 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In order to explore the influence of arbuscular mycorrhizal (AM) fungi in the rhizosphere of poisonous plants on the neighboring pasture grasses in the Tibetan Plateau Alpine meadow ecosystem, rhizosphere soils were collected from eight different poisonous plants in degraded grasslands and one from pasture grass in non-degraded grasslands (CK). The collected soils were used as inocula to assess the influence of indigenous AM fungi on the growth of two typical pasture grass species, Elymus nutans and Poa pratensis, in a bioassay experiment. Five growth parameters and two AM parameters were determined. The mycorrhizal responsiveness and the importance value were calculated. Significant differences between the eight poisonous plants and CK were observed. Compared to CK, rhizosphere soil from the eight poisonous plants had lower AM fungal spore densities. The growth of E. nutans and P. pratensis seedlings was depressed with the inoculation from poisonous plants rhizosphere soil. This study demonstrated that the presence of poisonous plants with grassland degradation altered inherent AM fungal community abundance, and could exert inhibition effects on the growth of pasture grasses. It may attribute to discover the important role of rhizosphere soil of different poisonous plants to AM fungal community on the Alpine meadow.
Introduction
Why poisonous plants are often more proliferative than pasture grasses in degraded range has been one major question in understanding grassland-degraded biology (Jin et al. Citation2011; Li et al. Citation2014a). Previous studies have provided various hypotheses to explain the increased competitive ability of poisonous plant species, including phenotypic plasticity (Harper Citation1977), livestock selective grazing behaviors (Cheng et al. Citation2014), climate changes (Gao et al. Citation2014), production of allelopathic compounds (Gai et al. Citation2012), evolution of increased competitive ability and the species richness of arbuscular mycorrhizal (AM) fungi (Cai et al. Citation2014). The first three hypotheses are traditionally considered as the key factors in helping poisonous plants to adapt to poor soil environments and compete against pasture grasses in the degraded grasslands. For example, phenotypic plasticity enabled the poisonous plant species Ligularia virgaurea to reproduce and rapidly colonize new areas (Xie et al. Citation2014). Similarly, the poisonous plants Saussurea japonica and Stellera chamaejasme exhibited pronounced morphological variation in different elevation gradients, allowing themselves to become dominant plants across diverse habitats in the grassland ecosystem (Hautier et al. Citation2009; Zhang, Zhang, and Sun Citation2011b). Moreover, poisonous plants are extremely harmful for animals (Wu et al. Citation2009). Allelopathy could also help the explanation of why some poisonous plant species could outcompete pasture grass in the degraded plant community (Shi et al. Citation2012; Dong et al. Citation2015a). On the other hand, in an undisturbed or healthy pasture ecosystem, the growth of grasses will be improved through the strategies of increasing forage grass functional group. At the same time, poisonous plants in these ecosystems will be inhabited with the limited soil C, N, P supplement (Wu et al. Citation2010).
Regarding the fifth hypothesis, i.e., AM fungi (Glomeromycota) are widely distributed in terrestrial ecosystems and could form symbiotic associations with more than 80% of vascular plants (Smith and Read Citation2008). In general, the host plants provide photosynthetic carbon for the growth of associated AM fungi, and the plant performance may also affect AM fungi community (Fellbaum et al. Citation2014). In return, AM fungi supply soil nutrients to host plants and hence could exert strong effects on plant communities and consequently affect ecosystem processes (Mikkelsen, Rosendahl, and Jakobsen Citation2008). It is generally accepted that AM associations, as critical links between the above- and below-ground biotic communities in ecosystems, are affected by plant species (Engelmoer and Kiers Citation2015). For instance, the presence of poisonous plants negatively influenced the diversity of AM fungi species in the Tibetan Plateau (Cai et al. Citation2014). Thus, the succession direction and course of plant communities in grassland can be determined by the interactions between soil microbial communities and plants; therefore, the soil microbes could have potential impacts on poisonous plants and alteration of the plant community structure (Inderjit and van der Putten Citation2010; Hiiesalu et al. Citation2014). Furthermore, altered AM fungi community composition and functioning in grassland degradation (i.e., presence of poisonous plants) may represent a limiting factor for restoration efforts even after removal of poisonous plants species, i.e., legacy effects on future generations of the dominant host species (Li et al. Citation2014a). In monoculture establishment areas of poisonous plants, the survival of pasture grass is compromised. The mechanisms underlying poisonous plants’ capacity to enter within intact pasture grass communities and proliferate in degraded grasslands are yet to be discovered. Previous studies have focused on the diversity of AM fungi in grasslands and their roles in the restoration of serious disturbed areas (Gai et al. Citation2009; Li et al. Citation2014a). These studies have shown that AM fungi exert a significant influence on plant community structure and dynamics in grasslands and other terrestrial ecosystems. However, the relationship between poisonous plants, AM fungi, and pasture grasses has been paid little attention in the Tibetan Plateau Alpine meadow.
The Tibetan Plateau Alpine meadow ecosystem is on the largest and highest plateau in the world, which extends over 0.88 million km2 (Yang et al. Citation2008). Based on its special terrain and landscape, the Alpine meadow is a fragile and sensitive terrestrial ecosystem (Cao et al. Citation2004). In recent years, the meadow ecosystems have been severely damaged by many disturbances in this area (Wen et al. Citation2013; Li et al. Citation2014b). The ecological impacts including the excessive defoliation of herbaceous vegetation, increasing the quantity of poisonous plants populations and reducing pasture plant species abundance and richness (Li et al. Citation2014b). Grassland degradation can also reduce the vigor and presence of dominant pasture plant species, favoring the spread of poisonous plants (Sternberg et al. Citation2000; Li et al. Citation2014b). The presence of a large number of poisonous plants populations could lead to huge economic losses for farmers and local governments (Jin et al. Citation2011). Thus, it is an urgent problem to determine the dynamics of poisonous plants under conditions of degraded grassland in the Tibetan Plateau Alpine meadow. Previous studies determined the effects of rodent (Lu et al. Citation2013), overgrazing (Peng et al. Citation2015), climate changes (Dong et al. Citation2015b) on the degraded grasslands, and a wide range of below-ground biotic interactions have been found to regulate plant distribution and ecosystem function (Wu et al. Citation2009). Therefore, knowledge of the interactions between plant and soil microbes is essential for the enhanced understanding of the complex dynamics of plant community succession in the course of poisonous plants’ development.
Plants and soil microbes often interact with each other and some of them are interdependent (Silva and Batalha Citation2008). In the present study, rhizosphere soils in the population of eight dominant poisonous species in the Tibetan Plateau Alpine meadow were examined to assess the relationship between poisonous plants, AM fungi, and pasture grass. The aims of this study were to test the following hypotheses: 1) presence of poisonous plants with grassland of degradation decreases AM fungi spore density, 2) rhizosphere soil of poisonous plants inhibits the growth of local pasture grasses, but promotes the growth and spread of poisonous plants.
Materials and methods
Field sites and sampling
The sampling sites were located at the natural Tibetan Plateau Alpine meadow in Hequ Farm, Maqu County, Gansu Province, China (N 33°40′, E 101°52′, altitude 3530 m a.s.l.). In this site, a sampling area (600 000 m2) was selected, covering the diversity of dominant plant species and soil patches completely colonized by the poisonous plants (Wang Citation2016). There were several different forages grasses in the Tibetan Plateau Alpine meadow ecosystem, thereinto, Elymus nutans and Poa pratensis were the two dominant forages species in this ecosystem. In order to ensure the experiments manageable, E. nutans and P. pratensis were selected as the local pasture grasses in this study. Firstly, the seeds of E. nutans and P. pratensis were collected from the natural Tibetan Plateau Alpine meadow in September 2014, and the seeds were kept in 4°C before used. Then a 10 m × 10 m sample plot was selected, which was further divided into 100 1 m×1 m quadrats with the adjacent lattice method. The number of plants, plant height, coverage index and shoot biomass of the poisonous plants within the 100 quadrats were measured. There were 29 species of poisonous plants belonging to 12 families mainly distributed within the Alpine grassland ecosystem (Chen and Zheng Citation1987; Shi Citation1997), among which many are either subordinate or dominant plant species with small number of plants (). The aim of this present study focused on the dominant poisonous plants which are most frequently distributed in the Tibetan Plateau Alpine meadow; thus, the importance value (IV) was used to determine which poisonous plants will be selected for further study. The importance values (IV) of poisonous plants were calculated by Lindsey (Citation1956) method:
Table 1. List of poisonous plants in the Alpine steppe ecosystem in Hequ Farm, Maqu County
According to , the following 8 perennial species with the highest IV were chosen for this study: Ligularia virgaurea (Asteraceae), Saussurea japonica (Asteraceae), Anaphalis lactea (Asteraceae), Leontopodium japonicum (Asteraceae), Lamiophlomis rotata (Lamiaceae), Anemone rivularis (Ranunculaceae), Polygonum viviparum (Polygonaceae), and Gentiana macrophylla (Gentianaceae). Rhizosphere soils in the population of each poisonous plant were collected in degraded grassland ranges. The rhizosphere soils which dominated by co-existing E. nutans and P. pratensis in the non-degraded grassland ranges were collected and treated as controls (CK). Each plant and CK has 5 replicates. Each site covered about 100 m2, and the distances between every neighboring site were 50–100 m. In each plant community, the samples of soil adhering to roots were collected with the depth from 0 to 20 cm. If one individual plant was not big enough to provide 500 g adhering soils, more individual plants from the same site were selected to collect the adhering soils, and then put together as one sample. Each plant species collected 500 g soil. All the samples and replicates for rhizosphere soil of the same plant were air dried at 13–24°C, and sieved (mesh size <2 mm) to remove coarse roots and fragments, then stored at 4°C until use. The rhizosphere soils were used directly as AM fungi inocula, which included spores and hyphal fragments.
Table 2. Distribution characteristics of plants in the study sites
AM fungi spore density measurement
Ten gram of the each air-dried soil samples were used to extract the spores of AM fungi by wet sieving and decanting method, followed by sucrose centrifugation (Sieverding, Friedrichsen, and Suden Citation1991) and recovery of the spores through 45 μm sieving of the supernatant. Then, AM fungi spores were counted on a grid-patterned dish under a binocular stereomicroscope.
Experiments with E. nutans or P. pratensis
The pot experiments were carried out in the Ecological Research Station, Lanzhou University from January to June 2015. The rhizosphere soil samples from eight poisonous plants and CK treatment were conducted to test the effects for E. nutans and P. pratensis. The seeds of E. nutans and P. pratensis were sterilized in 10% sodium hypochlorite for 15 min, and washed with sterilized water three times, then germinated on moist filter paper (sterilized water). Two germinated seeds of each species were planted in each of 180 plastic pots (25 cm diameter, 1000 ml) containing 3 kg substrate (dry weight) of an autoclaved (121°C, 30 min) soil mixture of loamy soil and quartz sand (1:1). The loamy soil originated from the grassland of E. nutans and P. pratensis native habitat where the seeds were collected. The substrate was generally low in nutrients: 3.9 ± 0.8 μg g−1 NO3-N, 5.65 ± 0.78 μg g−1 available P, 240 ± 12 μg g−1 potassium, 0.80 ± 0.07% organic matter, and pH of 7.5. Soils from the nine origin sites were stored at 4°C, and half of each soil sample was sterilized (121oC, 30 min) by autoclaving to perform greenhouse experiments as inocula. Before inoculated, the rhizosphere soils from each plant from 5 sites were combined together as one AM inoculum. The pots were treated with two inoculation treatments: 1) AM fungi treatment, each pot mixed with 20 g of rhizosphere soil; 2) non-AM fungi treatment: each pot mixed with 20 g of autoclaved (121°C, 30 min) rhizosphere soil. There were five replicates per treatment with a total size of 180 pots.
In order to introduce the bacterial community associated with the living AM inoculum to the non-AM fungi treatment pots, 100 g rhizosphere soil inoculum of each poisonous plants were suspended in 500 ml water, shaken for 5 min and then filtered through a filter paper (11 μm) and added to all of the non-AM fungi treatment pots (van der Heijden, Wiemken, and Sanders Citation2003; Asghari et al. Citation2005; Jin et al. Citation2010). Soils were incubated for 1 week to allow the bacterial community to equilibrate (Asghari et al. Citation2005). Pots were randomly placed in the greenhouse. The average greenhouse temperatures during the experiment were 28 ± 3 oC in daytime and 18 ± 3 oC at night, respectively. The daytime is 15 h and 9 h for night. The photosynthetic photon flux density was 810–920 μmol m−2 s−1. The pots were watered every 3 days with tap water without fertilizer.
After 5 months of growth, plants were harvested. The plants in each pot were washed to remove soil, and then the plants of E. nutans or P. pratensis were collected. Shoot height, root length and the number of tillers were measured firstly. Then, shoot and root of each plant was separated at the girth part of stem. Shoots were dried at 80°C for 48 h, and weighed to determine biomass. Roots were washed, weighed and then divided into two samples. One sample was analyzed for AM colonization rates. Another root sample was used to determine the fresh weight, and dry weight (80 oC for 48 h). The dry/wet weight ratio of the second root sample was used to calculate the total root biomass.
Determination of AM colonization rates
Roots of the second sub-sample were cleared and stained by the following method modified from Phillips and Hayman (Citation1970). The lateral fine roots (<1 mm) of E. nutans and P. pratensis were selected. The roots were cleared for 40 min in 10% KOH at 85°C, placed in 1% HCl for 2 min and then stained with glycerol-trypan blue (0.065%) at 90°C for 15 min. The stained root samples were examined at 40–100 × magnification and quantification of root colonization by AM fungi was estimated by the gridline-intersect method (Giovannetti and Mosse Citation1980).
Statistical analysis
All data sets were checked for normality prior to further analysis, and none of them was significantly different from a normal distribution, so no data transformation was required. Means of shoot biomass, root biomass, shoot height, root length, tillers per individual plant, AM spores and AM colonization rates were calculated according to the data using SPSS (v18.0). Multiple comparison procedures for one-way ANOVA were used to analyze the differences between AM treatment (original field soil) and non-AM (sterilized field soil) treatment. The means were separated using standard errors.
Mycorrhizal responsiveness was calculated by Menge, Johnson, and Platt (Citation1978) formula:
Pearson’s correlation using SPSS (v16.0) was used to test the relationship between AM colonization rate and shoot height, tillers, root length, shoot biomass, root biomass and total biomass of E. nutans and P. pratensis, respectively.
Results
Grassland degradation promoting the establishment of poisonous plant populations
The significant difference about the species abundance of poisonous plants was observed among the non-degraded grasslands and degraded grasslands treatments (). The importance values (IV) of eight poisonous plant species in degraded grasslands were higher than that in non-degraded grasslands treatments in Alpine meadow. The coverage of poisonous plants increased significantly (P < 0.05), while the coverage of non-poisonous plants decreased dramatically with grassland degradation (P < 0.05).
AM fungi inoculum potential and root colonization rates
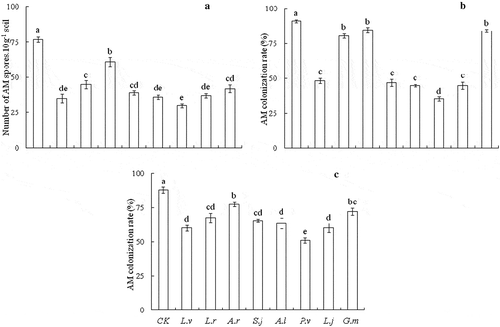
The AM fungi spore densities in the rhizosphere soil samples of dominant grass were higher than that in the rhizosphere soil of poisonous plants ()). Soil samples of dominant grass contained 77 spores per 10 g air-dried soil, and soils from P. viviparum had 30 spores per 10 g air-dried soil. Compared with soil samples of dominant grass, spore numbers were reduced by about 72% in rhizosphere soil samples of P. viviparum (P < 0.001). Our results demonstrated that inoculating rhizosphere soil of different plants could infect E. nutans and P. pratensis. There was no AM colonization in non-AM treatments. Thus, there were significant differences about AM colonization rates between AM treatments (un-sterilized) and non-AM treatments (sterilized). Root colonization rates of E. nutans and P. pratensis were significantly affected by the different soil origins. Compared with soil samples of poisonous plants, AM colonization rates of E. nutans and P. pratensis seedlings were significantly lower than those of the plants that were inoculated with rhizosphere soils of dominant grass ((b,c), P < 0.001). Root colonization rates of E. nutans varied from 35.25% to 91.02%, whilst those inoculated with rhizosphere soils of dominant grass had the highest root colonization rates, and those inoculated with rhizosphere soil of P. viviparum had the lowest. Root colonization rates of P. pratensis ranged from 51.06% to 87.76%, and those inoculated with the rhizosphere soil of P. viviparum have the lowest.
Figure 1. AM spore density (a), AM colonization rate of Elymus nutans (b) and AM colonization rate of Poa pratensis (c) inoculated with different poisonous plants rhizosphere soils. Bars indexed with different letters mean significant differences (P < 0.05)
Plant growth
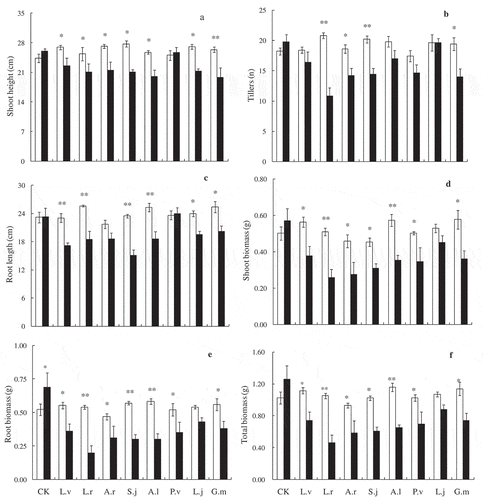
Our results showed that the shoot height, root length, tiller number and biomass of E. nutans and P. pratensis was significant affected by the soil origins (,). Compared with those inoculated with AM fungi from poisonous plants soils, the growth of E. nutans and P. pratensis seedlings were significantly lower than those inoculated with AM fungi from dominant grass soil. Shoot height of E. nutans significantly decreased when inoculated with AM fungi from L. virgaurea, L. rotata, S. japonica and P. viviparum soil ()). The number of tillers of E. nutans significantly decreased when inoculated with rhizosphere soil of L. rotata, S. japonica, A. lactea and G. macrophylla ()). Root length (except rhizosphere soil of L. japonicum and G. macrophylla) were significantly inhibited by inoculation with rhizosphere soil from poisonous plants ()). Plant biomass of E. nutans was negatively affected by AM fungi from poisonous plants, with both root biomass, shoot biomass (except AM fungi from L. virgaurea and L. japonicum) and total biomass (only except AM fungi from L. virgaurea) decreased significantly ((d,e,f)). Shoot heights and tillers of P. pratensis significantly decreased when inoculated with AM fungi from L. rotata, S. japonica, A. lactea and G. macrophylla ((a,b)). Furthermore, our results suggested that inoculating rhizosphere soil of L. rotata could significantly decreased the growth parameters of E. nutans and P. pratensis plants (,). Tillers and root length of P. pratensis were negatively affected by AM fungi inoculation ((b,c)). Plant biomass of P. pratensis was significantly decreased by AM fungi, with both root biomass and shoot biomass (only except AM fungi from L. japonicum) significantly decreased ((d,e,f)). There was no significant difference between the growth parameters (shoot heights, tillers, root lengths, and biomasses) of E. nutans and P. pratensis when they were inoculated with non-AM soil (,). Pearson’s correlation results showed that there were significantly negative correlation between AM colonization rates and plant growth indexes (except the root biomass and total biomass of E. nutans), means that compared with the sterilized rhizosphere soil of poisonous plants, the native rhizosphere soil of poisonous plants significantly inhibited the growth of E. nutans and P. pratensis (,, ). Moreover, mycorrhizal responsiveness indirectly reveals that the biomass response varied according to the type of inoculations. E. nutans and P. pratensis seedlings have shown mycorrhizal responsiveness when inoculated with rhizosphere soils of dominant grass, 36.43% and 18.45%, respectively. However, it is demonstrated that there was negatively influence for the growth of pasture grasses when inoculated with native AM fungi from poisonous plants rhizosphere soils ().
Table 3. Pearson’s correlation between AM fungal colonization rate and the plant growth parameters of E. nutans and P. pratensis.
Figure 2. Shoot height (a), tillers (b), root length (c), shoot biomass (d), root biomass (e), total biomass per pot (f) of Elymus nutans when inoculated with sterilized or un-sterilized rhizosphere soil from different poisonous plants. The filled columns are AM fungi inoculum treatments (un-sterilized) and the open columns are non-AM fungi treatments (sterilized). Bars represent means ± SE, * indicates p < 0.05, ** indicates p < 0.001
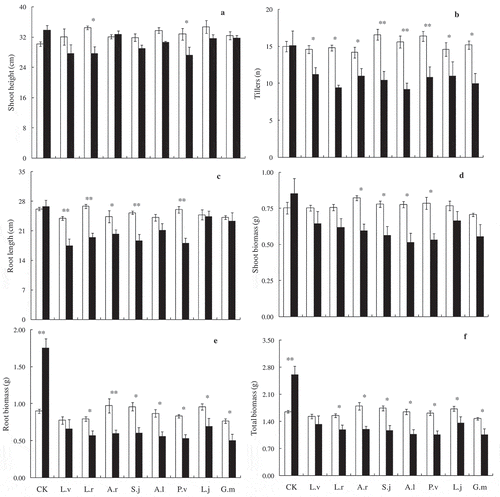
Figure 3. Shoot height (a), tillers (b), root length (c), shoot biomass (d), root biomass (e), total biomass per pot (f) of Poa pratensis when inoculated with sterilized or un-sterilized rhizosphere soil from different poisonous plants. The filled columns are AM fungi inocula treatments (un-sterilized) and the open columns are non-AM fungi treatments (sterilized). Bars represent means ± SE, * indicates p < 0.05, ** indicates p < 0.001
Discussion
Presence of poisonous plants and their relationship with AM fungi in degraded grasslands
It has been demonstrated that poisonous plants had the fastest spreading speed in degraded grasslands ecosystem (Li et al. Citation2014b). Most of the poisonous plants can be categorized as pioneer plants in degraded grasslands ecosystem, which could survive in barren environment to establish its population as dominance plants (Zuo et al. Citation2009). The reason may be that most of the poisonous plants have a developed root system and the ability for tolerance against biotic and abiotic stress (Cheng et al. Citation2014); thus, more water, soil, and other supporting resources could be obtained for poisonous plants. As a result, with these strong potential competitors, the growths of pasture grasses have been decreased and the ecological balance of Tibetan Plateau Alpine meadow was been destroyed (). In this study, the distribution of plant communities was significantly related to poisonous plants, especially of L. virgaurea (IV = 3.27). Several poisonous plants, including L. virgaurea and Stellera chamaejasme have been shown to reduce the richness of pasture grasses, plant diversity, and community productivity, causing grassland ecosystem instability (Jin et al. Citation2011; Cheng et al. Citation2014). Thus, the relatively higher poisonous plants diversity indicated that poisonous plants in degraded grassland was a big threat to the Alpine meadow ecosystem stability ().
AM symbioses have the capacity in determining plant species abundance, diversity, and richness in natural communities, and their function to provide various influences on plant growth with co-existing host plant, thus AM cause potential influences on the terrestrial ecosystem (Gai et al. Citation2009; Gianinazzi et al. Citation2010; Hiiesalu et al. Citation2014). For the reason that the responses are not equal among the different plant-fungal symbioses, the structure of plant communities may also have a feedback on the survival and growth of mycorrhizal fungi (Bever Citation2002; López-García, Azcón-Aguilar, and Barea Citation2014). Thus, the herbaceous plant species in grassland communities may also play an important role in determining the diversity and composition of AM fungi in natural habitats (Bever et al. Citation2009; Kiers et al. Citation2011; Jiang et al. Citation2018). Our results indicated that there is a significant difference in spore density and root colonization level in rhizosphere soil samples of different plants. It has been demonstrated that grassland degradation causes a decline in AM fungi community diversity of soil, leading to dramatic changes in both the structure of above-ground plant communities and the functioning of their below-ground ecosystem (Zhang et al. Citation2011a; Cai et al. Citation2014; Hiiesalu et al. Citation2014; Li et al. Citation2017). Gai et al. (Citation2006) also found that spore densities in the normal grassland were much higher than the degraded grasslands. Our study indicates that grasslands degeneration was one of the key factors determining the distribution of poisonous plants, by altering soil microbial community (especially AM fungi). Our results also showed that indirect disruption of these mutualistic associations between pasture grasses and AM fungi may be the main mechanism facilitating presence of poisonous plants.
Implications for E. nutans and P. pratensis growth reduction
The results indicated a significant growth reduction when pasture grasses were inoculated with AM from poisonous plant rhizosphere, compared to those uninoculated (,). There may be two possible reasons. One explanation could be that some poisonous plants produce secondary metabolites which cause negative effects on other plant growth (Shi et al. Citation2011, Citation2012; Yue et al. Citation2013). For example, the allelochemical monoterpene and norsesquiterpenoid released by L. virgaurea had allelopathic effects on the adjacent plants in the introduced fields (Dong et al. Citation2015a; Jin et al. Citation2017). In the current study, results were statistically non-significant differences in growth indexes of E. nutans and P. pratensis seedlings between sterilized CK treatments and sterilized rhizosphere soil of poisonous plants treatments (,). This was not consistent with the previous observation that the allelochemical inhibit the growth of pasture grasses (Vivanco et al. Citation2004; Ehlers Citation2011; Bostan et al. Citation2013). The possible reason would be the potential diluted allelopathy effects of a relatively small amount of rhizosphere soil used in this study. Another explanation would be the alteration in AM spore density and diversity by the presence of the poisonous plants (Hiiesalu et al. Citation2014). Therefore, the growth of seedling was significantly reduced, with the decrease of AM spores density and AM fungi community changes (Jin et al. Citation2017). Our results indicated that the suppressed growth was accompanied by critical reductions in root colonization. Moreover, poisonous plants’ presence could increase AM fungi diversity. Hence, fungal competition can be intensified. It has been demonstrated that initial colonization by AM fungi can prevent subsequent colonization by other fungi or micro-organisms (Werner and Kiers Citation2015). Cai et al. (Citation2014) found that AM fungi species diversity in moderately degraded grassland significantly decreased, but largely increased in lightly and severely degraded grassland. Interestingly, compared to AM treatments from poisonous plants, there was a significant growth increase when E. nutans and P. pratensis seedlings were uninoculated, but lower than the inoculated dominant grasses (,). It is also demonstrated that AM fungi is a large regulating factor, implying that AM fungi community in rhizosphere soil of poisonous plants inhibited the establishment and growth of pasture grasses, and aggravated grassland degradation (, Walder et al. Citation2012). Other researchers also demonstrated that host plants with different preferential allocation of photosynthate may have different effects on the growth of AM fungi (Eom, Hartnett, and Wilson Citation2000; Bever et al. Citation2009), since AM fungi species may vary in providing benefits to different host plants (van der Heijden, Wiemken, and Sanders Citation2003; Klironomos Citation2003; Moora et al. Citation2004). Thus, future work should identify AM fungi communities and distribution patterns, allelopathy in rhizosphere soil of poisonous plants, and the rhizosphere soil properties including physical, chemical and biological indicators.
In conclusion, this study demonstrated that the presence of poisonous plants with grassland degradation may influence the AM fungi community in rhizosphere soil, and could exert inhibition effects on the growth of pasture grasses (). It may attribute to discover the important role of rhizosphere soil of different poisonous plants to AM fungi community on Tibetan Plateau Alpine meadow.
Acknowledgments
This study was supported by National Natural Science Foundation of China (31270558), Natural Science Foundation of Shanghai (18ZR1425400) and the Research Funds for the Introduction of Talents of Shanghai Science and Technology Museum. We are grateful to the anonymous referees whose comments greatly improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Asghari, H. R., P. Marschner, S. E. Smith, and F. A. Smith. 2005. “Growth Response of Atriplex Nummularis to Inoculation with Arbuscular Mycorrhizal Fungi at Different Salinity Levels.” Plant and Soil 273: 245–256. doi:10.1007/s11104-004-7942-6.
- Bever, J. D. 2002. “Negative Feedback within a Mutualism: Host-specific Growth of Mycorrhizal Fungi Reduces Plant Benefit.” Proceedings of the Royal Society B London Biological Sciences 269: 2595–2601. doi:10.1098/rspb.2002.2162.
- Bever, J. D., S. C. Richardson, B. M. Lawrence, J. Holmes, and M. Watson. 2009. “Preferential Allocation to Beneficial Symbiont with Spatial Structure Maintains Mycorrhizal Mutualism.” Ecology Letters 12: 13–21. doi:10.1111/j.1461-0248.2008.01254.x.
- Bostan, C., M. Butnariu, M. Butu, A. Ortan, A. Butu, S. Rodino, and C. Parvu. 2013. “Allelopathic Effect of Festuca Rubra on Perennial Grasses.” Romanian Biotechnological Letters 18: 8190–8196.
- Cai, X. B., Y. L. Peng, M. N. Yang, T. Zhang, and Q. Zhang. 2014. “Grassland Degradation Decrease the Diversity of Arbuscular Mycorrhizal Fungi Species in Tibet Plateau.” Notulae Botanicae Horti Agrobotanici 42: 333–339.
- Cao, G. M., Y. H. Tang, W. H. Mo, Y. S. Wang, Y. N. Li, and X. Q. Zhao. 2004. “Grazing Intensity Alters Soil Respiration in an Alpine Meadow on the Tibetan Plateau.” Soil Biology and Biochemistry 36: 237–243. doi:10.1016/j.soilbio.2003.09.010.
- Chen, J. S., and S. Zheng. 1987. The Poisonous Plant in China. Beijing: Science Press.
- Cheng, W., G. Sun, L. Du, Y. Wu, Q. Y. Zheng, H. X. Zhang, L. Liu, and N. Wu. 2014. “Unpalatable Weed Stellera Chamaejasme L. Provides Biotic Refuge for Neighboring Species and Conserves Plant Diversity in Overgrazing Alpine Meadows on the Tibetan Plateau in China.” Journal of Mountain Science 11 (3): 746–754. doi:10.1007/s11629-013-2729-y.
- Dong, L. L., H. Y. Xiong, F. M. Qi, H. H. Li, G. X. Fan, J. M. Che, D. D. Guo, Z. X. Zhang, and D. Q. Fei. 2015a. “A New Norsesquiterpenoid from Ligularia Virgaurea.” Journal of Asian Natural Products Research 17: 415–419. doi:10.1080/10286020.2014.971018.
- Dong, S. K., X. X. Wang, S. L. Liu, Y. Y. Li, X. K. Su, L. Wen, and L. Zhu. 2015b. “Reproductive Responses of Alpine Plants to Grassland Degradation and Artificial Restoration in the Qinghai-Tibetan Plateau.” Grass and Forage Science 70: 229–238. doi:10.1111/gfs.2015.70.issue-2.
- Ehlers, B. K. 2011. “Soil Microorganisms Alleviate the Allelochemical Effects of a Thyme Monoterpene on the Performance of an Associated Grass Species.” PloS One 6 (11): e26321. doi:10.1371/journal.pone.0026321.
- Engelmoer, D. J. P., and E. T. Kiers. 2015. “Host Diversity Affects the Abundance of the Extraradical Arbuscular Mycorrhizal Network.” New Phytologist 205: 1485–1491. doi:10.1111/nph.13086.
- Eom, A. H., D. C. Hartnett, and G. W. T. Wilson. 2000. “Host Plant Species Effects on Arbuscular Mycorrhizal Fungal Communities in Tall Grass Prairie.” Oecologia 122: 435–444. doi:10.1007/s004420050050.
- Fellbaum, C. R., J. A. Mensah, A. J. Cloos, G. E. Strahan, P. E. Pfeffer, E. T. Kiers, and H. Bücking. 2014. “Fungal Nutrient Allocation in Common Mycorrhizal Networks Is Regulated by the Carbon Source Strength of Individual Host Plants.” New Phytologist 203: 646–656. doi:10.1111/nph.12827.
- Gai, J. P., P. Christie, X. B. Cai, J. Q. Fan, J. L. Zhang, G. Feng, and X. L. Li. 2009. “Occurrence and Distribution of Arbuscular Mycorrhizal Fungal Species in Three Types of Grassland Community of the Tibetan Plateau.” Ecological Research 24: 1345–1350. doi:10.1007/s11284-009-0618-1.
- Gai, J. P., G. Feng, X. B. Cai, P. Christie, and X. L. Li. 2006. “A Preliminary Survey of the Arbuscular Mycorrhizal Status of Grassland Plants in Southern Tibet.” Mycorrhiza 16: 191–196. doi:10.1007/s00572-005-0032-7.
- Gai, J. P., H. Tian, F. Y. Yang, P. Christie, X. L. Li, and J. N. Klironomos. 2012. “Arbuscular Mycorrhizal Fungal Diversity along a Tibetan Elevation Gradient.” Pedobiologia 55: 145–151. doi:10.1016/j.pedobi.2011.12.004.
- Gao, Q. Z., Y. Li, H. M. Xu, Y. F. Wan, and W. Z. Jiangcun. 2014. “Adaptation Strategies of Climate Variability Impacts on Alpine Grassland Ecosystems in Tibetan Plateau.” Mitigation and Adaptation Strategies for Global Change 19: 199–209. doi:10.1007/s11027-012-9434-y.
- Gianinazzi, S., A. Gollotte, M. N. Binet, D. van Tuinen, D. Redecker, and D. Wipf. 2010. “Agroecology: The Key Role of Arbuscular Mycorrhizas in Ecosystem Services.” Mycorrhiza 20: 519–530. doi:10.1007/s00572-010-0333-3.
- Giovannetti, M., and B. Mosse. 1980. “An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots.” New Phytologist 84: 489–500. doi:10.1111/j.1469-8137.1980.tb04556.x.
- Harper, J. L. 1977. Population Biology of Plants. London: Academic Press.
- Hautier, Y., C. F. Randin, J. Stöcklin, and A. Guisan. 2009. “Changes in Reproductive Investment with Altitude in an Alpine Plant.” Journal of Plant Ecology 2: 125–134. doi:10.1093/jpe/rtp011.
- Hiiesalu, I., M. Pärtel, J. Davison, P. Gerhold, M. Metsis, M. Moora, M. Öpik, M. Vasar, M. Zobel, and S. D. Wilson. 2014. “Species Richness of Arbuscular Mycorrhizal Fungi: Associations with Grassland Plant Richness and Biomass.” New Phytologist 203: 233–244. doi:10.1111/nph.12765.
- Inderjit, S., and W. H. van der Putten. 2010. “Impacts of Soil Microbial Communities on Exotic Plant Invasions.” Trends in Ecology and Evolution 25: 512–519. doi:10.1016/j.tree.2010.06.006.
- Jiang, S. J., J. B. Pan, G. X. Shi, T. Dorji, K. A. Hopping, J. Klein, Y. J. Liu, and H. Y. Feng. 2018. “Identification of Root-colonizing AM Fungal Communities and Their Responses to Short-term Climate Change and Grazing on Tibetan Plateau.” Symbiosis 74: 159–166. doi:10.1007/s13199-017-0497-0.
- Jin, L., X. W. Sun, X. J. Wang, Y. Y. Shen, F. J. Hou, S. H. Chang, and C. Wang. 2010. “Synergistic Interactions of Arbuscular Mycorrhizal Fungi and Rhizobia Promoted the Growth of Lathyrus Sativus under Sulphate Salt Stress.” Symbiosis 50: 157–164. doi:10.1007/s13199-010-0058-2.
- Jin, L., Q. Wang, Q. Wang, X. J. Wang, and A. C. Gange. 2017. “Mycorrhizal-induced Growth Depression in Plants.” Symbiosis 72: 81–88. doi:10.1007/s13199-016-0444-5.
- Jin, L., G. Q. Zhang, X. J. Wang, C. Y. Dou, M. Chen, S. S. Lin, and Y. Y. Li. 2011. “Arbuscular Mycorrhiza Regulate Inter-specific Competition between a Poisonous Plant, Ligularia Virgaurea, and a Co-existing Grazing Grass, Elymus Nutans, in Tibetan Plateau Alpine Meadow Ecosystem.” Symbiosis 55: 29–38. doi:10.1007/s13199-011-0141-3.
- Kiers, E. T., M. Duhamel, Y. Beesetty, J. A. Mensah, O. Franken, E. Verbruggen, C. R. Fellbaum, et al. 2011. “Reciprocal Rewards Stabilize Cooperation in the Mycorrhizal Symbiosis.” Science 333: 880–882. doi:10.1126/science.1208473.
- Klironomos, J. N. 2003. “Variation in Plant Response to Native and Exotic Arbuscular Mycorrhizal Fungi.” Ecology 84: 2292–2301. doi:10.1890/02-0413.
- Li, H. B., B. T. Liu, M. L. McCormack, Z. Q. Ma, and D. L. Guo. 2017. “Diverse Belowground Resource Strategies Underlie Plant Species Coexistence and Spatial Distribution in Three Grassland along a Precipitation Gradient.” New Phytologist 216: 1140–1150. doi:10.1111/nph.14710.
- Li, X. L., J. P. Gai, X. B. Cai, X. L. Li, P. Christie, F. S. Zhang, and J. L. Zhang. 2014a. “Molecular Diversity of Arbuscular Mycorrhizal Fungi Associated with Two Co-occurring Perennial Plant Species on a Tibetan Altitudinal Gradient.” Mycorrhiza 24: 95–107. doi:10.1007/s00572-013-0518-7.
- Li, Y. Y., S. K. Dong, S. Liu, X. Wang, L. Wen, and Y. Wu. 2014b. “The Interaction between Poisonous Plants and Soil Quality in Response to Grassland Degradation in the Alpine Region of the Qinghai-Tibetan Plateau.” Plant Ecology 215: 809–819. doi:10.1007/s11258-014-0333-z.
- Lindsey, A. A. 1956. “Sampling Methods and Community Attributes in Forest Ecology.” Forest Science 2: 287–296.
- López-García, Á., C. Azcón-Aguilar, and J. M. Barea. 2014. “The Interactions between Plant Life Form and Fungal Traits of Arbuscular Mycorrhizal Fungi Determine the Symbiotic Community.” Oecologia 176: 1075–1086. doi:10.1007/s00442-014-3091-7.
- Lu, H., S. S. Wang, Q. W. Zhou, Y. N. Zhao, and B. Y. Zhao. 2013. “Damage and Control of Major Poisonous Plants in the Western Grasslands of China – A Review.” The Rangeland Journal 34: 329–339. doi:10.1071/RJ12057.
- Menge, J. A., E. L. V. Johnson, and R. G. Platt. 1978. “Mycorrhizal Dependency of Several Citrus Cultivars under Three Nutrient Regimes.” New Phytologist 81: 553–559. doi:10.1111/j.1469-8137.1978.tb01628.x.
- Mikkelsen, B. L., S. Rosendahl, and I. Jakobsen. 2008. “Underground Resource Allocation between Individual Networks of Mycorrhizal Fungi.” New Phytologist 180: 890–898. doi:10.1111/j.1469-8137.2008.02623.x.
- Moora, M., M. Öpik, R. Sen, and M. Zobel. 2004. “Native Arbuscular Mycorrhizal Fungal Communities Differentially Influence the Seedling Performance of Rare and Common Pulsatilla Species.” Functional Ecology 18: 554–562. doi:10.1111/j.0269-8463.2004.00876.x.
- Peng, F., Y. Quangang, X. Xue, J. Guo, and T. Wang. 2015. “Effects of Rodent-induced Land Degradation on Ecosystem Carbon Fluxes in an Alpine Meadow in the Qinghai-Tibet Plateau, China.” Solid Earth 6: 303–310. doi:10.5194/se-6-303-2015.
- Phillips, J. M., and D. S. Hayman. 1970. “Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection.” Transactions of the British Mycological Society 55: 158–161. doi:10.1016/S0007-1536(70)80110-3.
- Shi, B., W. Liu, L. Gao, C. Chen, Z. Hu, and W. Wu. 2012. “Chemical Composition, Antibacterial and Antioxidant Activity of the Essential Oil of Anemone Rivularis.” Journal of Medicinal Plants Research 6: 4221–4224.
- Shi, X. M., X. G. Li, R. M. Wu, Y. H. Yang, and R. J. Long. 2011. “Changes in Soil Biochemical Properties Associated with Ligularia Virgaurea Spreading in Grazed Alpine Meadows.” Plant and Soil 347: 65–78. doi:10.1007/s11104-011-0818-7.
- Shi, Z. C. 1997. Important Poisonous Plants of China Grassland. Beijing: China Agriculture Press.
- Sieverding, E., J. Friedrichsen, and W. Suden. 1991. “Vesicular-arbuscular Mycorrhiza Management in Tropical Agrosystems.” Sonderpublikation Der GTZ 224: 339–365.
- Silva, D. M., and M. A. Batalha. 2008. “Soil-vegetation Relationships in Cerrados under Different Fire Frequencies.” Plant and Soil 311: 87–96. doi:10.1007/s11104-008-9660-y.
- Smith, S. E., and D. J. Read. 2008. Mycorrhizal Symbiosis. 3rd ed. New York: Academic Press.
- Sternberg, M., M. Gutman, A. Perevolotsky, E. D. Ungar, and J. Kigel. 2000. “Vegetation Response to Grazing Management in a Mediterranean Herbaceous Community: A Functional Group Approach.” Journal of Applied Ecology 37: 224–237. doi:10.1046/j.1365-2664.2000.00491.x.
- van der Heijden, M. G. A., A. Wiemken, and I. R. Sanders. 2003. “Different Arbuscular Mycorrhizal Fungi Alter Coexistence and Resource Distribution between Co-occurring Plant.” New Phytologist 157: 569–578. doi:10.1046/j.1469-8137.2003.00688.x.
- Vivanco, J. M., H. P. Bais, F. R. Stermitz, G. C. Thelen, and R. M. Callaway. 2004. “Biogeographical Variation in Community Response to Root Allelochemistry: Novel Weapons and Exotic Invasion.” Ecology Letters 7: 285–292. doi:10.1111/ele.2004.7.issue-4.
- Walder, F., H. Niemann, M. Natarajan, M. F. Lehmann, T. Boller, and A. Wiemken. 2012. “Mycorrhizal Networks: Common Goods of Plants Shared under Unequal Terms of Trade.” Plant Physiology 159: 789–797. doi:10.1104/pp.112.195727.
- Wang, Q. 2016. Studies on the AM Fungal Spores Identification in Poisonous Plants Rhizosphere and Its Regulation on Interactions between Plants on Tibetan Plateau Alpine Meadow. Lanzhou, China: Lanzhou University [D].
- Wen, L., S. K. Dong, Y. Y. Li, X. Y. Li, J. J. Shi, Y. L. Wang, D. M. Liu, and Y. S. Ma. 2013. “Effect of Degradation Intensity on Grassland Ecosystem Services in the Alpine Region of Qinghai-Tibetan Plateau, China.” PloS One 8 (3): e58432. doi:10.1371/journal.pone.0058432.
- Werner, G. D. A., and E. T. Kiers. 2015. “Order of Arrival Structures Arbuscular Mycorrhizal Colonization of Plants.” New Phytologist 205: 1515–1524. doi:10.1111/nph.13092.
- Wu, G. L., G. Z. Du, Z. H. Liu, and S. Thirgood. 2009. “Effect of Fencing and Grazing on a Kobresia-dominated Meadow in the Qinghai-Tibetan Plateau.” Plant and Soil 319: 115–126. doi:10.1007/s11104-008-9854-3.
- Wu, G. L., Z. H. Liu, L. Zhang, J. M. Chen, and T. M. Hu. 2010. “Long-term Fencing Improved Soil Properties and Soil Organic Carbon Storage in an Alpine Swamp Meadow.” Plant and Soil 332: 331–337. doi:10.1007/s11104-010-0299-0.
- Xie, T. P., G. F. Zhang, Z. G. Zhao, G. Z. Du, and G. Y. He. 2014. “Intraspecific Competition and Light Effect on Reproduction of Ligularia Virgaurea, an Invasive Native Alpine Grassland Clonal Herb.” Ecology and Evolution 4: 827–835. doi:10.1002/ece3.975.
- Yang, Y. H., J. Y. Fang, Y. H. Tang, C. J. Ji, C. Y. Zheng, J. S. He, and B. Zhu. 2008. “Storage, Patterns and Controls of Soil Organic Carbon in the Tibetan Grasslands.” Global Change Biology 14: 1592–1599. doi:10.1111/j.1365-2486.2008.01591.x.
- Yue, H. L., X. H. Zhao, L. J. Mei, and Y. Shao. 2013. “Separation and Purification of Five Phenylpropanoid Glycosides from Lamiophlomis Rotata (benth.) Kudo by a Macroporous Resin Column Combined with High-speed Counter-current Chromatography.” Journal of Separation Science 36: 3123–3129. doi:10.1002/jssc.201300268.
- Zhang, Y. F., P. Wang, Y. F. Yang, Q. Bi, S. Y. Tian, and X. W. Shi. 2011a. “Arbuscular Mycorrhizal Fungi Improve Reestablishment of Leymus Chinensis in Bare Saline-alkaline Soil: Implication on Vegetation Restoration of Extremely Degraded Land.” Journal of Arid Environments 75: 773–778. doi:10.1016/j.jaridenv.2011.04.008.
- Zhang, Z. Q., Y. H. Zhang, and H. Sun. 2011b. “The Reproductive Biology of Stellera Chamaejasme (thymelaeaceae): A Self-incompatible Weed with Specialized Flowers.” Flora-Morphology, Distribution, Functional Ecology of Plants 206: 567–574. doi:10.1016/j.flora.2011.01.008.
- Zuo, X. A., X. Y. Zhao, H. L. Zhao, T. Zhang, Y. Guo, Y. Li, and Y. Huang. 2009. “Spatial Heterogeneity of Soil Properties and Vegetation-soil Relationships following Vegetation Restoration of Mobile Dunes in Horqin Sandy Land, Northern China.” Plant and Soil 318: 153–167. doi:10.1007/s11104-008-9826-7.
