ABSTRACT
Objective
: To systematically review the use of drug-eluting stents (DES) and drug-coated balloons (DCB) in urology.
Materials and Methods
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. PubMed, Scopus, Web of science and Cochrane Library online databases were searched in February 2019. Experimental and clinical studies, which included the placement of a DES or dilatation with DCB for investigating their potential use in the urinary tract for the management of ureteric or urethral pathologies, were included. The primary endpoint was to evaluate the current use of DES and DCB in urology.
Results
A total of 29 articles were included in the systematic review. A total of 10 studies tested DES or DCB containing anti-proliferative agents (paclitaxel, zotarolimus, sirolimus, halofugione). Antibiotic agent-containing DES were tested in nine studies (triclosan, quinolones, teicoplanin, nitrofurantoin, silver sulfadiazine). A total of eight studies investigated the release of anti-inflammatory agents by DES (ketorolac, indomethacin, EW-7197). Another group studied heparin-eluting stents.
Conclusion
Despite the inconclusive outcomes of the three randomised controlled trials, drug-coated/eluting devices constitute a promising field in urology for the prevention of complications associated with conventional stents including pain and encrustation. Pre-clinical in vitro and in vivo studies have shown their ability to mitigate inflammation, inhibit re-stenosis and improve pain as indicated by declined use of anti-inflammatory drugs.
Abbreviations: DES: drug-eluting stents; DCB: drug-coated balloons; DCS: drug-coated stents; HF: halofungione; MCP-1: monocyte chemoattractant protein 1; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PTCA: percutaneous transluminal coronary angioplasty; RANTES: regulated on activation, normal T-cell expressed and secreted; RCT: randomised controlled trial; USSQ, Ureteric Stent Symptoms Questionaire.
Introduction
The ureteric stent is a useful tool to avoid kidney damage or sepsis in cases of obstructive uropathy due to stenoses, stones, and extrinsic compression. The ideal ureteric stent should demonstrate optimal flow characteristics and should be well tolerated by the patient, but conventional stents have significant comorbidities, including stent-associated infection, encrustation, migration, hyperplastic urothelial reaction, and patient’s discomfort [Citation1].
Another frequent condition affecting the lower urinary tract is urethral stricture formation related to high re-stenosis rates. A minimally invasive treatment for this is mechanical dilatation with a balloon or placing a urethral stent [Citation2], which are fraught with the same problems as ureteric stents, with rates of re-stenosis despite stent-placement being relatively high [Citation3].
Over recent years, the development of strategies to address ureteric stent-associated complications has been driven by progress made in cardiology. This is mostly due to the fact that vascular stenting, much like the ureter and urethra, involves maintaining patency of a tube whose main function is to conduct a liquid, and innovation in the cardiovascular space to prevent stenosis has progressed faster than in urology. In cardiology, percutaneous transluminal coronary angioplasty (PTCA) has become the main method of coronary re-vascularisation; however, re-stenosis remains a significant complication. Devices that release immunosuppressive agents to minimise benign tissue proliferation, which characterises intimal hyperplasia, proved to be successful over the years. These drug-eluting stents (DES) are devices that release a single or multiple bioactive agents in a controlled manner, depositing the agent on adjacent tissues, while drug-coated stents (DCS) are covered with a single or multiple pharmaceutical agents that provides additional properties to the stent [Citation4]. These devices are widely used in cardiology and vascular surgery, and have significantly reduced the re-stenosis rates after PTCA [Citation5,Citation6]. A more recent approach to further reduce re-stenosis rates after PTCA is the use of drug-coated balloons (DCB) that release the immunosuppresive agent directly at the site of the dilatated vascular stricture and reduce the re-stenosis rates without the need for an indwelling foreign material (i.e. stent) postoperatively. These balloons have reduced the need for DES and can also treat vascular sites that were otherwise unsuitable for stent insertion [Citation7].
The high success of the DES in cardiology and interventional radiology led to the adoption of the drug-eluting concept by endourological research in an attempt to prevent significant complications associated with indwelling ureteric stents [Citation8]. The concept of DCB for use in endourology has been proposed.
The present systematic review aimed to evaluate the literature and provide information about the use of DRE and DCB in urology.
Methods
Search strategy – Eligibility criteria
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. PubMed, Scopus and Cochrane Library online databases were searched in February 2019 for all relevant articles with no language restriction. The used search string was (elut* OR coat*) AND (stent OR balloon) AND (ureter* OR urethra*). The study was registered in International Prospective Register of Systematic Reviews (PROSPERO) with the reference number CRD42019121726 (). The eligibility criteria are presented in .
Table 1. Eligibility criteria of the systematic review
Data extraction
After de-duplication, the selected articles were screened by two authors (S.V., C.A.) independently according their titles and abstracts. Then, the full texts of the potentially eligible articles were retrieved and scrutinised using a standardised form. Disagreements were solved by consulting a supervisor (P.K.).
Types of study design included
All studies, either experimental or clinical, which included the placement of DES/DCS or dilatation with a DCB for investigating their potential use in the urinary tract for the management of ureteric or urethral pathologies, were included.
Population included
The population included in the clinical trials were humans (male and female), who underwent a stent insertion based on indications, and animals.
Types of outcomes
The primary endpoint was to review the literature about the current and potential use of DES/DCS and DCB in urology.
Results
Search results
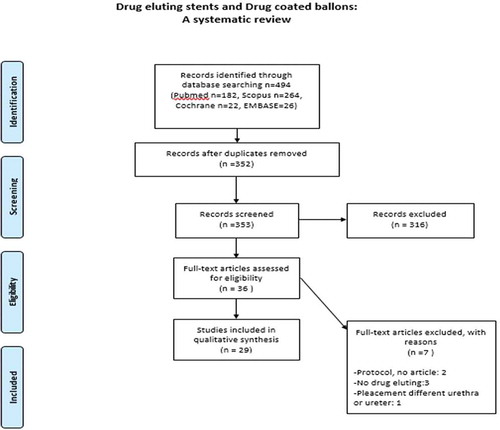
A total of 485 publications were screened for eligibility and 29 articles were eventually included in the systematic review ().
Study and population characteristics
[Citation9–17], [Citation3,Citation18–36] and [Citation3,Citation33] show a summary of the included studies. The available studies were published over a period of 18 years (2000–2019).
Table 2. Comparison of the experimental (in vitro) trials of DES/DCS
Table 3. Comparison of the clinical (in vivo) trials of DES/DCS
Table 4. Comparison of the clinical trials (in vivo) of DCBs
There were eight in vitro trials summarised in and 21 clinical trials (in vivo) summarised in .
Two of the clinical trials involved DCB, of which one studied the use of DCB in the urethra [Citation3], while the other investigated their use in the ureter [Citation33]. In all, 19 clinical studies focussed on DES/DCS, of which 12 were about the use of DES/DCS in the ureter [Citation12,Citation18–22,Citation24,Citation28,Citation29,Citation31,Citation32,Citation34] and seven in the urethra [Citation16,Citation23,Citation26,Citation30,Citation35–37].
Five clinical trials included humans [Citation18–20,Citation29,Citation34]; three randomised controlled trials (RCTs) [Citation18,Citation29,Citation34] and two comparative non-RCTs [Citation19,Citation20]. The remaining 16 clinical trials were performed using a variety of animal models (rabits, rats, pigs and dogs) [Citation3,Citation12,Citation16,Citation21–24,Citation26,Citation28,Citation30–33,Citation35–37].
Comparisons of interventions results
A total of 10 studies tested anti-proliferative agents containing DES/DCS or DCB [Citation3,Citation11,Citation24,Citation28,Citation30,Citation31,Citation33,Citation35-37]. Of these, seven investigated paclitaxel [Citation3,Citation11,Citation28,Citation31,Citation33,Citation35,Citation36], one zotarolimus [Citation24], one sirolimus [Citation37] and one halofugione (HF) [Citation30].
Antibiotic agent-containing DES/DCS were tested in nine studies [Citation12–15,Citation17-19,Citation22,Citation34], of which four studied triclosan [Citation12,Citation13,Citation19,Citation34], two the use of quinolones [Citation14,Citation17], two separate articles studied the use of either teicoplanin [Citation22] or nitrofurantoin [Citation15], and one studied the use of silver sulfadiazine [Citation18].
A total of eight studies investigated the release of anti-inflammatory agents by DES/DCS [Citation10,Citation16,Citation21,Citation23,Citation26,Citation27,Citation29,Citation32], of which four investigated ketorolac [Citation10,Citation21,Citation29,Citation32], three investigated indomethacin [Citation16,Citation26,Citation27], and one a TGF-β type 1 receptor kinase inhibitor, EW-7197 [Citation23]. Furthermore, another group studied heparin-eluting stents [Citation20].
Discussion
Given the success of DES/DCS and DCB in the prevention of re-stenosis in cardiology and the relevance to stricture formation and other relevant complications following endourological procedures [Citation13], the present systematic review was performed to assess their investigation in our field. Overall, the included studies could be divided into three major categories according to the drug used: anti-cancer, antibiotic, and anti-inflammatory agents.
DES/DCS containing-antibiotic agents
UTI is the most common hospital infection and is associated with the existence of foreign bodies in the urinary tract, e.g. catheters, stents, and nephrostomy tubes [Citation15]. This is because the foreign body in the urinary tract forms a surface easily colonised by bacteria, which over time form a highly resistant biofilm [Citation13–15,Citation17]. In an attempt to address this, research has focussed on coating ureteric stents with antibiotics to prevent bacterial adhesion and reduce associated infection rates [Citation13–15,Citation17].
Triclosan was studied in four articles [Citation12,Citation13,Citation19,Citation34]. It is an antimicrobial agent that intervenes with fatty-acid synthesis and hence the integrity of the bacterial cell-wall [Citation13]. Chew et al. [Citation13] tested the efficacy of a triclosan-eluting stent in artificial urine against a variety of common uropathogens (E. coli, E. faecalis, S. aureus, K. pneumoniae, P. mirabilis, P. aeruginosa) assessing bacterial growth and adherence. They concluded that triclosan DES/DCS halted the growth of most of the uropathogens, except P. aeruginosa. Cadieux et al. [Citation12] subsequently tested the efficacy of this stent using a rabbit UTI model in which the curls from triclosan DES/DCS and non-eluting controls were sutured in the bladder of rabbits, previously instilled with P. mirabillis. Culture of both urine and stent pieces revealed significantly reduced numbers in both samples from rabbits with a triclosan-eluting stent. In addition, the bladder of the triclosan group was less inflamed than the control group. Another study by Cadieux et al. [Citation19] investigated the long-term effiacy of the triclosan-eluting stent in eight patients stented due to cancer, ureteric stricture or retroperitoneal fibrosis. After an initial 3-month control period with conventional stenting, the stents were replaced by triclosan DES/DCS for 3 months. Patients were monitored over both periods with urine cultures and were prescribed antibiotics as indicated by UTI symptoms. Overall, there was no significant difference in the number of adherent bacteria to either the trilosan-eluting or non-eluting stents or in urine cultures. However, the need for antibiotic therapy was lower with a triclosan stent in place suggesting lower symptomatic UTIs. In a separate RCT, Mendez et al. [Citation34] studied the efficacy of the triclosan DES/DCS compared to a conventional stent in 20 patients (10 patients/group), who required short-term stenting. Overall, there was no difference in culture and encrustation between the groups; however, patients in the triclosan group reported lesser flank, abdominal or urethral pain during activity or urination.
Collectively, these studies indicate that despite promising effects with regard to the prevention of bacterial adhesion and development of UTI in in vitro and preclinical in vivo models, these same benefits were not seen in patients. That said, differences were observed in the use of antibiotics and lower overall dyscomfort, suggesting an effect on the development of symptomatic UTIs and patient discomfort. Despite these beneficial effects, this stent is no longer available on the market.
The antimicrobial effects of quinolones were studied in two articles [Citation14,Citation17]. Elayarajah et al. [Citation14] used stents impregnated with a mixture of ofloxocin and ornidazole. Using a simplistic agar diffusion test they showed the impregnated stents to be effective at killing E. coli and S. epidermidis. In addition, they tested efficacy against preventing bacterial adhesion in artificial urine and found the number on the DES/DCS to be significantly lower compared to the conventional stent. Ma et al. [Citation17] studied the characteristics and effiacy of three ciprofloxacin DES/DCS, differing in the composition of the antibiotic carrier. The stents remained in artificial urine for 120 days, over which time the coating degradation, antibiotic-release profile, the anti-bacterial activity, and cytotoxicity were assessed. It was found that ciprofloxacin release occured in three phases and was highly dependent on the degradation activity of the coating, except from the first 7 days (‘burst stage’). An inital burst release of antibiotics is important to ensure high enough concentrations to kill any introduced bacterial loads and prevent bacterial adhesion, colonisation and subsequent biofilm formation. Getting this amount right is critical, as the release of sub-optimal concentrations, especially over long periods of time, has a significant risk for inducing resistance. In general, it was shown that ciprofloxacin DES/DCS have good antibacterial activity against S. aureus and E. coli, and no cytotoxicity against human foreskin fibroblasts.
Johnson et al. [Citation15], on the other hand, studied the antimicrobial effects of urethral catheters coated with silver and nitrofurazone by using an inhibition zone and adherence assay. They compared two silver-coated and one nitrofurazone-coated marketed urethral catheters according to the inhibitory effect against E. coli (extended spectrum cephalosporin resistant and susceptible strains) and a P. aeruginosa. The inhibition activity was greater and lasted longer with the nitrofurazone-coated catheter for all E. coli strains. The silver-coated catheter showed no statistically significant inhibition, while none of the three catheters had any effect against P. aeruginosa. Moreover, El-Nahas et al. [Citation18] conducted an RCT comparing a silver sulfadiazine DCS to conventional stents in 126 patients after endoscopic lithotripsy. The authors compared the results from urine cultures collected before and after stenting (at removal), as well as the stenting-related symptoms using the Ureteric Stent Symptoms Questionaire (USSQ). The study concluded that there was no difference between the two groups regarding urine cultures and the USSQ scores. It is interesting that silver, a known antimicrobial currently used throughout medicine, was ineffective against both E. coli and P. aeruginosa, especially as it is known to be effective against these species. This may indicate that the concentration of silver released from these stents was not high enough to effectively kill the bacteria. This illustrates that while the ability to release a given agent from the surface of devices is important, it must reach high enough concentrations in the surrounding medium to be promising clincially.
Cirioni et al. [Citation22] used a different appraoch, developing a stent that released RNAIII-inhibiting peptide, which inhibits the formation of staphylococcal biofilm and the production of toxins, with or without teicoplanin, and tested the efficacy in a rat model of UTI. Interestingly, they found the combination of RNAIII-inhibiting peptide and teicoplanin to decrease the colonisation drastically with no bacterial counts (P < 0.001) in urine culture.
An experiment to reduce encrustation was conducted by Cauda et al. [Citation20], who did not use antibiotic agents, but heparin DES/DCS. Heparin is highly negatively charged and believed to prevent encrustation by repelling negatively charged crystals. They inserted a heparin DES/DCS and a conventional stent in five patients with bilateral obstruction and showed that the encrustation on heparinised stents were not as thick and extensive as the encrustation on the conventional one. Subsequent in vitro studies performed by Lange et al. [Citation38] showed no significant difference in bacterial adhesion between the heparin-coated and -uncoated stents.
Anti-inflammatory agent containing DES/DCS
One of the most common complications of indwelling stents is pain and discomfort felt by the patient. As a result, several studies have investigated the release of anti-inflammatory drugs by coating of stents with these agents [Citation10,Citation13,Citation29].
Ketorolac, which is a NSAID, was tested in four studies [Citation10,Citation21,Citation29,Citation32]. Barros et al. [Citation10] tested the release profile of ketolorac from two different types of biodegradable stents, in artificial urine. Ketorolac was found to be released within the first 72 h, which is the desirable duration in the case of postoperative stent insertion, where on average oedema resolves within 72 h.
Furthermore, the pharmakokinetics of ketorolac-eluting stents were studied in a porcine model by Chew et al. [Citation21], by measuring the distribution of the drug in plasma, urine and relevant tissues in pigs receiving the drug orally or via the insertion of a ureteric stent containing either 15%, 13% or 7% of the drug. Overall, most of the drug was released within 30 days and the highest levels of the drug were found in the ureteric and bladder tissues in the stented groups in a dose-dependent fashion. Lin et al. [Citation32] studied the delivery of lidocain and ketorolac of a DES/DCS in vitro and in vivo in rabbit models. They showed that the DES/DCS was able to obtain high doses of analgesic for at least 50 days in vitro and 30 days in vivo. Krambeck et al. [Citation29] went one step further and performed a RCT on post-ureteroscopy patients. The RCT included 276 patients who underwent ureteroscopy due to stone disease or for diagnostic reasons. The primary endpoint of this RCT was pain, defined as unscheduled Emergency Room visits, alteration in pain management and early removal of the stent, while secondary endpoints included intervention due to the stents, visual analogue scale pain assessment, medication use, satisfaction of patient and plasma concentration of ketorolac in the group with DES/DCS. None of the plasma samples taken from patients with DES/DCS had detectable levels of the drug, while differences in pain were identified only in male participants aged <45 years. This overall negative result is likely attributed to the fact that despite promising results in preclinical in vitro and in vivo large animal studies, not enough drug reached relevant tissues to elicit an effect. The sustained release of a high enough drug concentration to achieve effective concentrations within relevant tissues to have an effect remains a challenge in the urinary tract and will need to be overcome before DES can become a reality in urology.
Aside from urethral DES, a few studies have investigated the use of drug elution to address strcture formation in the urethra. Indomethacin-eluting urethral stents were investigated by Kotsar et al. [Citation16,Citation26,Citation27] in three studies. The first study investigated the degradation pattern of an absorbable urethral stent eluting indomethacin, dexamethasone, and ciprofloxacin. The stents were inserted in the posterior urethra of 16 rabbits, which were killed after 1 month at which point the urethras were harvested and histologically examined. The control and dexamethasone-coated stents were totally absorbed, while indomethacine and ciprofloxacine delayed the degradation process of the stent and caused epithelial hyperplasia. Histologically, there was no difference in either acute or chronic inflammation, or fibrosis [Citation16]. In a subsequent study, they investigated the production of cytokines and other inflammatory mediators caused by absorbable urethral DRE in vitro and in vivo. The eluted compounds were indomethacin, dexamethasone, and simvastatin. The stents were inserted in the posterior urethra of 18 rabbits, which were killed at 3 weeks or 3 months. Overall, only dexamethasone-eluting stents produced a significantly greater reaction than the control group, while at 3 months the reaction was resolved in all groups. Based on these results, they concluded that the drug elution on absorbable stents does not intervene significantly with the degradation process of the stent and is considered safe [Citation27]. In the last study, the group investigated the cytokine profiles induced by an absorbable indomethacin-eluting stent in vitro and in vivo in the rabbit urethra. Initial in vitro tests showed that indomethacin-eluting stents reduced the production of monocyte chemoattractant protein 1 (MCP-1) and RANTES (regulated on activation, normal T-cell expressed and secreted), while it had no influence on TGF-β production. Subsequent in vivo studies showed that at 3 months it caused less inflammation and calcification compared to the bare control stents, with no negative effects of drug elution on the degradation process of the stent [Citation26]. These absorbable urethral stents might be useful after a urethrotomy to reduce the chance of re-stenosis, but further testing is mandatory.
Moreover, Han et al. [Citation23] tested a nano-fibre covered self-expandable stent coated with EW-7197, which constitutes a TGF-β type 1 tyrosine kinase inhibitor, in a canine model over an 8-week period. The overall goal was to compare granulation tissue formation between DES/DCS and the control stent. Dogs with the DES/DCS had larger urethral luminal diameters and the pathology report revealed that in the DES/DCS group the papillary projection and submucosal fibrosis were less thick and the number of epithelial layers, as well as the degree of collagen deposition, was lower than in the control group. In addition, Krane et al. [Citation30] studied the use of HF, a collagen type I inhibitor, eluting catheters in the urethra of rats following the creation of a stricture using electrocautery. Interestingly, no new type I collagen was formed in the group with HF-eluting catheters.
DES/DCB and DCB containing anti-proliferative agent
DES/DCS releasing an anti-proliferative agent have been used with great success in interventional cardiology due to their ability to stop cell proliferation and hence the formation of hyperplastic or fibrotic tissue [Citation31,Citation35]. Paclitaxel is the most tested drug in this category [Citation3,Citation11,Citation28,Citation31,Citation33,Citation35,Citation36]. Barros et al. [Citation11] did an experimental trial with biodegradable ureteric stents eluting paclitaxel and doxorubicine to investigate the permeability of the drugs in three different membrane models: polyethersulfone membrane, human umbilical vein endothelial cells, and ex vivo porcine ureter. Overall, they found that the two drugs remained in the ureter and only a small proportion crossed all layers of the ureter. Kram et al. [Citation28] studied the hyperplastic proliferation of the ureter and whether it can be prevented by the use of paclitaxel DES/DCS in a rat model in which a ureteroureterostomy was performed and either a conventional or a paclitaxel DES was inserted. The histological report stated that the proliferation on the anastomosis side was lower in the paclitaxel group (laballing index 41.27 vs 51.58, P < 0.001). Likewise, Liatsikos et al. [Citation31] compared a conventional stent to a paclitaxel DES/DCS in the porcine ureter and concluded that after 21 days ureters with an indwelling paclitaxel DES/DCS remained more patent on urography due to the production of less inflammation or hyperplasia. Similar results were obtained by Shin et al. [Citation35] in the canine urethra.
Wang et al. [Citation36] tested a biodegradable paclitaxel DES/DCS in the urethra of rabbits, and observed that at 12 weeks indwelling time the stents were completely absorbed. In the DES/DCS group the urethral mucosa resembled normal mucosa, whereas in the control group, the urethra was tough and rigid.
In addition to DES/DCS, DCB have also been studied as a way to dilate urethral and ureteric strictures. Along these lines, Barbalias et al. [Citation3] studied the distribution of paclitaxel in the urethral layers of rabbits. For this, the posterior urethra was dilatated and a paclitaxel-coated balloon inflated at the same location. Paclitaxel was found to be distributed througout the urethral tissue upon histological analysis. Similarly, Liourdi et al. [Citation33] used the same setup in the ureter and found pactlitxel distributed in all layers of the ureter. While paclitaxel DES/DCS and DCB represent an interesting approach that has been validated in vitro and in vivo, it must be pointed out that this has been done in models that did not include a stricture. As a result further studies in more realistic in vivo or human clinical trials are required to determine usefulness of these devices in clinical practice.
Besides paclitataxel, other chemotherapeutic drugs have been used as well. Kallidonis et al. [Citation24] studied a zotarolimus DES/DCS in pigs and rabbits, and showed that anti-cancer DES/DCS prevent obstruction by reducing inflammation and hyperplasia.
Conclusions
There is no doubt that there is great potential for drug-coated/eluting devices to become useful at preventing complications associated with conventional stents, including pain and encrustation. Pre-clinical in vitro and in vivo studies have shown their ability to reduce inflammation, prevent re-stenosis, and improve pain as indicated by decreased use of anti-inflammatory drugs. However, three RCTs that included humans have shown ambiguous results. Therefore, human trials and testing in realistic clinical scenarios are still lacking and required to give insight into whether or not DES/DCS and DCB that have made a significant difference in cardiology will also do so in urology. One of the biggest challenges that will need to be achieved for DES/DCS and DCB to be effective is the sustained release of the drugs at concentrations high enough to overcome dilution in urine and elimination via urine flow and reach effective levels in the ureteric tissues.
Dislosure statement
None.
Support and Grant
None.
Aknowledgements
None.
References
- Al-Aown A, Kyriazis I, Kallidonis P. Ureteral stents: new ideas, new designs. Therapeutic Advances in Urology. 2010;2(2):85–92.
- Desai M, Vyas J, Ganpule A,et al.Balloon dilatation for male urethral strictures. “Revisited”. Urology Annals. 2013;5(4):245–248.
- Barbalias D, Lappas G, Ravazoula P, et al. Evaluation of the distribution of paclitaxel after application of a paclitaxel-coated balloon in the rabbit urethra. Journal of Endourology. 2018;32(5):381–386.
- Fattori R, Piva T. Drug-eluting stents in vascular intervention. The Lancet. 2003;361(9353):247–249.
- Ni L, Chen H, Luo Z, et al. Bioresorbable vascular stents and drug-eluting stents in treatment of coronary heart disease: a meta-analysis. Journal of Cardiothoracic Surgery. 2020;15(1):26.
- Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. New England Journal of Medicine. 2013;368(3):254–265.
- Nestelberger T, Kaiser C, Jeger R. Drug-coated balloons in cardiovascular disease: benefits, challenges, and clinical applications. Expert Opinion on Drug Delivery. 2020;17(2):201–211.
- Kallidonis PS, Georgiopoulos IS, Kyriazis ID, et al. Drug-eluting metallic stents in urology. Indian J Urol. 2014;30(1):8–12.
- Antimisiaris SG, Siablis D, Liatsikos E, et al. Liposome-Coated Metal Stents: an in Vitro Evaluation of Controlled-Release Modality in the Ureter. J Endourol. 2000;14(9):743–747.
- Barros AA, Oliveira C, Reis RL, et al. Ketoprofen-eluting biodegradable ureteral stents by CO2 impregnation: in vitro study. Int J Pharm. 2015;495(2):651–659.
- Barros AA, Oliveira C, Reis RL, et al. In Vitro and Ex Vivo Permeability Studies of Paclitaxel and Doxorubicin From Drug-Eluting Biodegradable Ureteral Stents. J Pharm Sci. 2017;106(6):1466–1474.
- Cadieux PA, Chew BH, Knudsen BE, et al. Triclosan loaded ureteral stents decrease proteus mirabilis 296 infection in a rabbit urinary tract infection model. J Urol. 2006;175(6):2331–2335.
- Chew BH, Cadieux PA, Reid G, et al. Second Prize : in-Vitro Activity of Triclosan-Eluting Ureteral Stents against Common Bacterial Uropathogens. J Endourol. 2006;20(11):949–58.
- Elayarajah E, Rajendran R, Venkatrajah B, et al. Biopolymer tocopherol acetate as a drug carrier to prevent bacterial biofilm formation on silicone ureteral stents. Int J Pharm Sci Rev Res. 2011;7:96–103.
- Johnson JR, Johnston BD, Kuskowski MA, et al. In vitro activity of available antimicrobial coated foley catheters against escherichia coli, including strains resistant to extended spectrum cephalosporins. J Urol. 2010;184(6):2572–2577.
- Kotsar A, Isotalo T, Uurto I, et al. Urethral in situ biocompatibility of new drug-eluting biodegradable stents: an experimental study in the rabbit. BJU International. 2009;103(8):1132–1135.
- Ma X, Xiao Y, Xu H, et al. Preparation, degradation and in vitro release of ciprofloxacin-eluting ureteral stents for potential antibacterial application. Mater Sci Eng C Mater Biol Appl. 2016;66:92–99.
- El-Nahas AR, Lachine M, Elsawy E, et al. A randomized controlled trial comparing antimicrobial (silver sulfadiazine)-coated ureteral stents with non-coated stents. Scand J Urol. 2018;52(1):76–80.
- Cadieux PA, Chew BH, Nott L, et al. Use of triclosan-eluting ureteral stents in patients with long-term stents. J Endourol. 2009;23(7):1187–1194.
- Cauda F, Cauda V, Fiori C, et al. Heparin Coating on Ureteral Double J Stents Prevents Encrustations: an in Vivo Case Study. J Endourol. 2008;22(3):465–472.
- Chew BH, Davoudi H, Li J, et al. An In Vivo Porcine Evaluation of the Safety, Bioavailability, and Tissue Penetration of a Ketorolac Drug-Eluting Ureteral Stent Designed to Improve Comfort. J Endourol. 2010;24(6):1023–1029.
- Cirioni O, Ghiselli R, Minardi D, et al. RNAIII-inhibiting peptide affects biofilm formation in a rat model of staphylococcal ureteral stent infection. Antimicrob Agents Chemother. 2007;51(12):4518–4520.
- Han K, Park J-H, Yang S-G, et al. EW-7197 eluting nano-fiber covered self-expandable metallic stent to prevent granulation tissue formation in a canine urethral model. PLoS One. 2018;13(2):e0192430.
- Kallidonis P, Kitrou P, Karnabatidis D, et al. Evaluation of zotarolimus-eluting metal stent in animal ureters. J Endourol. 2011;25(10):1661–16617.
- Kim SW, Park NC, Lee SW, et al. Efficacy and safety of a fixed-dose combination therapy of tamsulosin and tadalafil for patients with lower urinary tract symptoms and erectile dysfunction: results of a randomized, double-blinded, active-controlled trial. J Sex Med. 2017;14(8):1018–1027.
- Kotsar A, Nieminen R, Isotalo T, et al. Preclinical evaluation of new indomethacin-eluting biodegradable urethral stent. J Endourol. 2012;26(4):387–392.
- Kotsar A, Nieminen R, Isotalo T, et al. Biocompatibility of new drug-eluting biodegradable urethral stent materials. Urology. 2010;75(1):229–234.
- Kram W, Rebl H, Wyrwa R, et al. Paclitaxel-coated stents to prevent hyperplastic proliferation of ureteral tissue: from in vitro to in vivo. Urolithiasis. 2020;48(1):47–56.
- Krambeck AE, Walsh RS, Denstedt JD, et al. A novel drug eluting ureteral stent: a prospective, randomized, multicenter clinical trial to evaluate the safety and effectiveness of a ketorolac loaded ureteral stent. J Urol. 2010;183(3):1037–1042.
- Krane LS, Gorbachinsky I, Sirintrapun J, et al. Halofuginone-coated urethral catheters prevent periurethral spongiofibrosis in a rat model of urethral injury. J Endourol. 2011;25(1):107–112.
- Liatsikos EN, Karnabatidis D, Kagadis GC, et al. Application of paclitaxel-eluting metal mesh stents within the pig ureter: an experimental study. Eur Urol. 2007;51(1):217–223.
- Lin Y-C, Liu K-S, Lee D, et al. In Vivo and In Vitro Elution of Analgesics from Multilayered Poly(D,L)-lactide- co -glycolide Nanofibers Incorporated Ureteral Stents. J Nanomater. 2018;2018:4943210.
- Liourdi D, Kallidonis P, Kyriazis I, et al. Evaluation of the distribution of paclitaxel by immunohistochemistry and nuclear magnetic resonance spectroscopy after the application of a drug-eluting balloon in the porcine ureter. J Endourol. 2015;29(5):580–589.
- Mendez-Probst CE, Goneau LW, MacDonald KW, et al. The use of triclosan eluting stents effectively reduces ureteral stent symptoms: a prospective randomized trial. BJU International. 2012;110(5):749–754.
- Shin JH, Song H-Y, Choi CG, et al. Tissue Hyperplasia: influence of a Paclitaxel-eluting Covered Stent—Preliminary Study in a Canine Urethral Model. Radiology. 2005;234(2):438–444.
- Wang Z-X, Hong B-F, Zhang X, et al. New biodegradable drug-eluting stents for urethral strictures in a rabbit model. J Bioact Compat Pol. 2011;26(1):89–98.
- Kim KY, Park J-H, Kim DH, et al. Sirolimus-eluting Biodegradable Poly- l -Lactic Acid Stent to Suppress Granulation Tissue Formation in the Rat Urethra. Radiology. 2018;286(1):140–148.
- Lange D, Elwood CN, Choi K, et al. Uropathogen interaction with the surface of urological stents using different surface properties. J Urol. 2009;182(3):1194–1200.