ABSTRACT
Objective
: To review the debate about the routine use of cryopreserved testicular sperm for intracytoplasmic sperm injection (ICSI) from patients with non-obstructive azoospermia (NOA), as some authors suggest repeating sperm retrieval in such cases due to poorer ICSI results when frozen–thawed testicular sperm is used compared with fresh sperm.
Methods
: A systematic literature review was performed in August 2020 using the Medical Literature Analysis and Retrieval System Online (MEDLINE), Web of Science databases and the Excerpta Medica dataBASE (EMBASE), and we included 26 studies that were considered eligible for this systematic review.
Results
: In all, 1189 publications were screened and 26 articles were included in the systematic review. Three meta-analysis reviews were included and they all concluded that the use of fresh and frozen sperms for ICSI from patients with NOA showed comparable fertilisation and pregnancy rates.
Conclusion
: The use of frozen testicular sperm from men with NOA results in fertilisation and clinical pregnancy rates similar to those of fresh sperm. This may encourage fertility centres to use frozen testicular sperm samples, as this policy has certain advantages that would help with organising their workflow.
Abbreviations: CPR: clinical pregnancy rate; 2PN%: two pronuclei % fertilisation rate; ICSI: intracytoplasmic sperm injection; NOA: non-obstructive azoospermia; OA, obstructive azoospermia; SCO: Sertoli cell-only syndrome; (micro-)TESE: (microsurgical) testicular sperm extraction
Introduction
Azoospermia is defined by a complete absence of sperm in the ejaculate after centrifugation of two semen specimens and affects about 1–2% of males and 10–15% of the infertile males [Citation1,Citation2].
Obstructive azoospermia (OA) is the absence of spermatozoa in the ejaculate secondary to a transport failure between the testis and urethra [Citation3]. While two-thirds of azoospermic cases are categorised as non-obstructive azoospermia (NOA) caused by spermatogenic failure, which means failure to produce sperm in the testes, with a spectrum of various causes of intrinsic testicular impairment, but fortunately focal areas of sperm production can be found in some of these men with NOA [Citation4].
An isolated diagnostic testicular biopsy should rarely be indicated, as it will not provide definitive proof of whether sperm will be found during sperm retrieval, particularly in Sertoli cell-only syndrome (SCO) and maturation arrest cases [Citation5–10]. Histopathological evaluation by removal of rare spermatogenesis foci may jeopardise future retrieval attempts [Citation10]. So diagnostic biopsies are indicated if the differential diagnosis between OA and NOA cannot be established based on clinical and endocrine parameters, and also for screening for carcinoma in situ in patients with azoospermia [Citation11].
A suitable treatment for NOA is microsurgical testicular sperm extraction (micro-TESE) followed by intracytoplasmic sperm injection (ICSI). A testicular biopsy can be performed on the day of oocyte retrieval and fresh sperm can be used to fertilise the oocytes [Citation12].
For those cases with anticipated difficult sperm retrieval, it is better to start testicular sperm retrieval at least 8 h before ovum retrieval to avoid post-maturity oocyte damage [Citation3]. However, this can cause scheduling conflicts (operating room availability and the urologist may change his time schedule). Another option is to perform the surgical sperm retrieval independent of the ovum retrieval day and freeze the testicular sperm. An advantage of this is that the couple will know in advance that testicular sperm is available and therefore not worry about the possibility of futile ovarian stimulation and financial loss. Cryopreserved sperm also allows the embryologist to know whether viable spermatozoa are available for ICSI before oocyte retrieval. Cryopreservation of testicular sperm is generally recommended for fear of future failure in order to obtain suitable spermatozoa for ICSI in such patients [Citation11].
Some authors suggest repeating the sperm retrieval in such cases due to poorer ICSI results when frozen–thawed testicular sperm samples are used compared with fresh sperm samples, as they are convinced that during the process of freezing and thawing spermatozoa are subjected to a series of drastic changes in their environment. Phase transitions of the lipids in the plasma membrane may impair the function of membrane proteins that are needed for ion metabolism and structural integrity. Freezing may also lead to extracellular ice nucleation producing osmotic changes with efflux of water from the cells, with loss of stability of the lipid bilayer. Further consequences may also include denaturation of proteins and structural deformation of the cell organelles [Citation13,Citation14].
The aim of the present systematic review was to review the debate about the routine use of cryopreserved testicular sperm in ICSI in patients with NOA, as some authors suggest repeating the sperm retrieval in such cases due to poorer ICSI results when frozen–thawed testicular sperm samples are used compared with fresh sperm samples.
Methods
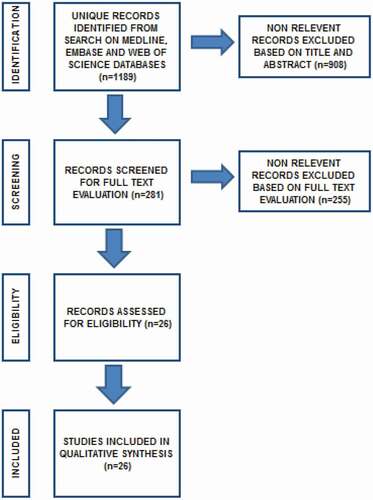
A systematic literature review was performed in August 2020 using the Medical Literature Analysis and Retrieval System Online (MEDLINE), Web of Science databases, and the Excerpta Medica dataBASE (EMBASE). Review articles, congress abstracts and editorials were excluded. Search terms included ‘non-obstructive azoospermia’ in combination with the term ‘cryopreservation’ OR in combination with the term ‘intracytoplasmic sperm injection’. The search was limited to the English literature. References cited in selected articles and in review articles retrieved in the search were used to identify other studies and articles that were not included in the initial searches. We included the articles that provided the highest level of evidence. The systematic review was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation15] ().
Results
In all, 1189 publications were screened and 26 of them were included in this systematic review based on our inclusion criteria and were considered eligible for this study. Duplicate studies and abstracts were excluded. Only full-text articles in the English language were included. Non-relevant records were excluded based on title and abstract (n = 908). Non-relevant records were excluded based on full-text evaluation (n = 255). Three meta-analysis reviews were included and they all concluded that the use of fresh and frozen sperm samples in ICSI in patients with NOA showed comparable fertilisation and pregnancy rates.
Discussion
Cryopreservation of testicular sperm is routinely recommended for fear of future failure to obtain motile spermatozoa in NOA cases. Some authors suggest repeating the sperm retrieval in such cases due to poorer ICSI results when frozen–thawed testicular sperm samples are used compared with fresh sperm samples. The present systematic review focussed on this debate. A systematic literature review was performed in August 2020 using MEDLINE, Web of Science databases and EMBASE. We screened 1189 publications and 26 articles and three meta-analysis reviews were included.
The success of testicular sperm cryopreservation and their further use in ICSI was first reported in the 1990s. We may refer to for a comparison of ICSI outcomes between fresh and frozen testicular sperm from patients with NOA to glean confidence in the frozen sperm results in ICSI [Citation16–37]. also shows that the cryopreservation protocol varied between the laboratories in the different studies. It is obvious that there are two choices for testicular sperm preservation, freezing the sperm-containing suspensions or the testicular tissue sample, and both options are used in the selected studies. In most of the studies dealing with sperm cryopreservation, the most commonly used method was freezing in liquid nitrogen vapour for 15–30 min without the use of any device () that would control the freezing slope, which was previously documented as being crucial for the healthiness of the sperm after thawing [Citation38].
Table 1. Comparison of ICSI cycles using fresh vs frozen–thawed surgical retrieved testicular sperm
Some fertility specialists are convinced that during the process of freezing and thawing, spermatozoa are harmed and so they recommend repeating the sperm retrieval procedure in such cases to avoid poorer ICSI results when frozen–thawed testicular sperm samples are used. A potential risk to be considered is testicular sperm loss after freezing and thawing, as survival of frozen–thawed samples is not uniform in all centres (20–90%), while repeating the sperm retrieval procedure cannot be done before 3 [Citation39] or 6 months [Citation40].
So to settle the debate about the success rates of fresh vs frozen testicular sperm use, three meta-analysis reviews were conducted. They all concluded that fresh and frozen sperms in ICSI from patients with NOA showed comparable fertilisation and pregnancy rates.
The first meta-analysis review article by Nicopoullos et al. [Citation41] in 2004 from the Chelsea and Westminster Hospital, London, compared the use of fresh vs frozen testicular sperm ICSI in men with azoospermia (OA and NOA). They reviewed the available data to determine if frozen testicular sperm samples were associated with decreased fertilisation and pregnancy rates. The authors identified a total of 17 studies and data were analysed from 1476 cycles. No difference in fertilisation, clinical pregnancy rate (CPR) and ongoing pregnancy rate was noted when the testicular cycles were analysed separately, but implantation was significantly impaired using frozen–thawed sperm.
In the second meta-analysis review article by Ohlander et al. [Citation42] in 2014 from the University of Illinois, Chicago, a total of 224 studies were identified, 11 of which met criteria for inclusion. Data were analysed from 274 cycles with fresh and 299 cycles with frozen testicular sperm (in only men with NOA to be more specific than the first meta-analysis). Fertilisation rates (two pronuclei % [2 PN%]) were similar when comparing fresh vs frozen sperm (52.9% and 54%, respectively). In addition, the CPR was similar when comparing fresh vs frozen sperm (28.7% and 28.1%, respectively).
In the third meta-analysis review article by Yu et al. [Citation43] in 2018 from Huazhong University in China, a total of 997 studies were reviewed, of which 17 met the inclusion criteria. Data were analysed from 1261 cycles. Fertilisation, good embryo, CPR, implantation rate and live-birth rate were similar when comparing fresh vs frozen sperm () [Citation41–43].
Table 2. The three meta-analyses studying the effects of testicular sperm freezing on the ICSI outcome in NOA
Surgical retrieval of rare sperms can be achieved in clinical practice. Conventional cryopreservation cannot help those patients due to its technical limitations. More complicated technologies have been developed over the years to manage this situation. A number of devices have been suggested to cryopreserve rare spermatozoa like empty zona, Cryolock, Cell Sleepers, Petri dishes, the novel sperm vitrification device (SpermVD) and other biological or non-biological devices. None of these have achieved widespread use due to technical requirements and cost concerns [Citation44].
Conclusion
Use of frozen testicular sperm from men with NOA results in fertilisation rates similar to that of fresh sperm samples. In addition, the CPR was found to be similar when comparing fresh vs frozen sperm use in that group of patients. This may encourage fertility centres to use frozen testicular sperm samples, as this policy has certain advantages that would help organising the workflow.
Summary and key points
Significant progress has been made over the past few years in our understanding of male infertility. This understanding, as well as rapid technological progress, has played a great role in managing males with azoospermia. In view of these findings, sperm cryopreservation has to be considered in every surgical sperm retrieval operation to guarantee the best use of those valuable sperms. Patients with azoospermia need to be reassured that frozen–thawed viable spermatozoa are as good as the fresh ones, and they should be adequately counselled before any surgical procedure ( for proper patient counselling [Citation3,Citation39,Citation40,Citation45,Citation46]).
Table 3. A comparison between the different policies while dealing with a NOA case
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics (Sao Paulo). 2011;66:691–700.
- Aziz N. The importance of semen analysis in the context of azoospermia. Clinics (Sao Paulo). 2013;68(Suppl 1):35–38.
- Nelson K, Schlegel P. Obstructive and Nonobstructive Azoospermia. In: Patton PE, Battaglia DE, editors. Office Andrology. Contemporary Endocrinology. Totowa, NJ, USA: Humana Press Inc; 2005. p. 201–213.
- Esteves SC, Agarwal A. Reproductive outcomes, including neonatal data, following sperm injection in men with obstructive and nonobstructive azoospermia: case series and systematic review. Clinics (Sao Paulo). 2013;68(Suppl 1):141–150.
- Tournaye H, Verheyen G, Nagy P, et al. Are there any predictive factors for successful testicular sperm recovery in azoospermic patients? Hum Reprod. 1997;12(1):80–86. .
- Su LM, Palermo GD, Goldstein M, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. J Urol. 1991;161:112–116.
- Esteves SC, Prudencio C, Seol B, et al. Comparison of sperm retrieval and reproductive outcome in azoospermic men with testicular failure and obstructive azoospermia treated for infertility. Asian J Androl. 2014;16(4):602–606. .
- Guler I, Erdem M, Erdem A, et al. Impact of testicular histopathology as a predictor of sperm retrieval and pregnancy outcome in patients with nonobstructive azoospermia: correlation with clinical and hormonal factors. Andrologia. 2016;48(7):765–773. .
- Vloeberghs V, Verheyen G, Haentjens P, et al. How successful is TESE-ICSI in couples with non-obstructive azoospermia? Hum Reprod. 201530: 1790–1796.
- Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–583.
- Esteves SC. Management of infertile men with nonobstructive azoospermia due to spermatogenic failure. In: Gunasekaran K, Pandiyan N, editors. Male Infertility: a Clinical Approach. New Dehli, India: Springer Nature; 2017. p. 107–134.
- Verheyen G, Popovic-Todorovic B, Tournaye H. Processing and selection of surgically-retrieved sperm for ICSI: a review. Basic Clin Androl. 2017;27:6.
- Gómez-Torres MJ, Medrano L, Romero A, et al. Effectiveness of human spermatozoa biomarkers as indicators of structural damage during cryopreservation. Cryobiology. 2017;78:90–94.
- Oehninger S, Duru NK, Srisombut C, et al. Assessment of sperm cryodamage and strategies to improve outcome. Mol Cell Endocrinol. 2000;169:3–10.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
- Friedler S, Raziel A, Soffer Y, et al. Intracytoplasmic injection of fresh and cryopreserved testicular spermatozoa in patients with nonobstructive azoospermia – a comparative study. Fertil Steril. 1997;68:892–897.
- Ben-Yosef D, Yogev L, Hauser R, et al. Testicular sperm retrieval and cryopreservation prior to initiating ovarian stimulation as the first line approach in patients with non-obstructive azoospermia. Hum Reprod. 1999;14:1794–1801.
- Habermann H, Seo R, Cieslak J, et al. In vitro fertilization outcomes after intracytoplasmic sperm injection with fresh or frozen thawed testicular spermatozoa. Fertil Steril. 2000;73:955–960.
- Fukunaga N, Haigo K, Kyono K, et al. Efficiency of using frozen-thawed testicular sperm for multiple intracytoplasmic sperm injections. J Assist Reprod Genet. 2001;18:634–637.
- Friedler S, Raziel A, Strassburger D, et al. Factors influencing the outcome of ICSI in patients with obstructive and nonobstructive azoospermia: a comparative study. Hum Reprod. 2002;17:3114–3121.
- Sousa M, Cremades N, Silva J, et al. Predictive value of testicular histology in secretory azoospermic subgroups and clinical outcome after microinjection of fresh and frozen-thawed sperm and spermatids. Hum Reprod. 2002;17:1800–1810.
- Verheyen G, Vernaeve V, Van Landuyt L, et al. Should diagnostic testicular sperm retrieval followed by cryopreservation for later ICSI be the procedure of choice for all patients with non-obstructive azoospermia? Hum Reprod. 2004;19:2822–2830.
- Hauser R, Yogev L, Amit A, et al. Severe hypospermatogenesis in cases of nonobstructive azoospermia: should we use fresh or frozen testicular spermatozoa? J Androl. 2005; 26(6):772–8.
- Wu B, Wong D, Lu S, et al. Optimal use of fresh and frozen-thawed testicular sperm for intracytoplasmic sperm injection in azoospermic patients. J Assist Reprod Genet. 2005;22(11–12):389–394. .
- Konc J, Kanyó K, Cseh S. The effect of condition/state of testicular spermatozoa injected to the outcome of TESE–ICSI–ET cycles. Eur J Obstet Gynecol Reprod Bio. 2008;141: 39–43.
- Akarsu C, Caglar G, Vicdan K, et al. Pregnancies achieved by testicular sperm recovery in male hypogonadotrophic hypogonadism with persistent azoospermia. Reprod Biomed Online. 2009;18:455–459.
- Kalsi J, Thum MY, Muneer A, et al. Analysis of the outcome of intracytoplasmic sperm injection using fresh or frozen sperm. BJU Int. 2011;107:1124–1128.
- Karacan M, Alwaeely F, Erkan S, et al. Outcome of intracytoplasmic sperm injection cycles with fresh testicular spermatozoa obtained on the day of or the day before oocyte collection and with cryopreserved testicular sperm in patients with azoospermia. Fertil Steril. 2013;100(4):975–980. .
- Abdel Raheem A, Rushwan N, Garaffa G, et al. Factors influencing intracytoplasmic sperm injection (ICSI) outcome in men with azoospermia. BJU Int. 2013;112:258–264.
- Tavukcuoglu S, Al-Azawi T, Al-Hasani S, et al. Using fresh and frozen testicular sperm samples in couples undergoing ICSI-MicroTESE treatment. J Reprod Infertil. 2013;14:79–84.
- Madureira C, Cunha M, Sousa M, et al. Treatment by testicular sperm extraction and intracytoplasmic sperm injection of 65 azoospermic patients with non-mosaic Klinefelter syndrome with birth of 17 healthy children. Andrology. 2014;2(4):623–631. .
- Hessel M, Robben JC, D’Hauwers KW, et al. The influence of sperm motility and cryopreservation on the treatment outcome after intracytoplasmic sperm injection following testicular sperm extraction. Acta Obstet Gynecol Scand. 2015;94:1313–1321.
- Park YS, Lee SH, Lim CK, et al. Effect of testicular spermatozoa on embryo quality and pregnancy in patients with non-obstructive azoospermia. Syst Biol Reprod Med. 2015;61:300–306.
- Schachter-Safrai N, Karavani G, Levitas E, et al. Does cryopreservation of sperm affect fertilization in nonobstructive azoospermia or cryptozoospermia? Fertil Steril. 2017;107(5):1148–1152. .
- Okuyama N, Obata R, Oka N, et al. Long-term clinical outcomes of testicular sperm extraction and intracytoplasmic sperm injection for infertile men. Reprod Med Biol. 2018;17:82–88.
- Falah KM. Intracytoplasmic sperm injection with fresh versus cryopreserved testicular sperm in azoospermic patients. Middle East Fertil Soc J. 2019;24:11.
- Zhang HL, Mao JM, Liu DF, et al. Clinical outcomes of microdissection testicular sperm extraction-intracytoplasmic sperm injection with fresh or cryopreserved sperm in patients with nonobstructive azoospermia. Asian J Androl. 2021;23(2):211–214. .
- Paz G, Yogev L, Gottreich A, et al. The use of an electric freezer in human semen banking. Eur J Obstet Gynecol Reprod Biol. 1991;38(2):141–144. .
- Amer M, El Haggar S, Moustafa T, et al. Testicular sperm extraction: impact of testicular histology on outcome, number of biopsies to be performed and optimal time for repetition. Human Reprod. 1999;14:3030–3034.
- Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Human Reprod. 1997;12:1688–1692.
- Nicopoullos JD, Gilling-Smith C, Almeida PA, et al. Use of surgical sperm retrieval in azoospermic men: a meta-analysis. Fertil Steril. 2004;82:691–701.
- Ohlander S, Hotaling J, Kirshenbaum E, et al. Impact of fresh versus cryopreserved testicular sperm upon intracytoplasmic sperm injection pregnancy outcomes in men with azoospermia due to spermatogenic dysfunction: a meta-analysis. Fertil Steril. 2014;101:344–349.
- Yu Z, Wei Z, Yang J, et al. Comparison of intracytoplasmic sperm injection outcome with fresh versus frozen-thawed testicular sperm in men with nonobstructive azoospermia: a systematic review and meta-analysis. J Assist Reprod Genet. 2018;35:1247–1257.
- Liu S, Li F. Cryopreservation of single-sperm: where are we today? Reprod Biol Endocrinol. 2020;18:41.
- Amer M, Ateyah A, Zohdy W, et al. Preoperative and intraoperative factors that predict difficult testicular sperm retrieval in patients with non-obstructive azoospermia. Fertil Steril. 2002;78:646–647.
- Gangrade BK. Cryopreservation of testicular and epididymal sperm: techniques and clinical outcomes of assisted conception. Clinics (Sao Paulo). 2013;68(Suppl 1):131–140.