ABSTRACT
Drugs related to morphine represent not only large range of important therapeutic applications for the relief of moderate to severe pain but also give rise to a relatively large series of novel opioids that mimic the action of this naturally occurring analgesic. Most of these are based on fentanyl structures that are much more potent, and dangerous, than fentanyl itself. This publication reviews reports of fatalities attributed to 15 novel opioids with the view to assessing mortality associated with their misuse as well as reviewing published analytical procedures that would be able to detect these and other novel opioids. These drugs include reports of deaths to acetylfentanyl, acrylfentanyl, butr(yl)fentanyl, carfentanil, 2- and 4-fluorofentanyls, 4-fluorobutyrfentanyl, 4-fluoroisobutyrfentanyl, furanylfentanyl, α- and 3-methylfentanyls, 4-methoxyfentanyl, ocfentanil, as well as AH-7921, U-47700 and MT-45. Most of these cases reporting a drug-caused death involved other drugs in addition to the opioid. No obvious minimum fatal concentration was discerned for any of the opioids for which details were provided, however, the more potent members required detection limits well under 1 ng/mL and often even well below 0.1 ng/mL requiring use of the most sensitive mass spectral detection procedures, particularly when screening specimens using a non-targeted mode. Four other novel opioids have been reported in admissions to hospitals include 4-chloroisobutryfentanyl, cyclopentylfentanyl and tetrahydrofuranfentanyl, all of which are likely to have the potential to cause death. It is also likely that other analogues will appear with time.
Introduction
Morphine with its many of semi-synthetic and synthetic analogues including codeine, oxycodone, hydrocodone, hydromorphone, dihydrocodeine, ethylmorphine, methadone are widely used as analgesics to treat moderate to severe pain. Heroin, the diacetyl analogue of morphine has been, and continues to be, a major illicit opiate with estimates of usage of about 17 million users worldwide [Citation1]. According to the United Nations Office of Drug Control prescription, opioids are used by a greater number (about 35 million, or 0.7% of the population). While much of this opioid usage relates to misuse of prescribed drugs, increasingly fentanyl and a number of potent analogues based on fentanyl, or other drugs acting on the opioid receptors, have been detected and have led to hospital admissions and even death [Citation1–6]. This publication complements other recent reviews covering opioids and prevalence of sudden death [Citation2,Citation3].
There have been hundreds of deaths from fentanyl since deaths from its abuse were first reported over 30 years ago. These include those first reported in California [Citation5], to other states including Illinois [Citation6,Citation7], Michigan [Citation8,Citation9], Florida [Citation10], Kansas [Citation11], Maryland [Citation12], Massachusetts [Citation13], Minnesota [Citation14], New Mexico [Citation15], and also in other parts of the world including clusters in Canada [Citation16] and Sweden [Citation17]. In 2015, the USA Centre for Disease Control (CDC) reported 33 091 opioid deaths, of which almost 10 000 were due to synthetic opioids other than methadone (including fentanyl and related drugs), an increase of 72% over 2014 [Citation18].
Fentanyl is about 100 times more potent on the mu-opioid receptor than morphine and has now been detected in batches of heroin. This is a particularly dangerous combination and has led to increased risk of sudden drug-caused death [Citation8,Citation19].
This publication reviews reports of these novel psychoactive opioids that have led to fatalities and provides an overview of their concentrations and circumstances in reported fatalities, and the detectability of these drugs in biological specimens.
Methods
Publications in the English language that reported fatalities from use of a fentanyl derivative or any other novel opioid were searched in PubMed as well as Scopus. Publications not captured in the initial searches but cited in publications were also retrieved and included, where relevant. Published methods were included in this publication where the procedure was targeted to measuring a number of fentanyls and other novel opioids or a number of fentanyls and novel opioids were included in a wider analytical procedure designed for blood and/or urine.
Results
Structural characteristics
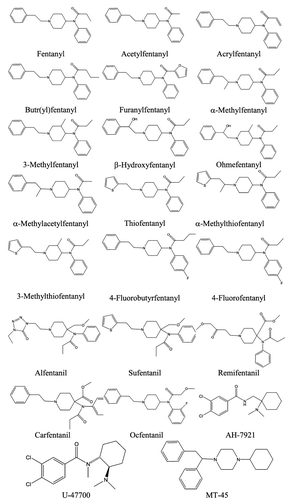
Synthetic opioids related to the phenylheptylamines and phenylpiperidines show significant differences in their apparent 2-D structures, although they do bind to the opioid receptors, particularly the mu-subtype, sometimes with higher affinity than morphine itself. Examples include methadone, pethidine (meperidine), propoxyphene, fentanyl and l-α-acetylmethadol (LAAM).
Fentanyl is a piperidinyl derivative with moieties on the nitrogen and the 4-position. Alfentanil, remifentanil and sufentanil are short acting analogues of fentanyl (with piperidinyl ring) and have been approved for use in humans for some time for induction of anaesthesia ().
The original (designer) fentanyl derivatives included α-methylfentanyl and 3-methylfentanyl [Citation20]. China White, or 3-methylfentanyl, is some 6 000 times more potent than morphine and is active from a few micrograms. They are fentanyl analogues, e.g. a methyl substitution on fentanyl. More recently, replacement of the propionyl moiety of fentanyl with acetyl (acetylfentanyl), acryl (acrylfentanyl), butyryl (butyrfentanyl) or furanyl (furanylfentanyl) has led to a series of novel opioids (). Other variations of the fentanyl molecule include replacement of the phenethyl moiety with β-hydroxy substituted form (with and without other substitutions) (β-hydroxyfentanyl, ohmefentanyl, 2-thiophene-ethyl, thiofentanyl, 3-methylfentanyl, α-methylthiofentanyl), and substitutions on the N-phenyl ring, often with 4-flouro (4-fluorofentanyl, 4-fluorobutyrfentanyl, 4-fluoroisobutyrfentanyl).
Carfentanil, with modifications at the 4-piperidinyl end is possibly the most potent commercially available fentanyl derivative that also has a veterinary use for analgesia in large animals [Citation21].
Newer novel opioids that have more structural variations to the fentanyl structure and have been detected in recent opioid-caused deaths include AH-7921 and the isomer U-47700, and MT-45 ().
A more detailed review of the structure-activity relationships of a larger series of fentanyl analogues can be seen in the following publications [Citation22,Citation23].
Fentanyl fatalities
summarizes the papers that describe fatalities arising from novel opioids including a sampling of those that have arisen from fentanyl itself.
Table 1. Publications reporting fatalities from fentanyl and other novel opioids.
It is metabolized primarily to the inactive norfentanyl (removal of the phenethyl moiety on the piperazine nitrogen) predominately by P450 3A4, and less so to inactive hydroxyfentanyl and hydroxynorfentanyl [Citation24], with the latter two further converted to conjugates [Citation25]. Despropionylfentanyl has also been detected in plasma, but not urine [Citation26].
Numerous publications have described fatalities associated with its misuse [Citation5–7,Citation9–17,Citation27–29]. As with most drug-caused deaths other drugs have also been used and often also misused in combination. Peripheral blood concentrations range from near 1 ng/mL to well over 20 ng/mL with a median somewhere between 5 and 10 ng/mL, depending on degree of tolerance and presence of other significant drugs. Doses vary significantly depending on the route of administration and degree of tolerance [Citation30]. For example, typical carfentanil doses are of the order of several micrograms, compared to fentanyl that range up to about 1 mg and less potent opioids may be up to about 10 mg; however, the dose will also depend on the degree of tolerance to opioids. Intravenous use, smoking and nasal insufflation provide a rapid absorption and pharmacological effect, whereas as oral or intramuscular administration will provide a slower onset of action. A common report was misuse of transdermal patches either by applying multiple patches on the body or injecting the contents of a used patch [Citation31–35].
Novel opioid fatalities
also summarizes reported fatalities of other fentanyl-like opioids and some other novel opioids.
Acetylfentanyl
This has been the most common novel opioid with 11 publications with reported deaths from drug toxicity in at least several European countries and across much of the USA. It appears to be threefold less potent than fentanyl itself [Citation36] and death has occurred when used by insufflation as well as intravenously and orally [Citation37]. Blood concentrations in fatalities show a very large range from 0.1 to 2 100 ng/mL, although the median is about 10 ng/mL. Almost all cases are fatalities from multiple drugs. In the three reported mono-intoxication cases the blood concentrations were 170 ng/mL (femoral) [Citation38], 260 ng/mL [Citation39] and 270 ng/mL (heart) [Citation40], respectively.
Acrylfentanyl
This fentanyl sometimes known as simply acryloylfentanyl is slightly more potent than fentanyl in displacing labelled naloxone from the mu-opioid receptor (IC50 1.4 nmol/L) [Citation41]. Deaths from this opioid have largely been restricted to Sweden and other Nordic countries with cited publications listing numerous drug-caused deaths with blood concentrations ranging from 0.01 to 5 ng/g with a median blood concentration of about 0.2 ng/g [Citation42–44]. These deaths included evidence of nasal insufflation of both sprays and crushed tablets. This drug was believed to enter Sweden and neighbouring countries of Denmark, Estonia, Finland and Latvia as well as Slovenia as a powder [Citation45]. More recently acrylfentanyl was detected in the deaths of three male drug users in the USA; all of whom had blood concentrations less than 1 ng/mL [Citation46].
Butyrfentanyl
Sometimes also termed butyrylfentanyl is less potent than fentanyl (Ki 32 nmol/L cf 1 nmol/L) but has also caused deaths in Europe and the USA. Two case reports report fatalities attributed to this opioid with femoral blood concentrations of 58 and 66 ng/mL [Citation47,Citation48]. In one of these acetylfentanyl was also detected as well as cocaine with levamisole [Citation47]. In another publication this opioid was found in deaths with other fentanyl or fentanyl analogues, such as carfentanil [Citation49]. By comparison, two publications reported survival from use of this opioid [Citation50,Citation51]. Helander et al.[Citation43] reported serum concentrations in the three cases ranging from 0.6 to 66 ng/mL; showing considerable range in concentrations and overlap with concentrations than those found in the fatalities.
Carfentanil
This opioid is used primarily as an incapacitating agent for large animals, and it is even more potent than 3-methylfentanyl. It is some 100 times more potent than fentanyl [Citation52] and was reportedly used in combination with another opioid, remifentanil, in the Melnikov street theatre (Moscow) siege of 2002 as an aerosol to subdue terrorists that claimed over 100 lives [Citation53]. A large series (n = 355) of carfentanil deaths were reported from the USA with blood concentrations ranging from 0.1 to 14 ng/mL [Citation54]. Two deaths were reported in one case report from Florida, one of which was in combination with furanylfentanyl and gave postmortem heart blood concentrations of 0.12 and 1.3 ng/mL [Citation55]. In another Florida report, two more deaths were reported from this opioid, with blood concentrations of 0.12 and 1.3 ng/mL; one of which (highest concentration of carfentanil) also involved furanylfentanyl [Citation55]. This ultra-potent opioid has also been seen in the UK in which 25 deaths were reported with blood concentrations ranging from 0.09 to 4 ng/mL, often in combination with other fentanyls and morphine [Citation49].
Furanylfentanyl
This opioid which is about five times less potent than fentanyl has been reported in deaths in Canada, Sweden and the USA [Citation55–59]. Mohr reported eight cases with furanylfentanyl, of which five were in combination with another opioid, U-47700 [Citation56] while Papsun reported a series of nine deaths in USA from this opioid in combination with other drugs [Citation60]. Blood concentrations in these two series ranged from 2 to almost 76 ng/mL with a median of about 10 ng/mL. In a Swedish series of seven fatalities, blood concentrations ranged from 0.38 to 2.7 ng/mL, again with most using other drugs [Citation57]. Almost all cases had oedematous lungs recorded at autopsy. Four also had pregabalin detected. There was some evidence provided that this fentanyl was reasonably stable in vitro. The Canadian case report described two women that died using counterfeit tablets labeled as Perocet; blood concentrations were 0.68 and 1.1 ng/mL [Citation58] and in another case a young man died following ingestion of a blue pill resembling oxycodone [Citation59]. In one case of survival reported by the Swedes, the serum concentration was 148 ng/mL [Citation37].
Methylfentanyls
3-Methylfentanyl, known as “China White”, was first reported to cause hospitalizations and many deaths in California (and some in neighbouring States) and Pennsylvania in the 1980s as well as a number of other fentanyl derivatives [Citation20,Citation61]. The more active cis-isomer is about 7 000 times more potent as an opioid as morphine. In 1980s, a death from use of α–methylfentanyl [Citation62] was also reported. The occurrence of methylfentanyls is characterized by relatively short-lived epidemics. This is probably due to the high potency of the drug with low doses and subsequent dilution problems, causing a significant risk of overdosing as well as closure of clandestine laboratories [Citation61].
A series of 16 3-methylfentanyl deaths was also reported from Allegheny County in Pennsylvania [Citation63]. Over a decade ago, there were three deaths reported by use of 3-methylfentanyl in Finland [Citation64] and later an epidemic in neighbouring Estonia [Citation65]. Blood concentrations were often less than 1 ng/mL. In the Estonian series of over 100 fatalities, the cis-3-methylfentanyl blood concentrations ranged up to about 2 ng/mL with a median of about 1 ng/mL [Citation65].
Using LC-MS/MS, it was for the first time possible to determine cis-3-methylfentanyl in the blood of victims of fatal overdose, the mean concentration being 0.5 g/L (range 0.3–0.9 g/L). These values are significantly lower than the levels reported above for α-methylfentanyl and fentanyl. Despite the presence of other drugs, poisoning by 3-methylfentanyl was in each case considered the underlying cause of death. This was due to death appearing to occur immediately following injection of the drug. The victims’ ages, ranged from 30 to 41 years, were higher than those typically found in fatal heroin poisonings in Finland; half of the victims of heroin fatalities have been younger than 25 years.
4-Methoxybutyrfentanyl
Four deaths due in part to 4-methoxybutyrfentanyl were also from the Swedish group, all of whom had low serum concentrations (1.3–11 ng/mL) of this fentanyl but who had other drugs also present [Citation37]. Again, symptoms were suggestive of opioid overdose.
Ocfentanil
Two case reports describe deaths of young men using this opioid by nasal insufflation with postmortem femoral blood concentrations of 9.1 and 15 ng/mL [Citation66,Citation67]. In both cases, no other drugs were apparently involved. This opioid is about twice as potent as fentanyl.
AH-7921
This opioid was first reported in deaths in Sweden in 2014 in which nine cases were described with blood concentrations ranging from 0.08 to almost 1 mg/L [Citation68]. It has a similar potency on the μ-opioid receptor as morphine. In the same year, a fatality in USA [Citation69] and two deaths in Norway [Citation70] were reported, followed by five deaths in the UK (blood concentrations 0.05 to 4.46 mg/L) [Citation71] and a case report from Germany [Citation72]. While low concentrations can be expected particularly if death is delayed or other significant drugs are operative blood concentrations tend not to be that low with a recorded median of about 0.3–0.4 mg/L. This drug was the subject of a Critical Review Report to the United Nations in 2014 [Citation73] and a review [Citation74].
MT-45
This opioid was first detected in Sweden in late 2013 and resulted in 28 deaths from November 2013 to July 2014 leading to a risk assessment conducted by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [Citation75]. It is a disubstituted N,N’-piperazine also known as I-C6 and is taken in much the same way as other fentanyl-like opioids (nasal insufflation, oral, smoking) with a potency somewhat higher than morphine. Doses around 15–75 mg are used depending on route and degree of tolerance. In these 28 deaths, almost all were due to drug toxicity with the median blood concentration was 0.35 μg/g and as other opioids most involved use of other drugs. In eight cases, the cause of death was listed as MT-45 intoxication, however, the median was actually higher at 0.8 μg/g (range 0.2–1.9 μg/g). In nine non-fatal intoxications the blood concentrations ranged from 6–157 ng/mL (median 47 ng/mL) [Citation76]. In the peer-reviewed literature a further two publications involving single case reports of young males in which death occurred from use of this opioid in conjunction with other drugs giving blood concentrations of 0.52 and 0.66 mg/L [Citation72,Citation77]. It was also reported as present in illegal products in Japan in 2014 [Citation78].
U-47700
This opioid is a structural isomer of AH-7921 (see earlier). While less potent than fentanyl numerous deaths resulting from its use have been reported worldwide including the USA, UK and Belgium. Fatal toxicity was first reported in 2016 in the UK of a young male snorting this drug [Citation79]. In the same year, a death was reported from Belgium of a young man inhaling fumes, in combination with fentanyl [Citation80] and a series of 16 cases from the USA in which five also involved furanylfentanyl [Citation56]. These and seven other publications in 2017 found blood concentrations of U-47700 ranging from 0.4 ng/mL to 1.46 mg/L with a median of around 0.3 mg/L (). As with other opioids most had other drugs present including the case report from the USA in which the stimulant 3-fluorophenmetrazine was also detected [Citation81], and in another, of a morbidly obese man with an enlarged heart [Citation82]. Survival from use of this opioid has also been reported: all patients showed classical signs of opioid toxicity [Citation83,Citation84]. In one case, serum concentration of this opioid during hospitalization was 394 ng/mL and the desmethylated and hydroxylated metabolites were also detected in urine [Citation84]. In the 2 years to end 2016, the DEA reported at least 46 fatalities linked to the use of U-47700, largely in New York and North Carolina [Citation85].
Other novel opioids
4-Fluorobutyrfentanyl was detected in two drug-related fatalities in Poland in a young male and young female. The male at least presumably smoked this drug through an e-cigarette (with nicotine) [Citation6] ().
A young intravenous drug user was admitted to emergency but died some time later. He was found to have 4-fluoroisobutyrfentanyl with a serum concentration of 38 ng/mL a few hours after admission [Citation43].
Recently two 4-fluorofentanyl and one 2-fluorofentanyl deaths were reported in Germany and Sweden, respectively [Citation86,Citation87]. The 4-fluorofentanyl cases were both suicides, while the Swedish case initially involved two young men who had previously been admitted to hospital following toxicity to snorting 2-fluorofentanyl but recovered after supportive care and treatment for opioid overdose and were subsequently released one day later. Unfortunately, one of these was found deceased a few days later due to drug toxicity, largely from this fentanyl (blood concentration 2.4 ng/mL) in the presence of three benzodiazepines, cannabis and GHB.
More recently, one death was reported from Tennessee (USA) of an anaesthetist who died from self-injection of sufentanil and midazolam [Citation88]. In this case, the heart blood concentration of sufentanil was 1.1 ng/mL.
Other novel opioids based on fentanyl have been identified in emergency hospital admissions that did not lead to death. These were 4-chloroisobutryfentanyl, cyclopentylfentanyl and tetrahydrofuranfentanyl [Citation43].
Pathology findings
The most common and consistent findings on autopsy of persons that have died from novel opioid toxicity is that seen in heroin and other deaths from opiates, notably heavy lungs associated with pulmonary oedema and hyperaemia, with pneumonia also seen in some cases, particularly in cases where death process was relatively long [Citation42,Citation50,Citation57,Citation62,Citation67,Citation72,Citation89,Citation90]. Often cerebral oedema and congestion in liver and other organs is also noted. This is consistent with death from depression of the central nervous system. In hospital admissions high heart rate, high blood pressure with signs of apnea and miosis is also commonly seen [Citation37].
However, some unusual individual pathology has been observed. MT-45 has been linked to bilateral hearing loss, a side effect also reported elsewhere for this drug [Citation72,Citation77] and in one case a known heroin user developed significant haemoptysis, acute lung injury, hypoxic respiratory failure and diffuse alveolar haemorrhage following use of butyrfentanyl [Citation50]. He survived and was released from hospital after 1 week [Citation50]. Diffuse alveolar haemorrhage was also seen in a intranasal user of fentanyl [Citation91] and is probably a rarer side effect of opioid toxicity [Citation92].
Other pathology is sometimes noted, and may also contribute to death, but the drug does not cause this directly, rather, is present for other reasons, such as coronary artery disease.
Methods of analysis
Before the widespread use of tandem MS (MS/MS) and/or high-resolution MS (HR-MS) specimens were analysed by immunoassay and if positive by GC-MS with selected ion monitoring (SIM). Immunoassays tend to have a limit of detection from about 0.25 ng/mL to about 2 ng/mL, and have been able to detect the presence of some other fentanyls due to cross-reactivity [Citation93–95]. Depending on the antibody used this includes acetylfentanyl, butyrfentanyl, furanylfentanyl, 4-methylfentanyl, 4-fluorofentanyl, but not alfentanil or carfentanil [Citation94–96]. It is possible, but not confirmed, that related structures involving N-alkylated piperazines may also cross-react including risperidone and 9-hydroxyrisperidone (paliperidone) [Citation94].
The Randox biochip platform enables detection of acetylfentanyl, carfentanil, furanylfentanyl, ocfentanil, remifentanil and sufentanil, as well as AH-7921, MT-45 and U-47700 with cut-offs ranging from 0.25 (carfentanil) to 10 ng/mL (U-47700) in urine [personal communication].
Before the widespread availability and use of MS/MS and HR-MS GC-MS, operating in the SIM mode was used to detect fentanyl and even some of the other fentanyls, however, modern GC-MS instruments appear to have sufficient sensitivity to detect sub-nanogram per millilitre concentrations of many of the synthetic opioids, such as those reported in some recent publications [Citation46,Citation82,Citation97].
Fentanyls and related designer opioids are usually well extracted in organic solvents from basified blood/plasma/serum or another liquidified specimen. Butyl chloride has been used for this purpose [Citation31,Citation65], however, isooctane has also been used [Citation17]. Solid phase extraction (SPE) has also been used successfully [Citation33]. Detection limits of fentanyl have ranged from 0.5 to 2 ng/mL and have often also included norfentanyl.
summarizes procedures published since 2000 that were validated to detect 3 or more fentanyls. These procedures were also designed to quantify opioids in blood/serum and urine, and sometimes also other specimens that can be collected postmortem. These nine publications were validated to detect three [Citation56,Citation98], five [Citation99], six [Citation100,Citation101], nine [Citation102], 10 [Citation103], 13 [Citation104] and 14 fentanyls [Citation105], respectively. All bar two used SPE and all bar one used either LC-MS/MS or ion trap MS instruments. The one that used GC-MS (operated in SIM mode) used a pentafluorobenzamide derivative that also gave the best limit of detection of 0.002 5 ng/mL (or 2.5 fg/mL). The LOD of other procedures varied from 0.003 to 0.5 ng/mL. The higher grade MS/MS or MS instruments will usually provide a higher level of sensitivity. The desired LOD/LLOQ will depend on the opioid. For example, carfentanil one of the most potent fentanyls will require limits down to at least 0.01 ng/mL to be reasonably able to detect use in cases.
Table 2. Analysis details for published methods targeting two or more fentanyls.
Discussion
Since the beginning of the 1990s, a large number of reports on the abuse of fentanyl analogues appeared in the scientific literature, with numerous reported deaths. This occurrence may, in part, have been due to the temporary reduction in the availability of heroin decreasing drastically during the Afghanistan crisis. Some of these were summarized in with the majority occurring in the USA and Western Europe [Citation8,Citation10,Citation28,Citation31,Citation33,Citation68,Citation80,Citation106]. Initially these were caused by abuse or misuse of fentanyl by practitioners with access to legal supplies of the drug, however, more recently the opioid has become more widely available, largely through skin patches for transdermal absorption [Citation31,Citation33] and also more recently possible deliberate doping of heroin with a fentanyl [Citation10,Citation19,Citation28]. Clandestine manufacture of fentanyl is known and contributes to the availability and misuse of this opioid [Citation107].
Simultaneously, fatalities due to prescription opioids began to rise over the last 2 decades, with alarming mortality, particularly from oxycodone, although other legal opioids have contributed [Citation108–110].
In most of these cases, as it is in most drug-caused deaths, other contributing drugs are detected alongside fentanyl. These are often another opiate or opioid, alcohol, amphetamines, cocaine or one or more of the benzodiazepines. Due to the presence of other drugs, the degree of tolerance to opioids and sometimes the presence of significant natural disease there are no clearly defined minimum fatal concentration of fentanyl with concentrations contributing or causing death from as low as about 0.2 ng/mL, although the median peripheral blood concentrations have tended to be about 10–20 ng/mL [Citation28,Citation111].
The first reports of other fentanyl derivatives causing death was in the 1980's by α-methylfentanyl in California [Citation62] and later from 3-methylfentanyl, or China White, as it was known in which 16 deaths were reported from Allegheny county, Pennsylvania [Citation63]. It was not until about 10 years ago when fentanyl derivatives causing death began to appear on a more regular basis, starting with clusters of 3-methylfentanyl deaths in Finland and Estonia [Citation64,Citation65], and then acetylfentanyl fatalities in the USA a few years later [Citation112].
In 2002, fentanyls gained notoriety in connection with the Moscow Dubrovka theater siege, when the Russian military used a knockout gas to incapacitate Chechen rebels, leading to the loss of more than 100 rebels and hostages. The available evidence suggests that a combination of an aerosolized fentanyl derivative, such as carfentanil, and an inhalation anesthetic, such as halothane, was used [Citation113], although it seems that remifentanil may also have been present in the aerosol [Citation53].
In the last few years, numerous deaths have been reported from 16 novel opioids in various publications including acetylfentanyl, acrylfentanyl, butr(yl)fentanyl, carfentanil, 2- and 4-fluorofentanyls, 4-fluorobutyrfentanyl, 4-fluoroisobutyrfentanyl, furanylfentanyl, α- and 3-methylfentanyls, 4-methoxyfentanyl, ocfentanil, as well as AH-7921, U-47700 and MT-45, reports of which have been summarized in .
While the concentrations detected bear some relation to the potency of the drug to the opioid receptor(s) with the weaker agonists having concentrations well above the nanogram per millilitre level (e.g. acetylfentanyl, butyrfentanyl and furanylfentanyl) many are substantially more potent than fentanyl and require detection limits well below 1 ng/mL. For example, the most potent of the opioids listed here, carfentanil, in a recent publication was able to be detected down as low as 5 pg/mL (5 ng/L) with many cases positive at near this concentration. Only the best MS instruments would be able to detect (and provide sufficient number of confirmatory ions or ion transitions) at such low concentrations in biological matrices.
Unfortunately, on review of the publications listed here (reports of deaths from use of a novel opioid), there is no concentration that could be considered a minimum that can cause death. This is not surprising since there is no such minimum fatal concentration for other opioids, such as morphine (including from use of heroin), methadone and oxycodone. The use of other significant drugs, such as other opiates or opioids, and other illicit drugs, as well as alcohol and benzodiazepines to list some, will also contribute to toxicity. Moreover, tolerance is a substantial factor, or lack of it in some cases, that cannot be assessed from the often scant history obtained in a routine death investigation. Additionally, the route and consequently the rate of administration into the body (mostly to brain stem) and posture if a collapse occurs will often also be a factor that can mean the difference between life and death.
Given that fentanyls and probably most, if not all, the related opioids are lipid soluble and can penetrate tissues much better than the water-soluble morphine there is surprisingly little difference in concentration whether blood was drawn from the heart or a peripheral site (e.g. femoral, iliac, sub-clavian). Often drugs with high lipid solubility have higher tissue concentrations leading to diffusion into pooled blood giving give to redistribution phenomenon postmortem [Citation114]. The lack of concentration changes postmortem may be due to tight binding of these drugs to tissue structures. In the 16 examples provided in where there were paired peripheral and central blood quantitative results the median ratio of central to peripheral blood concentration was 1.3 and only nine of the cases displayed a higher concentration in the central site (range of ratios were 0.4–3.1). Other research has found increases in fentanyl concentrations in blood over one day from pre-autopsy to autopsy [Citation115], which is not surprising given that the drug will have higher concentrations in tissues surrounding blood allowing diffusion into pooled blood after death. More generally, there can be significant variation in the quality of blood taken from a deceased person even when no putrefactive processes are evident.
Nevertheless, given the wide variability in concentrations producing a toxic response relative small artefactual changes in concentration are not likely to allow any concentration (other than perhaps extremely high from intentional misuse) to be used as a predictor of toxicity without recourse to the context of the case and other relevant findings including the pathology.
Of vital importance in any death investigation is the overall reliance on the testing laboratory to be able to detect novel opioids, and indeed other potentially toxic substances. For this reason alone, and the knowledge that there are so many toxic substances available to the wider community, a laboratory must be able to detect unknown substances at very low concentrations, rather than just relying on targeted detections, particularly for cases in which the circumstances suggest another substances may have been used, or the cause of a possible drug-caused death is equivocal.
In conclusion, there have been at least 16 novel opioids reported in death investigations and a number more identified in admissions to emergency departments that also have the potential to cause death, most of which are related structurally to fentanyl. Laboratories engaged in identifying poisons will need to beware of these drugs as well as other novel psychoactive substances (NPS) and of course the more widely available illicit drugs and prescription drugs that are encountered in the community.
Compliance with ethical standards
This paper does not contain any studies with human participants or animals performed by any of authors.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- World Drug Report 2017. Vienna: United Nations; 2017 [cited 2017 September 16]. Available from: http://www.unodc.org
- Pichini S, Solimini R, Berretta P, et al. Acute intoxications and fatalities from illicit fentanyl and analogues: an update. Ther Drug Monit. 2018;40:38–51.
- Armenian P, Vo KT, Barr-Walker J, et al. Fentanyl, fentanyl analogs and novel synthetic opioids: a comprehensive review. Neuropharmacology. 2017. doi: 10.1016/j.neuropharm.2017.10.016
- Ventura L, Carvalho F, Dinis-Oliveira RJ. Opioids in the frame of new psychoactive substances network: a complex pharmacological and toxicological issue. Curr Mol Pharmacol. 2018:11:97–108.
- Henderson GL. Fentanyl-related deaths: demographics, circumstances, and toxicology of 112 cases. J Forensic Sci. 1991;36:422–433.
- Schumann H, Erickson T, Thompson TM, et al. Fentanyl epidemic in Chicago, Illinois and surrounding Cook County. Clin Toxicol (Phila). 2008;46:501–506.
- Denton JS, Donoghue ER, McReynolds J, et al. An epidemic of illicit fentanyl deaths in Cook County, Illinois: September 2005 through April 2007. J Forensic Sci. 2008;53:452–454.
- Algren DA, Monteilh CP, Punja M, et al. Fentanyl-associated fatalities among illicit drug users in Wayne County, Michigan (July 2005–May 2006). J Med Toxicol. 2013;9:106–115.
- Tamburro LP, Al-Hadidi JH, Dragovic LJ. Resurgence of fentanyl as a drug of abuse. J Forensic Sci Med. 2016;2:111–114.
- Lee D, Chronister CW, Broussard WA, et al. Illicit fentanyl-related fatalities in Florida: toxicological findings. J Anal Toxicol. 2016;40:588–594.
- Okic M, Cnossen L, Crifasi JA, et al. Opioid overdose mortality in Kansas, 2001-2011: toxicologic evaluation of intent. J Anal Toxicol. 2013;37:629–635.
- Smialek JE, Levine B, Chin L, et al. A fentanyl epidemic in Maryland 1992. J Forensic Sci. 1994;39:159–164.
- Hull MJ, Juhascik M, Mazur F, et al. Fatalities associated with fentanyl and co-administered cocaine or opiates. J Forensic Sci. 2007;52:1383–1388.
- Thompson JG, Baker AM, Bracey AH, et al. Fentanyl concentrations in 23 postmortem cases from the hennepin county medical examiner's office. J Forensic Sci. 2007;52:978–981.
- Krinsky CS, Lathrop SL, Crossey M, et al. A toxicology-based review of fentanyl-related deaths in New Mexico (1986–2007). Am J Forensic Med Pathol. 2011;32:347–351.
- Martin TL, Woodall KL, McLellan BA. Fentanyl-related deaths in Ontario, Canada: toxicological findings and circumstances of death in 112 cases (2002-2004). J Anal Toxicol. 2006;30:603–610.
- Kronstrand R, Druid H, Holmgren P, et al. A cluster of fentanyl-related deaths among drug addicts in Sweden. Forensic Sci Int. 1997;88:185–193.
- Increases in fentanyl drug confiscations and fentanyl-related overdose fatalities [cited 2017 November 13]. Available from: https://www.cdc.gov/drugoverdose/opioids/fentanyl.html
- Rodda LN, Pilgrim JL, Di Rago M, et al. A cluster of fentanyl-laced heroin deaths in 2015 in Melbourne, Australia. J Anal Toxicol. 2017;41:318–324.
- Henderson GL. Designer drugs: past history and future prospects. J Forensic Sci. 1988;33:569–575.
- Lust EB, Barthold C, Malesker MA, et al. Human health hazards of veterinary medications: information for emergency departments. J Emerg Med. 2011;40:198–207.
- Vardanyan RS, Hruby VJ. Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications. Future Med Chem. 2014;6:385–412.
- Vuckovic S, Prostran M, Ivanovic M, et al. Fentanyl analogs: structure-activity-relationship study. Curr Med Chem. 2009;16:2468–2474.
- Labroo RB, Paine MF, Thummel KE, et al. Fentanyl metabolism by human hepatic and intestinal cytochrome P450 3A4: implications for interindividual variability in disposition, efficacy, and drug interactions. Drug Metab Dispos. 1997;25:1072–1080.
- Goromaru T, Matsuura H, Yoshimura N, et al. Identification and quantitative determination of fentanyl metabolites in patients by gas chromatography–mass spectrometry. Anesthesiology. 1984;61:73–77.
- Van Rooy HH, Vermeulen MP, Bovill JG. The assay of fentanyl and its metabolites in plasma of patients using gas chromatography with alkali flame ionisation detection and gas chromatography-mass spectrometry. J Chromatogr. 1981;223:85–93.
- Coopman V, Cordonnier J, Pien K, et al. LC-MS/MS analysis of fentanyl and norfentanyl in a fatality due to application of multiple Durogesic transdermal therapeutic systems. Forensic Sci Int. 2007;169:223–227.
- Marinetti LJ, Ehlers BJ. A series of forensic toxicology and drug seizure cases involving illicit fentanyl alone and in combination with heroin, cocaine or heroin and cocaine. J Anal Toxicol. 2014;38:592–598.
- Sutter ME, Gerona RR, Davis MT, et al. Fatal fentanyl: one pill can kill. Acad Emerg Med. 2017;24:106–113.
- Lotsch J, Walter C, Parnham MJ, et al. Pharmacokinetics of non-intravenous formulations of fentanyl. Clin Pharmacokinet. 2013;52:23–36.
- Anderson DT, Muto JJ. Duragesic® transdermal patch: postmortem tissue distribution of fentanyl in 25 cases. J Anal Toxicol. 2000;24:627–634.
- Bakovic M, Nestic M, Mayer D. Death by band-aid: fatal misuse of transdermal fentanyl patch. Int J Legal Med. 2015;129:1247–1252.
- Kuhlman JJ Jr., McCaulley R, Valouch TJ, et al. Fentanyl use, misuse, and abuse: a summary of 23 postmortem cases. J Anal Toxicol. 2003;27:499–504.
- Prosser JM, Jones BE, Nelson L. Complications of oral exposure to fentanyl transdermal delivery system patches. J Med Toxicol. 2010;6:443–447.
- Serghini I, Qamouss Y, Zoubir M, et al. Fatal intoxication caused by the application of the multiple transdermals patchs of fentanyl. Pan Afr Med J. 2015;20:21.
- Higashigawa Y, Suzuki S. Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds. VI. Structure–analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other analogues. Forensic Toxicol. 2008;26:1–5.
- Helander A, Backberg M, Beck O. Intoxications involving the fentanyl analogs acetylfentanyl, 4-methoxybutyrfentanyl and furanylfentanyl: results from the Swedish STRIDA project. Clin Toxicol (Phila). 2016;54:324–332.
- Dwyer JB, Janssen J, Luckasevic TM, et al. Report of increasing overdose deaths that include acetyl fentanyl in multiple counties of the southwestern region of Pennsylvania in 2015–2016. J Forensic Sci. 2017;63:195–200.
- McIntyre IM, Trochta A, Gary RD, et al. An acute acetyl fentanyl fatality: a case report with postmortem concentrations. J Anal Toxicol. 2015;39:490–494.
- Takase I, Koizumi T, Fujimoto I, et al. An autopsy case of acetyl fentanyl intoxication caused by insufflation of ‘designer drugs’. Leg Med (Tokyo). 2016;21:38–44.
- Maryanoff BE, Simon EJ, Gioannini T, et al. Potential affinity labels for the opiate receptor based on fentanyl and related compounds. J Med Chem. 1982;25:913–919.
- Guerrieri D, Rapp E, Roman M, et al. Acrylfentanyl: another new psychoactive drug with fatal consequences. Forensic Sci Int. 2017;277:e21–e29.
- Helander A, Backberg M, Signell P, et al. Intoxications involving acrylfentanyl and other novel designer fentanyls — results from the Swedish STRIDA project. Clin Toxicol (Phila). 2017;55:589–599.
- Ujvary I, Jorge R, Christie R, et al. Acrylfentanyl, a recently emerged new psychoactive substance: a comprehensive review. Forensic Toxicol. 2017;35:232–243.
- European drug report: European monitoring centre for drugs and drug addiction; 2016 [cited 2017 October 26, 2017]. Available from: http://www.emcdda.europa.eu/edr2016
- Butler DC, Shanks K, Behonick GS, et al. Three cases of fatal Acrylfentanyl Toxicity in the United States and a review of literature. J Anal Toxicol. 2018;42:e6–e11.
- McIntyre IM, Trochta A, Gary RD, et al. An acute butyr-fentanyl fatality: a case report with postmortem concentrations. J Anal Toxicol. 2016;40:162–166.
- Staeheli SN, Baumgartner MR, Gauthier S, et al. Time-dependent postmortem redistribution of butyrfentanyl and its metabolites in blood and alternative matrices in a case of butyrfentanyl intoxication. Forensic Sci Int. 2016;266:170–177.
- Hikin L, Smith PR, Hudson S, et al. Multiple fatalities in the North of England associated with synthetic fentanyl analogue exposure; detection and quantitation in a case series from early 2017. Forensic Sci Int. 2018;282:179–183.
- Cole JB, Dunbar JF, McIntire SA, et al. Butyrfentanyl overdose resulting in diffuse alveolar hemorrhage. Pediatrics. 2015;135:e740–e7433.
- Backberg M, Beck O, Jonsson KH, et al. Opioid intoxications involving butyrfentanyl, 4-fluorobutyrfentanyl, and fentanyl from the Swedish STRIDA project. Clin Toxicol (Phila). 2015;53:609–617.
- George AV, Lu JJ, Pisano MV, et al. Carfentanil–an ultra potent opioid. Am J Emerg Med. 2010;28:530–532.
- Riches JR, Read RW, Black RM, et al. Analysis of clothing and urine from Moscow theatre siege casualties reveals carfentanil and remifentanil use. J Anal Toxicol. 2012;36:647–656.
- Papsun D, Isenschmid D, Logan BK. Observed carfentanil concentrations in 355 blood specimens from forensic investigations. J Anal Toxicol. 2017;41:777–778.
- Swanson DM, Hair LS, Strauch Rivers SR, et al. Fatalities involving carfentanil and furanyl fentanyl: two case reports. J Anal Toxicol. 2017;41:498–502.
- Mohr AL, Friscia M, Papsun D, et al. Analysis of novel synthetic opioids U-47700, U-50488 and furanyl fentanyl by LC-MS/MS in postmortem casework. J Anal Toxicol. 2016;40:709–717.
- Guerrieri D, Rapp E, Roman M, et al. Postmortem and toxicological findings in a series of Furanylfentanyl-related deaths. J Anal Toxicol. 2017;41:242–249.
- Milroy CM, Kepron C. Two deaths associated with Furanylfentanyl toxicity. Forensic Sci Int. 2017; IAFS special issue:88.
- Martucci HFM, Ingle EA, Hunter MD, et al. Distribution of furanyl fentanyl and 4-ANPP in an accidental acute death: a case report. Forensic Sci Int. 2018;283:e13–e17.
- Papsun D, Hawes A, Mohr AL, et al. Case series of novel illicit opioid-related deaths. Acad Forensic Pathol. 2017;7:477–486.
- Martin M, Hecker J, Clark R, et al. China white epidemic: an eastern United States emergency department experience. Ann Emerg Med. 1991;20:158–164.
- Gillespie TJ, Gandolfi AJ, Davis TP, et al. Identification and quantification of alpha-methylfentanyl in post mortem specimens. J Anal Toxicol. 1982;6:139–142.
- Hibbs J, Perper J, Winek CL. An outbreak of designer drug–related deaths in Pennsylvania. JAMA. 1991;265:1011–1013.
- Ojanpera I, Gergov M, Rasanen I, et al. Blood levels of 3-methylfentanyl in 3 fatal poisoning cases. Am J Forensic Med Pathol. 2006;27:328–331.
- Ojanpera I, Gergov M, Liiv M, et al. An epidemic of fatal 3-methylfentanyl poisoning in Estonia. Int J Legal Med. 2008;122:395–400.
- Coopman V, Cordonnier J, De Leeuw M, et al. Ocfentanil overdose fatality in the recreational drug scene. Forensic Sci Int. 2016;266:469–473.
- Dussy FE, Hangartner S, Hamberg C, et al. An acute ocfentanil fatality: a case report with postmortem concentrations. J Anal Toxicol. 2016;40:761–766.
- Kronstrand R, Thelander G, Lindstedt D, et al. Fatal intoxications associated with the designer opioid AH-7921. J Anal Toxicol. 2014;38:599–604.
- Vorce SP, Knittel JL, Holler JM, et al. A fatality involving AH-7921. J Anal Toxicol. 2014;38:226–230.
- Karinen R, Tuv SS, Rogde S, et al. Lethal poisonings with AH-7921 in combination with other substances. Forensic Sci Int. 2014;244:e21–e24.
- Elliott S, Bodenschaft C, Beran D. AH-7921: critical review report. Geneva: World Health Organization; 2014. p. 22.
- Fels H, Krueger J, Sachs H, et al. Two fatalities associated with synthetic opioids: AH-7921 and MT-45. Forensic Sci Int. 2017;277:e30–e35.
- Elliott S, Bodenschatz C, Beran D. AH-7921. Critical review report. Geneva: United Nations; 2014.
- Katselou M, Papoutsis I, Nikolaou P, et al. AH-7921: the list of new psychoactive opioids is expanded. Forensic Toxicol. 2015;33:195–201.
- Evans-Brown E, Cunningham A, Gallegos A, et al. MT-45 risk assessment. Lisbon: EMCDDA; 2014.
- Helander A, Backberg M, Beck O. MT-45, a new psychoactive substance associated with hearing loss and unconsciousness. Clin Toxicol (Phila). 2014;52:901–904.
- Papsun D, Krywanczyk A, Vose JC, et al. Analysis of MT-45, a novel synthetic opioid, in human whole blood by LC-MS-MS and its identification in a drug-related death. J Anal Toxicol. 2016;40:313–317.
- Uchiyama N, Matsuda S, Kawamura M, et al. Identification of two new-type designer drugs, piperazine derivative MT-45 (I-C6) and synthetic peptide Nooppet (GVS-111), with synthetic cannabinoid A-834735, cathinone derivative 4-methoxy-alpha-PVP, and phenethylamine derivative 4-methylbuphedrine from illegal products. Forensic Toxicol. 2014;32:9–18.
- Elliott SP, Brandt SD, Smith C. The first reported fatality associated with the synthetic opioid 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide (U-47700) and implications for forensic analysis. Drug Test Anal. 2016;8:875–879.
- Coopman V, Blanckaert P, Van Parys G, et al. A case of acute intoxication due to combined use of fentanyl and 3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide (U-47700). Forensic Sci Int. 2016;266:68–72.
- Ellefsen KN, Taylor EA, Simmons P, et al. Multiple drug toxicity involving novel psychoactive substances, 3-fluorophenmetrazine and U-47700. J Anal Toxicol. 2017;41:765–770.
- Rohrig TP, Miller SA, Baird TR. U-47700: a not so new opioid. J Anal Toxicol. 2017;42:e12–e14.
- Domanski K, Kleinschmidt KC, Schulte JM, et al. Two cases of intoxication with new synthetic opioid, U-47700. Clin Toxicol (Phila). 2017;55:46–50..
- Jones MJ, Hernandez BS, Janis GC, et al. A case of U-47700 overdose with laboratory confirmation and metabolite identification. Clin Toxicol (Phila). 2017;55:55–59.
- DEA Headquarters News. Washington (DC): Drug Enforcement Administration; 2016 [cited 2017 October 23]. Available from: https://www.dea.gov/divisions/hq/2016/hq111016.shtml
- Helland A, Brede WR, Michelsen LS, et al. Two hospitalizations and one death after exposure to ortho-fluorofentanyl. J Anal Toxicol. 2017;41:708–709.
- Strehmel N, Vejmelka E, Kastner K, et al. NPS-findings in forensic toxicology – three case reports. Toxichem Krimtech. 2017;84:199–204.
- Ferslew KE, Hagardorn AN, McCormick WF. Postmortem determination of the biological distribution of sufentanil and midazolam after an acute intoxication. J Forensic Sci. 1989;34:249–257.
- Cunningham SM, Haikal NA, Kraner JC. Fatal intoxication with acetyl fentanyl. J Forensic Sci. 2016;61 Suppl 1:S276–S280.
- Giorgetti A, Centola C, Giorgetti R. Fentanyl novel derivative-related deaths. Hum Psychopharmacol. 2017;32:1–11.
- Ruzycki S, Yarema M, Dunham M, et al. Intranasal fentanyl intoxication leading to diffuse alveolar hemorrhage. J Med Toxicol. 2016;12:185–188.
- Porter R, O'Reilly H. Pulmonary hemorrhage: a rare complication of opioid overdose. Pediatr Emerg Care. 2011;27:742–744.
- Tiscione NB, Wegner K. Validation of the Neogen(R) Fentanyl ELISA Kit for blood and urine. J Anal Toxicol. 2017;41:313–317.
- Wang BT, Colby JM, Wu AH, et al. Cross-reactivity of acetylfentanyl and risperidone with a fentanyl immunoassay. J Anal Toxicol. 2014;38:672–675.
- Ruangyuttikarn W, Law MY, Rollins DE, et al. Detection of fentanyl and its analogs by enzyme-linked immunosorbent assay. J Anal Toxicol. 1990;14:160–164.
- Schueler HE. Emerging synthetic fentanyl analogs. Acad Forensic Pathol. 2017;7:36–40.
- Pearson J, Poklis J, Poklis A, et al. Postmortem toxicology findings of acetyl fentanyl, fentanyl, and morphine in heroin fatalities in Tampa, Florida. Acad Forensic Pathol. 2015;5:676–689.
- Van Nimmen NF, Poels KL, Veulemans HA. Highly sensitive gas chromatographic-mass spectrometric screening method for the determination of picogram levels of fentanyl, sufentanil and alfentanil and their major metabolites in urine of opioid exposed workers. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;804:375–387.
- Eckart K, Rohrich J, Breitmeier D, et al. Development of a new multi-analyte assay for the simultaneous detection of opioids in serum and other body fluids using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;1001:1–8.
- Cooreman S, Deprez C, Martens F, et al. A comprehensive LC-MS-based quantitative analysis of fentanyl-like drugs in plasma and urine. J Sep Sci. 2010;33:2654–2662.
- Sofalvi S, Schueler HE, Lavins ES, et al. An LC-MS-MS method for the analysis of carfentanil, 3-Methylfentanyl, 2-Furanyl Fentanyl, acetyl fentanyl, fentanyl and norfentanyl in postmortem and impaired-driving cases. J Anal Toxicol. 2017;41:473–483.
- Gergov M, Nokua P, Vuori E, et al. Simultaneous screening and quantification of 25 opioid drugs in post-mortem blood and urine by liquid chromatography-tandem mass spectrometry. Forensic Sci Int. 2009;186:36–43.
- Shaner RL, Kaplan P, Hamelin EI, et al. Comparison of two automated solid phase extractions for the detection of ten fentanyl analogs and metabolites in human urine using liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;962:52–58.
- Wang L, Bernert JT. Analysis of 13 fentanils, including sufentanil and carfentanil, in human urine by liquid chromatography-atmospheric-pressure ionization-tandem mass spectrometry. J Anal Toxicol. 2006;30:335–341.
- Shoff EN, Zaney ME, Kahl JH, et al. Qualitative identification of fentanyl analogs and other opioids in postmortem cases by UHPLC-Ion Trap-MSn. J Anal Toxicol. 2017;41:484–492.
- Alexander RT, Hedrick CW, Alexander SD, et al. Epidemic fentanyl deaths in Maryland: A public health intervention involving geographic information systems and collaboration with the drug enforcement administration. Acad Forensic Pathol. 2016;6:301–314.
- Griswold MK, Chai PR, Krotulski AJ, et al. Self-identification of nonpharmaceutical fentanyl exposure following heroin overdose. Clin Toxicol (Phila). 2018;56:37–42.
- Sgarlato A, deRoux SJ. Prescription opioid related deaths in New York City: a 2 year retrospective analysis prior to the introduction of the New York State I-STOP law. Forensic Sci Med Pathol. 2015;11:388–394.
- Murphy Y, Goldner EM, Fischer B. Prescription opioid use, harms and interventions in Canada: a review update of new developments and findings since 2010. Pain Physician. 2015;18:E605–E614.
- van Amsterdam J, van den Brink W. The misuse of prescription opioids: a threat for Europe? Curr Drug Abuse Rev. 2015;8:3–14.
- Al-Saffar Y, Stephanson NN, Beck O. Multicomponent LC-MS/MS screening method for detection of new psychoactive drugs, legal highs, in urine-experience from the Swedish population. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;930:112–120.
- Centers for Disease Control and Prevention. Acetyl fentanyl overdose fatalities–Rhode Island, March-May 2013. MMWR Morb Mortal Wkly Rep. 2013;62:703–704.
- Wax PM, Becker CE, Curry SC. Unexpected “gas” casualties in Moscow: a medical toxicology perspective. Ann Emerg Med. 2003;41:700–705.
- Drummer OH. Postmortem toxicology of drugs of abuse [Review]. Forensic Sci Int. 2004;142:101–113.
- Krinsky CS, Lathrop SL, Zumwalt R. An examination of the postmortem redistribution of fentanyl and interlaboratory variability. J Forensic Sci. 2014;59:1275–1279.
- Lozier MJ, Boyd M, Stanley C, et al. Acetyl fentanyl, a novel fentanyl analog, causes 14 overdose deaths in Rhode Island, March-May 2013. J Med Toxicol. 2015;11:208–217.
- Poklis J, Poklis A, Wolf C, et al. Two fatal intoxications involving butyryl fentanyl. J Anal Toxicol. 2016;40:703–708.
- Rojkiewicz M, Majchrzak M, Celinski R, et al. Identification and physicochemical characterization of 4-fluorobutyrfentanyl (1-((4-fluorophenyl)(1-phenethylpiperidin-4-yl)amino)butan-1-one, 4-FBF) in seized materials and post-mortem biological samples. Drug Test Anal. 2017;9:405–414.
- Yonemitsu K, Sasao A, Mishima S, et al. A fatal poisoning case by intravenous injection of “bath salts” containing acetyl fentanyl and 4-methoxy PV8. Forensic Sci Int. 2016;267:e6–e9.
- Fort C, Curtis B, Nichols C, et al. Acetyl fentanyl toxicity: two case reports. J Anal Toxicol. 2016;40:754–757.
- Dziadosz M, Klintschar M, Teske J. Postmortem concentration distribution in fatal cases involving the synthetic opioid U-47700. Int J Legal Med. 2017;131:1555–1556.
- Ellefsen KN, Taylor EA, Simmons P, et al. Multiple drug-toxicity involving novel psychoactive substances, 3-Fluorophenmetrazine and U-47700. J Anal Toxicol. 2017;41:765–770.
- McIntyre IM, Gary RD, Joseph S, et al. A fatality related to the synthetic opioid U-47700: postmortem concentration distribution. J Anal Toxicol. 2017;41:158–160.
- Acryloylfentanyl. EMCDDA – Europol joint report on a new psychoactive substance. Lisbon: EMCDDA; 2017.
- Shanks KG, Behonick GS. Detection of Carfentanil by LC-MS-MS and reports of associated fatalities in the USA. J Anal Toxicol. 2017;41:466–472.