic studies. Int Neurourol J. 2016;20:214–223.
- Shapiro E. Urodynamics in children. Rev Urol. 2012;14:36–38.
- Koff SA. Estimating bladder capacity in children. Urology. 1983;21:248.
- Nevéus T, von Gontard A, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the standardization committee of the international children’s continence society (ICCS).Neurourol Urodyn. 2007;26:90–102.
- Hoebeke P, Bower W, Combs A, et al. Diagnostic evaluation of children with daytime incontinence. J Urol. 2010;183:699–703.
- Watson PF, Petrie A. Method agreement analysis: a review of correct methodology. Theriogenology. 2010;73:1167–1179.
- Schäfer W, Abrams P, Liao L, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies.Neurourol Urodyn. 2002;21:261–274.
- Suskind AM, Clemens JQ, Kaufman SR, et al. Patient perceptions of physical and emotional discomfort related to urodynamic testing: a questionnaire-based study in men and women with and without neurologic conditions. Urology. 2015;85:547–551.
- Hogan S, Gammie A, Abrams P. Urodynamic features and artefacts. Neurourol Urodyn. 2012;31:1104–1117.