ABSTRACT
Objective
The concentration of albumin and globulin in the body can serve as indicators of both nutritional status and inflammation. The predictive significance of the albumin-to-globulin ratio (AGR) has been documented in multiple cancer types. Consequently, a meta-analysis was conducted in order to investigate the prognostic impact of AGR on survival outcomes among individuals diagnosed with renal cell carcinoma (RCC).
Methods
A systematic search was conducted across four electronic databases to identify pertinent studies that evaluated the predictive significance of pre-treatment albumin-to-globulin ratio (AGR) in patients with renal cell carcinoma (RCC). The main outcome of interest in this study was overall survival (OS), whereas additional outcomes included cancer-specific survival (CSS), progression-free survival (PFS), and disease-free survival (DFS). The researchers utilized random-effect models to summarize the time-to-event outcomes, presenting the results as adjusted hazard ratios (aHR) along with their corresponding 95% confidence intervals (CI).
Results
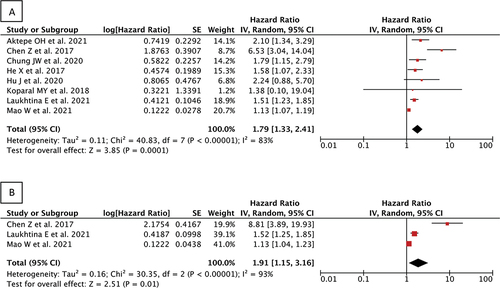
A total of 5,732 RCC patients in eight studies were included. Cut-off for AGR value varies among studies, with AGR higher than 1.1–1.47 regarded as normal. Pooled analysis from these studies showed that low AGR value was associated with shorter OS (aHR 1.84 (95% CI 1.35–2.51), p = 0.0001) and CSS (aHR 1.91 (95% CI 1.15–3.16), p = 0.01).
Conclusions
This study suggests the role of AGR in predicting the OS and CSS of RCC. AGR values can be used in the risk stratification of patients with RCC, where a low AGR value indicates poorer prognosis.
Introduction
Renal cell carcinoma (RCC) is a neoplastic condition that arises from the epithelial cells lining the renal tubules. It represents the predominant form of kidney malignancies, constituting nearly 90% of all cases. Consequently, it is regarded as the most prevalent solid lesion observed in the renal organ. The development of this particular malignancy mainly occurs in individuals aged 40 to 70, with a male-to-female ratio of 1.5–2 to 1 [Citation1,Citation2]. Over the past two decades, there has been a consistent annual rise of 2% in the global incidence of RCC according to the GLOBOCAN 2018 data [Citation3]. As the seventh most prevalent form of cancer in developed countries, it is estimated that around 403 thousand patients each year are diagnosed with kidney cancer. Moreover, the mortality rate associated with this disease surpasses 175,000 deaths annually [Citation4]. This observation suggests that there exists a considerable burden associated with RCC.
The current body of academic literature has demonstrated that there exists a correlation between the development, growth, and metastasis of tumors and systemic inflammation, which is defined by an elevation in inflammatory cells, acute-phase proteins, and pro-inflammatory cytokines [Citation5]. In addition to inflammation, nutritional factors also contribute to the progression of cancer, since malnutrition, anorexia, and cachexia have been linked to worse outcomes and increased mortality rates [Citation6,Citation7]. Albumin and globulin are the primary plasma proteins that have the ability to concurrently indicate both nutritional status and inflammation [Citation8]. The albumin-to-globulin ratio (AGR) that is derived by dividing the albumin concentration by the difference between the total protein and globulin concentrations may effectively describe the patient’s inflammatory and nutritional status [Citation8]. Multiple meta-analysis studies have demonstrated a correlation between low AGR levels and unfavorable outcomes in terms of overall survival (OS) and disease-free survival (DFS) across a range of cancer types, including lung [Citation9], gastric [Citation10], colorectal [Citation11], and head and neck malignancies [Citation12].
The prognostic significance of AGR in renal cell carcinoma (RCC) has been examined in multiple studies [Citation13,Citation14]. Unfortunately, the results of these inquiries demonstrate inconsistent findings [Citation13,Citation14]. An investigation conducted by Chen Z et al. [Citation13] demonstrated that lower values of the AGR were independently linked to poorer overall survival (OS) and cancer-specific survival (CSS). In contrast, a separate investigation conducted by Koparal MY et al. [Citation14] yielded inconclusive results regarding the correlation between low AGR and unfavorable OS in the multivariable analysis. Hence, it is imperative to conduct a comprehensive review and meta-analysis in order to address these existing gaps in the research. The objective of our study is to conduct a comprehensive analysis of the predictive capacity of AGR in determining the prognosis of RCC.
Materials and methods
Eligibility criteria
This is a systematic review and meta-analysis of observational studies, registered on PROSPERO database (registration number CRD42022365915). The publications were deemed eligible for inclusion in this study if they met the predetermined criteria for inclusion, which are outlined as follows: (1) studies’ population were patients diagnosed with renal cell carcinoma in any grade or any histopathological results; (2) having the laboratory data of serum albumin–globulin ratio (AGR) which were taken during the day of hospital admission; (3) reporting the primary outcome: overall survival (OS), with/without secondary outcomes as follows: cancer-specific survival (CSS), progression-free survival (PFS), and disease-free survival (DFS); (4) all of the data for the outcomes of interest were reported in adjusted hazard ratio (aHR), given that HR values reported has already been adjusted for statistically significant baseline characteristics; (5) studies with observational design, specifically cohort or case-control; (6) the articles are accessible in their full-text form. Articles will be omitted from the study if they meet any of the exclusion criteria: (1) scholarly papers with a specific focus on the pediatric population; (2) articles focusing on urothelial carcinoma in general/mixed populations; (3) articles that have been reported in languages other than English; (4) articles besides observational studies (randomized or non-randomized clinical trials, case-series, case-report, cross-sectional studies); (5) study that has not been published or abstract; and (6) non-primary research.
Search strategy and study selection
A systematic search of databases was conducted to identify papers that met the criteria of being written in English. The search was performed across four databases, namely PubMed, Science Direct, Scopus, and Cochrane Library. The search strategy employed on each database is adjusted accordingly, but always include the following terms: ‘(albumin OR globulin OR albumin-globulin ratio OR AGR) AND (renal cell carcinoma OR RCC OR kidney cancer OR kidney adenocarcinoma OR renal cancer)’. The search was conducted until 8 October 2022, to identify relevant papers. The first stage involved the selection of acceptable publications by conducting a thorough screening of titles and abstracts, which was carried out by two reviewers. Further assessment of the citations from the identified suitable research was undertaken in order to identify other potential articles. The removal of duplicate items was carried out. In the last stage, two reviewers conducted an independent screening of the full-text articles. Any differences that arose were resolved by discussion between the reviewers. The study utilized the PRISMA guidelines [Citation15].
Data extraction and quality assessment
The data extraction was undertaken by two writers. The development of an extraction form aimed to compile pertinent details on the study, including the names of the authors, the year of the study, the study design, the sample size, type of intervention, histopathological type, tumor stage, Fuhrman grade, AGR cut-off value, age, gender, as well as the findings of interest.
Two writers independently assessed the methodological quality of the studies that were included. The assessment of the quality of each observational study included in this analysis was conducted using the Newcastle-Ottawa Scale (NOS). The assessment procedure encompassed an evaluation of the comparability, selection, and outcome of each study, followed by the assignment of a numerical score ranging from 0 to 9 for each individual research endeavor. Research is considered to be of high quality if it has a score of 7 or higher [Citation16].
Statistical analysis
The meta-analysis was conducted utilizing the Review Manager 5.4 software, developed by the Cochrane Collaboration. The study utilized the Generic Inverse-Variance formula to combine the adjusted hazard ratio (aHR) and its corresponding 95% confidence interval (95% CI) for all outcomes, regardless of heterogeneity, by employing random-effect models. The assessment of heterogeneity among papers in this meta-analysis was conducted using I-squared (I2), also known as Inconsistency. The I2 statistic is commonly used to assess the degree of heterogeneity in a meta-analysis. A number below 25% is generally regarded as indicative of a low degree of heterogeneity, while a range of 26–50% suggests a moderate degree of heterogeneity. Conversely, a value beyond 50% is typically thought to indicate a high degree of heterogeneity [Citation17]. The evaluation of publication bias using a funnel plot was deemed appropriate when the meta-analysis involved the pooling of more than 10 studies [Citation18]. Sensitivity analysis is later conducted to evaluate the influence of studies on the overall result of the forest plot using STATA 27 (StataCorp).
Results
Study selection and characteristics
The initial search of the database resulted in a total of 501 research articles. After screening the titles and abstracts and removing duplicate entries, a total of 24 papers were deemed appropriate for further analysis. Out of the studies that met the eligibility criteria, a total of 16 papers were subsequently discarded following a comprehensive evaluation of their full-text content. Nine articles were not done specifically in renal cell carcinoma populations (consisting of mixed populations), five articles were review articles, two articles did not have data on the outcome of interest, thus resulting in the final number of eight observational studies [Citation13,Citation14,Citation19–24], which included a total of 5,732 renal cell carcinoma patients for the analysis (). All of the included studies have retrospective cohort design. Sample sizes ranged from 162 to 2,970. The majority of the samples in the included studies have undergone surgical procedures in the form of radical nephrectomy or partial nephrectomy as intervention for their renal cell carcinoma [Citation21–27]. The dominant histopathological type of renal cell carcinoma in all of the included studies was clear cell carcinoma, followed by chromophobe carcinoma, papillary carcinoma, multilocular carcinoma, and oncocytoma. The TNM stage, Fuhrman grade, and AGR cut-off values were varied among the included studies and can be seen in detail in .
Figure 1. PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta-analysis.
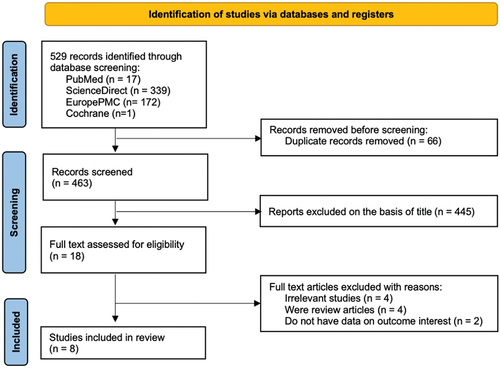
Table 1. Characteristics of the included studies.
Quality of study assessment
All of the cohort studies included in this study were deemed to possess high quality based on the assessment scale of the Newcastle Ottawa Scale (NOS). All studies were considered appropriate for inclusion in the meta-analysis. A summary evaluation of the risk of bias is displayed in .
Table 2. Newcastle-Ottawa quality assessment of observational studies.
Primary outcome
Overall survival (OS)
Eight studies reported on the overall survival outcome. Our pooled analysis of adjusted hazard ratio (aHR) from included studies showed that low albumin–globulin ratio (AGR) was significantly associated with shorter overall survival (OS) among renal cell carcinoma patients when compared with high AGR, and therefore can be used as independent predictor of poor OS [aHR 1.84 (95% CI 1.35–2.51), p = 0.0001, I2 = 84%, random-effect modelling] ().
Secondary outcome
Cancer-specific survival (CSS)
Three studies reported on the cancer-specific survival outcome. Our pooled analysis of adjusted hazard ratio (aHR) from included studies showed that low albumin–globulin ratio (AGR) was significantly associated with shorter cancer-specific survival (CSS) among renal cell carcinoma patients when compared with high AGR, therefore can also be used as independent predictor of poor CSS [aHR 1.91 (95% CI 1.15–3.16), p = 0.01, I2 = 93%, random-effect modelling] ().
Progression-free survival (PFS)
Only one study [Citation19] reported on the progression-free survival outcome. That corresponding study has indicated that low AGR was significantly associated with shorter PFS when compared with high AGR [aHR 1.83 (95% CI 1.27–2.65), p = 0.001].
Disease-free survival (DFS)
Only one study [Citation14] reported on the disease-free survival (DFS) outcome. That corresponding study has indicated that AGR value was not significantly associated with DFS [aHR 2.34 (95% CI 0.36–15.10), p = 0.372].
Publication bias
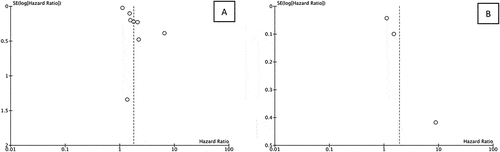
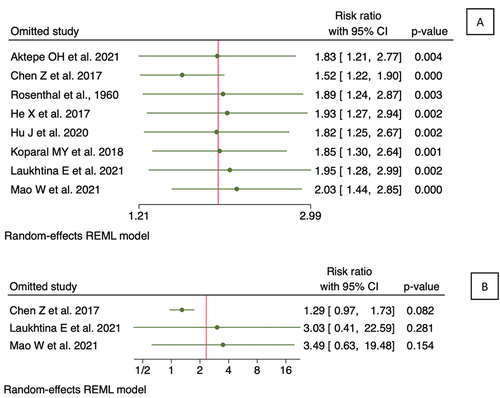
The reliability of detecting publication bias using either funnel plots or statistical tests is compromised when the number of studies is less than 10, in comparison to cases when there are more than 10 studies included [Citation18,Citation25]. Since the number of papers included in each outcome of interest was less than 10, the test for publication bias was not conducted in this analysis. However, funnel plot analysis was still conducted as shown in . Comparison of the precision from the included study showed a symmetrical pattern from funnel plot analysis for both OS and CSS, suggesting low publication bias. A sensitivity was then shown in using leave-one-out plot. As shown in , studies included in OS analysis were equally influential to the meta-analysis in general, with only one study by Chen et al.. (2017) having a higher influence in increasing the hazard ratio relative to the rest of the studies. Similarly, sensitivity analysis of CSS showed that the study by Chen et al.. (2017) has a higher influence on the study compared to the rest.
Discussion
This study presents the results of a systematic review and meta-analysis encompassing a total of eight research articles. The findings of this study provide evidence supporting the prognostic value of the albumin–globulin ratio (AGR) in predicting unfavorable outcomes among individuals diagnosed with renal cell carcinoma (RCC). Specifically, there is a substantial correlation between a low AGR score and decreased overall survival (OS), cancer-specific survival (CSS), and progression-free survival (PFS) in RCC. Meanwhile, the relationship between AGR and disease-free survival (DFS) yields inconclusive results.
Albumin is a protein synthesized by the liver and constitutes the primary protein component of plasma, accounting for approximately 55–60% of the total plasma proteins [Citation26]. Albumin serves several important roles in the body, including the maintenance of capillary oncotic pressure, the removal of free radicals from the bloodstream, the inhibition of systemic inflammatory reactions, and its utility as an indicator of nutritional status [Citation26]. Malnutrition is frequently observed in individuals diagnosed with cancer, a condition that can escalate to cachexia and subsequently expedite the advancement of the disease [Citation27,Citation28]. The state of malnutrition leads to a reduction in protein reserves and a decrease in the synthesis of albumin, resulting in the development of hypoalbuminemia [Citation27,Citation28]. In addition, it is important to note that within the context of cancer, there exists an inflammatory state that arises not only from the presence of tumor cells but also as a result of tissue remodelling and angiogenesis [Citation29,Citation30]. Consequently, there is a notable elevation in the concentrations of pro-inflammatory cytokines, including tumor necrosis factor (TNF), interleukin (IL)-1, IL-6, IL-8, and vascular endothelial growth factor (VEGF) [Citation29,Citation30]. This upregulation of cytokines serves to facilitate the progression of tumor development and the spread of cancer cells to distant sites in the body [Citation29,Citation30]. Hence, the growth of tumors towards malignancy is known to facilitate the occurrence of malnutrition and exacerbate inflammation, and vice versa [Citation29,Citation30]. In the context of inflammatory conditions, it has been observed that there is a notable elevation in the permeability of capillaries [Citation28]. This increased permeability leads to the leakage of albumin from the vascular system into the interstitial space, thereby resulting in a state of hypoalbuminemia [Citation28]. Furthermore, the existence of pro-inflammatory cytokines, particularly TNF, IL-1, and IL-6, may disrupt the process of albumin synthesis in the liver, hence potentially inducing hypoalbuminemia [Citation31].
Meanwhile, globulin has a crucial function in modulating the immune system and regulating inflammatory processes within the human body [Citation32,Citation33]. The globulin component of albumin-to-globulin ratio (AGR) comprises a range of proinflammatory proteins, including immunoglobulins, C-reactive protein (CRP), complement components, α-2 macroglobulin, fibrinogen, prothrombin, and serum amyloid A (SAA) [Citation32,Citation33]. Hyperglobulinemia can arise in patients with significant hepatic dysfunction due to poor clearance of immunoglobulins, which are largely metabolized in the liver [Citation34]. Elevated levels of this globulin may also serve as an indicator for the existence of acute or chronic inflammatory conditions [Citation32,Citation33]. Given the provided explanation, it is reasonable to assert that the incorporation of albumin and globulin components in the AGR renders it a dependable prognostic indicator for cancer, particularly renal cell carcinoma (RCC). However, the underlying mechanism between both albumin and globulin with tumor aggressiveness remains unclear as there has been no previous study on biomechanical function.
The present investigation is not devoid of limitations. The analysis observed significant heterogeneity in the outcome of interest, which is likely attributable to variations in the type, stage, and management of renal cell carcinoma (RCC) across the included studies. Furthermore, the studies included in the analysis employed varying thresholds to classify individuals as having low or high AGR, as there are currently no universally accepted or international consensus criteria for establishing the cut-off point of AGR in relation to cancer. In addition, a significant proportion of the studies used in the analysis exhibit limited sample sizes, namely including fewer than 500 individuals. Finally, our analysis is based on a retrospective cohort study, which is susceptible to the influence of information and selection bias, and therefore must be interpreted with care. Further validation of the findings from our investigation necessitates the execution of large, multicenter, prospective observational studies.
Conclusions
The findings from our systematic review and meta-analysis suggest a potential role for AGR in predicting the prognosis of patients with RCC. Those patients diagnosed with RCC with low AGR values may be at a higher risk of worse survival and disease progression, as indicated by shorter OS and CSS. Additional studies on PFS are required to establish how it is affected by low AGR values. The use of AGR in these patients may help in the stratification of patients to identify high-risk individuals so that an effective therapeutic approach can be made.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017 Mar 9;3(1):17009. doi: 10.1038/nrdp.2017.9
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022 Oct;82(4):399–410. doi: 10.1016/j.eururo.2022.03.006
- Bukavina L, Bensalah K, Bray F, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022 Nov;82(5):529–542. doi: 10.1016/j.eururo.2022.08.019
- Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of renal cell carcinoma. World J Oncol. 2020 Jun;11(3):79–87. doi: 10.14740/wjon1279
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009 May;12(3):223–226. doi: 10.1097/MCO.0b013e32832a7902
- Hariyanto TI, Kurniawan A. Appetite problem in cancer patients: pathophysiology, diagnosis, and treatment. Cancer Treat Res Commun. 2021;27:100336. doi: 10.1016/j.ctarc.2021.100336
- Van Cutsem E, Arends J. The causes and consequences of cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S51–63. doi: 10.1016/j.ejon.2005.09.007
- Lv GY, An L, Sun XD, et al. Pretreatment albumin to globulin ratio can serve as a prognostic marker in human cancers: a meta-analysis. Clin Chim Acta. 2018 Jan;476:81–91. doi: 10.1016/j.cca.2017.11.019
- Li J, Wang Y, Wu Y, et al. Prognostic value of pretreatment albumin to globulin ratio in lung cancer: a meta-analysis. Nutr Cancer. 2021;73(1):75–82. doi: 10.1080/01635581.2020.1737155
- Wei C, Yu Z, Wang G, et al. Low pretreatment albumin-to-globulin ratio predicts poor prognosis in gastric cancer: insight from a meta-analysis. Front Oncol. 2021 Jan 26;10:623046. doi: 10.3389/fonc.2020.623046
- Quan L, Jiang X, Jia X, et al. Prognostic value of the albumin-to-globulin ratio in patients with colorectal cancer: a meta-analysis. Nutr Cancer. 2022;74(9):3329–3339. doi: 10.1080/01635581.2022.2076890
- Wang YT, Kuo LT, Lai CH, et al. Low pretreatment albumin-to-globulin ratios predict poor survival outcomes in patients with head and neck cancer: a systematic review and meta-analysis. J Cancer. 2023 Jan 9;14(2):281–289. doi: 10.7150/jca.80955
- Chen Z, Shao Y, Yao H, et al. Preoperative albumin to globulin ratio predicts survival in clear cell renal cell carcinoma patients. Oncotarg. 2017 Jul 18;8(29):48291–48302. doi: 10.18632/oncotarget.15162
- Koparal MY, Polat F, Çetin S, et al. Prognostic role of preoperative albumin to globulin ratio in predicting survival of clear cell renal cell carcinoma. Int braz j urol. 2018 Sep–Oct;44(5):933–946. doi: 10.1590/S1677-5538.IBJU.2018.0012
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71
- Margulis AV, Pladevall M, Riera-Guardia N, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle–Ottawa scale and the RTI item bank. Clin Epidemiol. 2014 Oct 10;6:359–368. doi: 10.2147/CLEP.S66677
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557
- Thornton A. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000 Feb;53(2):207–216. doi: 10.1016/s0895-4356(99)00161-4
- Aktepe OH, Güner G, Güven DC, et al. Impact of albumin to globulin ratio on survival outcomes of patients with metastatic renal cell carcinoma. Turk J Urol. 2021 Mar;47(2):113–119. doi: 10.5152/tud.2021.20377
- Chung JW, Park DJ, Chun SY, et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with non-metastatic renal cell carcinoma undergoing partial or radical nephrectomy. Sci Rep. 2020 Jul 20;10(1):11999. doi: 10.1038/s41598-020-68975-3
- He X, Guo S, Chen D, et al. Preoperative albumin to globulin ratio (AGR) as prognostic factor in renal cell carcinoma. J Cancer. 2017 Jan 15;8(2):258–265. doi: 10.7150/jca.16525
- Hu J, Chen J, Li H, et al. A preoperative nomogram predicting the pseudocapsule status in localized renal cell carcinoma. Transl Androl Urol. 2020 Apr;9(2):462–472. doi: 10.21037/tau.2020.01.26
- Laukhtina E, Pradere B, D’Andrea D, et al. Prognostic effect of preoperative serum albumin to globulin ratio in patients treated with cytoreductive nephrectomy for metastatic renal cell carcinoma. Transl Androl Urol. 2021 Feb;10(2):609–619. doi: 10.21037/tau-20-1101
- Mao W, Zhang N, Wang K, et al. Combination of albumin-globulin score and sarcopenia to predict prognosis in patients with renal cell carcinoma undergoing laparoscopic nephrectomy. Front Nutr. 2021 Sep 23;8:731466. doi: 10.3389/fnut.2021.731466
- Terrin N, Schmid CH, Lau J, et al. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003 Jul 15;22(13):2113–2126. doi: 10.1002/sim.1461
- Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000 Oct;85(4):599–610. doi: 10.1093/bja/85.4.599
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010 Dec 22;9(1):69. doi: 10.1186/1475-2891-9-69
- Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019 Feb;43(2):181–193. doi: 10.1002/jpen.1451
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008 Jul 24;454(7203):436–444. doi: 10.1038/nature07205
- Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013 Nov;13(11):759–771. doi: 10.1038/nrc3611
- Cabrerizo S, Cuadras D, Gomez-Busto F, et al. Serum albumin and health in older people: review and meta analysis. Maturitas. 2015 May;81(1):17–27. doi: 10.1016/j.maturitas.2015.02.009
- Deng Y, Pang Q, Miao RC, et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther. 2016 Aug 24;9:5317–5328. doi: 10.2147/OTT.S109736
- Suh B, Park S, Shin DW, et al. Low albumin-to-globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol. 2014 Nov;25(11):2260–2266. doi: 10.1093/annonc/mdu274
- Tanaka S, Okamoto Y, Yamazaki M, et al. Significance of hyperglobulinemia in severe chronic liver diseases–with special reference to the correlation between serum globulin/IgG level and ICG clearance. Hepatogastroenterology. 2007 Dec;54(80):2301–2305.