ABSTRACT
Ratoon is the stub or root of a perennial plant that is commonly retained after harvest to produce a following crop. This paper presents a review of ratoon cotton in relation to a broader framework that has been examining perennialization of agriculture for the benefit of ecology and economy. Cotton is botanically indeterminate, but has been treated as an annual after domestication, yet the habit of perenniality is retained and the plants begin to resprout after the first harvest. In some cropping systems, this tendency is exploited using the “ratooning” practice (i.e. growing one or more crops on the rootstock of the first). Ratooning has declined for various reasons such as an increase in the prevalence of pests and diseases and overwintering risk. However, ratooning has many benefits such as no annual tillage before sowing, a well-established root system, and high yield. The three methods of ratooning offer flexibility to balance the environmental and economic benefits in agriculture. The greatest environmental benefits arise from perennial ratoon cropping of semi-wild cotton, and the greatest economic benefit is obtained from biannually cropping modern annual cultivars. However, an optimum solution would be provided by perennial cropping annual cultivars. To realize both environmental and economic benefits, research is needed in the following main areas: preventing the buildup of pests and diseases, breeding the most suitable cotton cultivars for ratooning, and developing light and simplified cultivation (LSC) systems for ratoon cultivation.
Introduction
A major current development in agriculture is to realize environmental, economic, and social benefits simultaneously and sustainably (Wang et al. Citation2011; Chitakira, Torquebiau, and Ferguson Citation2012; Liu et al. Citation2019). Perennial agriculture, a form of ancient agriculture that appeared in primitive society, is thought to have begun with cultivating perennial fruit trees 11,000 years ago, and is defined as the cultivation of crop species that live longer than two years, without being replanted annually (Potts Citation2012). However, with the progress of human civilization, perennial agriculture has declined. To survive, humans domesticated low-yielding perennial species into high-yielding annual species via artificial selection and planted the latter yearly, causing annual-dominated agriculture that increases soil erosion, water loss, and deforestation in its required land (Lambin and Geist Citation2003; Smaje Citation2015). However, perennial crops have great potential in reducing soil erosion, improving agro-ecosystems and economic benefits, and have increasingly attracted attention from breeders and ecologists (Cox et al. Citation2002; DeHaan, Van Tassel, and Cox Citation2005; Zhang et al. Citation2012a; Migicovsky and Myles Citation2017; Crews and Cattani Citation2018).
Cotton (Gossypium) is an important economic crop that provides natural fiber and other industrial raw materials. It originated from perennial shrubs or trees in tropical and subtropical zones (Wendel, Brubaker, and Percival Citation1992; Alkhafaji Citation2016). After long-term introduction and domestication by humans, only four Gossypium species were transformed from dozens of photoperiod-sensitive perennial species into day-neutral annual species. Annual cotton plants can survive cold winters in greenhouses (Mihail, Brown, and Nelson Citation1987; Percy et al. Citation2014), meaning that temperature is the main factor affecting the overwintering ability of annual cotton (Abdurakhmonov Citation2007). In most cases, annual cotton can survive the overwinter in the open fields in near tropics, where the average temperature of the coldest month is not lower than 10°C (Zhang et al. Citation2013). However, after the annual cotton plants have completed one growth cycle in a winter with more rain and lower temperature than normal year, some plants can completely survive, some plants survive as underground rootstocks with a dead upper section, and a few plants die (Zhang et al. Citation2015a). In the spring of the next year, the axillary buds and adventitious buds on the overwintered cotton stubs can sprout to form renewed plants. The regrowth process can be extended over many years to form renewed cotton plants, which are called perennial cotton, ratoon (rattoon) cotton, or stub cotton plants (Sachs and Zilkah Citation1985; Silva, Bezerra, and Silva Citation2008).
Ratoon cropping is the production of an indeterminate crop without sowing, and it is common in crops such as rice (He et al. Citation2019), sugarcane (Castro et al. Citation2019), pigeon pea (Grabowski et al. Citation2019) and sorghum (Snapp et al. Citation2019), as well as cotton (Komala, Kumar, and Ganesan Citation2019). Ratoon cotton is advantageous for sustainable development, and has been cultivated in many cotton-growing countries, such as Australia, Brazil, China, Guyana, Kenya, India, Israel, Pakistan, Peru, South Africa and the USA (Plucknett, Evenson, and Sanford Citation1970; Khan and Shabbir Citation1974; Bullen, Granger, and Persaud Citation1982; Macharia Citation2013; Khader and Prakash Citation2014; Zhang et al. Citation2015a). In comparison to sown cotton, ratoon cotton has three major advantages: (1) no-tillage cultivation, which can cause less disturbance; more biodiversity (Risch, Andow, and Altieri Citation1983; Perdikis, Fantinou, and Lykouressis Citation2011); and less soil, nutrient, water loss (DeHaan, Van Tassel, and Cox Citation2005; Zhang et al. Citation2013), and pesticide usage (Luttrell et al. Citation1994; Khader and Prakash Citation2014) without destroying cotton stalks (Mubvekeri et al. Citation2014), thereby saving plowing and seed expenses (Macharia Citation2013; Zhang et al. Citation2013) and lowering labor costs (Zhang et al. Citation2015a, Citation2015b); (2) a well-established root system, which results in less weed threat (Evenson Citation1970; Khader and Prakash Citation2014), a lower demand for fertilizer (Haughton et al. Citation2009; Bunzel et al. Citation2014), plants that are more adaptable to irregular rainfall (Fontes et al. Citation2006; Macharia Citation2013), and the ability to sell crops earlier as a result of an earlier harvest; and (3) an indeterminate flowering habit, which is advantageous for longer flowering and fruiting (Macharia Citation2013), thus resulting in a higher yield (Chen et al. Citation2008; Zhang et al. Citation2015a) or less yield loss without pesticide application (Ramalho Citation1994). However, ratooning cotton is associated with a range of problems, such as (1) encouraging the accumulation of pests and diseases over years, including serving as a host for pests and diseases in winter (Bergman Citation1985; Showler Citation2009), resulting in earlier pest attack (Macharia Citation2013). Obvious protuberances at the base of stubs that may be caused by diseases invading through the pruning wound (Zhang et al. Citation2015b), and negative plant-soil feedback (changes in soil properties caused by plants that affect plant performance); which is particularly evident when plants promote soil pathogens that are harmful to their own growth (Liu et al. Citation2008; Vukicevich et al. Citation2016); and (2) the risk of damage in severe winter (Zhang, Zhou, and Lou Citation2008; Chen et al. Citation2010a). A clearer description is given in .
Table 1. Benefits and problems of ratoon cotton vs sown cotton
Ratoon cotton cropping methods
In this paper, “ratoon cotton” is a term used to indicate a form of production in which more than one harvest is taken from one planted crop. The crop can be a perennial form, in which case the harvests are taken over several years after regrowing from the original root stock (perennial ratooning) (Silva, Bezerra, and Silva Citation2008) or an annual form, which is stem-pruned after the first harvest to encourage a second fruiting cycle in the same year (biannual ratooning) (Macharia Citation2013; Khader and Prakash Citation2014). According to its senescence characteristics, ratoon cotton can be divided into two types of cotton species: annual species (used for re-perennialization) and perennial species. All modern cultivars of four cultivated species are annual forms, and all wild species are perennial forms. In the tropics, annual species and perennial species of ratoon cotton are significantly different from one another; the annual species tend to lose their leaves and enter dormancy during winter. Most modern cultivated strains of cotton are so-called annual species that can overwinter safely and become ratoon cotton in frost-free or snow-free areas throughout the year (Geng Citation2015).
Semi-wild landraces of cultivated cotton species (semi-wild cotton) can be used for ratoon cultivation. In this paper, perennial species were divided into semi-wild (semi-domesticated or semi-perennial) species and undomesticated (fully wild) species. The perennial semi-wild landraces (having the same genome type as their corresponding cultivars according to molecular genetics classification) of the four cultivated species () include five landraces of African cotton (G. herbaceum L.), six landraces of Asian cotton (G. arboreum L.), seven landraces of upland cotton (G. hirsutum L.), and three landraces of island cotton (G. barbadense L.). Among them, G. herbaceum var. africanum is thought to be the first cultivated perennial cotton, which escaped from cultivation and was rediscovered as a semi-perennial relict (Baker Citation1962). In addition, there are some other perennial genotypes, such as G. purpurascens Poir. (Abdurakhmonov et al. Citation2012) and G. darwinii Watt (Khadi, Santhy, and Yadav Citation2010; Xu et al. Citation2012); however, whether they are landraces of cultivated species is controversial.
Table 2. Perennial semi-wild landraces of cultivated cotton species
According to many years of observation and research in tropical and near tropical regions, perennial species hold advantages such as better overwintering status, longer lifecycle with less recession, and stronger resistance to harmful environments compared to annuals (Zhang et al. Citation2012b; Boopathi et al. Citation2014). However, the yield of perennial species is lower; in particular, there is almost no yield during the sowing year due to their strong short-day habits and long vegetative growth period; survival traits are negatively correlated with yield in perennial cotton species (De Souza and Da Silv Citation1987), and it is generally accepted that the establishment and persistence of long-lived perennial species depends on a relatively high investment in roots, along with less and later fruit growth. A survival strategy thought to exist among perennial species is restricted reproductive growth, which is a response to growth under multiple stresses including drought, low temperature, shortage of soil nitrogen, and herbivory (Sadras Citation1996) that affect resource allocations of the plant. In contrast, annuals have a weaker overwintering ability, shorter lifecycle, greater ease of recession, and weaker resistance to environmental stress, but higher yield (Zhang et al. Citation2013, Citation2015a). When cultivating by grafting or breeding ratoon cultivars by hybridization, the advantages of perennial cotton and annual cotton can be combined.
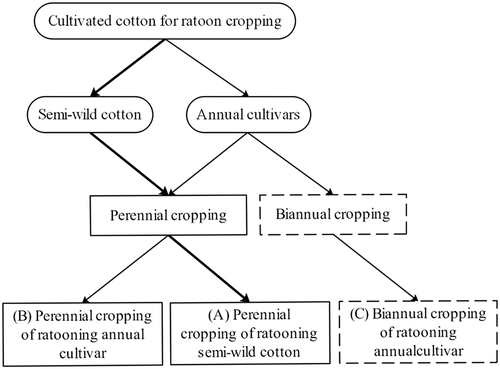
The differences between annual cotton cultivars and perennial semi-wild landraces of cultivated cotton determine their performance and application in ratoon cultivation. In fact, annual cotton cultivars are suitable for both perennial and biannual ratoon cropping, and perennial semi-wild landraces of cultivated species are usually used for perennial cropping. Therefore, three methods are used in cropping ratoon cotton ().
Figure 1. Three methods of growing ratoon cotton: (A) perennial cropping for the semi-wild cotton (following the bold line to the solid box), (B) perennial cropping for annual cotton cultivars left after the first year (following the solid line to the solid box), and (C) biannual cropping for annual cotton cultivars (following the solid line to the dotted box)
Status of the cropping of ratoon cotton
Ratoon croppingof semi-wild cotton landraces used for perennial cropping
Since all wild cottons are perennial species, we can speculate that annual cultivated species were domesticated from perennial wild species. Perennial landraces of the four cultivated Gossypium species are still found in the tropics and subtropics (D’Eeckenbrugge and Lacape Citation2014; Bourgou et al. Citation2017; Singh Citation2017). Among the four cultivated Gossypium species, both G. herbaceum L. and G. arboretum L. are commonly called “Old World” cottons, whereas G. hirsutum L. and G. barbadense L. are commonly known as “New World” cottons (Gumber et al. Citation2016). In southern Africa, the perennial G. herbaceum landrace africanum is the wild ancestor of the cultivated diploid (Genome A, 2n = 2x = 26) cotton (Chen et al. Citation2017). Until the 13th century, all Indian cottons were perennial species (Boopathi et al. Citation2014). Recently, G. arboreum L. and G. herbaceum L. have been cultivated in Africa, India and South-East Asian countries, because of their favorable traits such as early growth and resistance to drought, pests and diseases (Stewart Citation1994; Boopathi et al. Citation2014). G. hirsutum L. and G. barbadense L. originated in Central America and northern South America, the Caribbean, and even distant islands in the Pacific (Wendel and Grover Citation2015).
Upland cotton, G. hirsutum L., is the source of more than 90% of the raw cotton in the world, and is also the main cultivated ratoon cotton. Mocó cotton (Gossypium hirsutum L. landrace marie-galante (Watt) Hutch.), a semi-perennial upland cotton form, is restricted to the semi-arid region of northeastern Brazil, where it was cultivated as early as the 1880s on a semi-domesticated basis as a local variety (Pickersgill, Spencer, and Andrade-Lima Citation1975; De Souza and Da Silv Citation1987; Pinto De Menezes et al. Citation2010). In the 1970s, more than 2 million hectares were planted in Brazil. However, today, mocó cotton is planted on only 9,000 ha in the driest regions of Brazil and is usually used for three years (Fontes et al. Citation2006). In addition, bourbon cotton (classified as G. purpurascens Poir. by I. E. Webber in 1938 but as G. hirsutum var. punctatum (Schum.) Hutch in 1947) is an extremely hardy and vigorous cotton tree found in both wild and cultivated forms in the West Indies, and is also known as “four-season cotton” in China because its cultivated forms can reproduce indefinitely in the tropics (Harland Citation1937; Lee Citation1986; Yang, Cai, and Deng Citation1990).
The perennial landrace of the island cotton G. barbadense var. barbadense is very suitable for mixing with the shorter annual upland cotton fibers in the textile industry because of its long fibers. It was planted as a main commercial crop once per year over 2 million hectares in Brazil, where it occupied a niche in the market (Sampaio et al. Citation2017). In southern China, G. barbadense was introduced earlier than G. hirsutum, and was planted mainly in Yunnan Province as a perennial cultivar (Jia, Sun, and Du Citation2014). In 1936, Feng Zefang, the founder of modern cotton science in China, noticed that there were two semi-domesticated perennial arboreal G. barbadense landraces in Yunnan, China. One was called seed-jointed arboreal cotton, which is a kidney cotton (G. barbadense var. brasiliense) with low yield and short fibers, so named for its lint-less seeds in each locule that are clustered together into a kidney-shaped mass. The other called seed-isolated arboreal cotton is a landrace of G. barbadense, its fibers are soft and silky and can be spun into 32–62 yarns (Zeng Citation2012; Zeng and Wang Citation2013; Geng Citation2015). The perennial form of seed-isolated arboreal cotton has a lifespan of up to 20 years and a height of up to 3 m. It is worth noting that the yield of each perennial plant could reach 2.5 kg, and their fiber lengths increase annually. The fiber lengths of 3-year-old, 10-year-old and 20-year-old seed-isolated arboreal cotton were 33.09 mm, 35.27 mm and 37.10 mm, respectively (Zeng Citation2012; Zeng and Wang Citation2013). From 1939 to 1946, the arboreal cotton was cultivated on more than 3,300 ha in Yunnan (Liu Citation1998; Zeng and Wang Citation2013). Unfortunately, the cultivation history of the arboreal cotton in Yunnan was interrupted for a variety of reasons. To date, these two forms of arboreal cotton can be found in southern China.
Ratooning annual cotton cultivars for perennial cropping
There are great differences between perennial and annual cotton species when they are perennially cultivated. In Brazil, a five-year study on five types of perennial cotton species at five sites showed that all perennial species had good yield stability (De Souza and De Holanda Citation1993). However, ratooned annual cotton cultivars for perennial cropping resulted in high yield but poor yield stability.
Annual species can be perennially cultivated in southern China (), and some studies in Guangxi showed that the lint yield of 2-year-old perennially cultivated annual cottons (upland cotton, island cotton and their interspecific hybrids) was 14.57–94.11% higher than that of the annuals, and its highest kapas yield was up to 7127.25 kg/ha, with no significant difference in fiber quality (Chen et al. Citation2008; Zhang et al. Citation2012b). A six-year study in south Sichuan showed that seed cotton yields of 2-year-old to 6-year-old forms were 10.3%, 5.9%, −2.4%, −9.4% and −14.0% higher/lower than those of annuals in the same year, respectively, while there was no significant difference between the 4-year-old forms and their associated annuals (Zhang et al. Citation2015a). In Pakistan, growers in Dera Ghazi Khan District believed that ratoon cotton could escape the attack of black-headed crickets and achieve a higher yield than sown cotton. A local experiment involved planting ‘American Cotton 134ʹ; the ratoon crops were unpruned, detopped 28 inches from the ground, trimmed back to the first internode in January, and compared to cotton resown in mid-April and mid-May, with yields being 2469.39 kg/ha, 2211.55 kg/ha, 1911.19 kg/ha, 1407.51 kg/ha and 396.47 kg/ha, respectively (Khan and Shabbir Citation1974). The results showed that the yields of ratoon cotton were much higher than those of sown cotton, and it was interesting that the yield of regenerated cotton without pruning was the highest. In addition, no serious pest or disease problems were observed.
Figure 2. Two photos of ratoon cotton regrowing for a second lifecycle were taken in Nanning, Guangxi, China. Note that the ratoon cotton with lots of buds was cut at an approximate height of 45 cm in the left photo. Subsequently, the weaker buds were removed, leaving only about three stronger buds on each plant to grow in the right photo

Because the yield of ratoon cotton is influenced by the variety, culture, and climate, there are also a few reports indicating that the yield of ratoon cotton in perennial cropping is lower than that of sown cotton. In coastal, eastern, and central Kenya, approximately 14% of cotton planters cultivate ratooned annual cultivars of upland cotton. Macharia (Citation2013) reported the highest seed cotton yield of the ratooned cultivar `HART 89M’ was 561.0 kg/ha, which was lower than the 675.0 kg/ha yield of its sown control and much lower than its potential seed cotton yield of 2500 kg/ha (Pkania et al. Citation2014).
It must be mentioned that the fear of pests being able to survive safely over winter in ratoon cotton has caused legislation to be passed against the perennial cropping of ratoon cotton. However, commercially ratooned cotton has existed in the Ord River region of northwest Australia since 1965, and no serious pest outbreaks directly attributable to ratoon cotton have been recorded (Plucknett, Evenson, and Sanford Citation1970). Remarkably, in some countries with mid-season droughts and relatively low labor costs such as the Republic of Zimbabwe, perennial cropping of ratoon cotton, although prohibited by closed-season legislation, is becoming popular due to the price and unavailability of cotton seed, labor savings, and the fact that existing roots grow into mature plants earlier than sown cotton; thus, planters are likely to sell crops earlier from an earlier harvest without destroying cotton stalks (Mubvekeri et al. Citation2014).
Ratooning annual cotton cultivars for biannual cropping
Countries that historically banned or currently ban the perennial cropping of ratoon cotton include Australia (Plucknett, Evenson, and Sanford Citation1970), Egypt (Evenson Citation1970), the USA (Bergman Citation1985; Showler Citation2009), Zambia (Macharia Citation2013) and Zimbabwe (Mubvekeri et al. Citation2014). Although perennial cropping of ratoon cotton has many long-term ecological and economic benefits, the fear of carrying or accumulating the spiny bollworm (Earius insulana), pink bollworm (Pectinophora gossypiella) and cotton bollworm (Helicoverpa armigera) serves to hinder its usage. According to the legislation of these countries, cotton crops should be terminated by December, and crop residues and stalks must be removed immediately after harvest (Sarwar Citation2017). Templeton (Citation1925) was skeptical about the legislative reasons for banning ratoon cotton cultivation in Egypt because his results indicated that ratoon cotton was less affected by bollworm than sown cotton (Plucknett, Evenson, and Sanford Citation1970). In addition, developments during the 1990s have successfully controlled bollworms in ratoon cotton (Rajendran, Birah, and Burange Citation2018). Even so, in the production status of ratoon cotton in Brazil, where it is not banned, has not been positive since the 1990s due to the increasing cost of labor and the pursuit of increased cropping profits. This has led to a sharp decline in the perennial cropping of ratoon cotton (Sampaio et al. Citation2017) and has even caused ratoon cotton to be removed as a dangerous weed in annual cropping systems (Wilson et al. Citation2008).
In the tropics, ratooning annual cotton cultivars for biannual cropping in a single season can prevent closed-season legislation and provide additional yield to increase income. According to a field test during the Kharif season of 2012–2013 in India, ratoon cotton used in biannual cropping started from pruning the main stem 45 cm above the ground surface after the third harvest in a single season. New buds of ratoon cotton sprouted within 8–10 days, squaring initiated within 40 days, and bolling began within 70 days. The average seed cotton yield of the ratoon upland cotton cultivar “Suraj,” and the island cotton cultivar “Suvin,” in the second fruiting cycle were found to be 2010 kg/ha and 1250 kg/ha, equivalent to 63.81% and 64.10% of the sown cotton, respectively. Also, the fiber quality of both ratoon cottons was not significantly different from that of sown cottons (Khader and Prakash Citation2014). In addition, with the wide application of short-season cotton in production, the biannual cropping of annual cultivars would have great potential and prospects.
Analysis of the ecological advantages of ratoon cotton
Lower fertilizer demand
Ratoon cotton, especially semi-wild cotton, grows longer and has higher yield than sown cotton in the same year, and the fertilizer consumption of the former is lower. The main reasons for this phenomenon are as follows: First, sown cotton needs more fertilizer than ratoon cotton for growth and root morphogenesis. Second, ratoon cotton is attributed to fewer disturbances since less soil and nutrient elements are lost in the no-tillage cultivation of ratoon cotton. Third, ratoon cotton has a more powerful tap root system that penetrates to a depth roughly equal to the height of the stem, which allows it to extract fertilizer from deeper soil. Fourth, considering that the abundant light, heat, and microbial resources in the tropics contribute to the rapid mineralization of organic matter, and that the fertility of the soil is the result of its alkalinity and richness (Sampaio et al. Citation2017), the methods used for cultivating ratoon cotton in the tropics are performed to minimize interference to the biological environment, and enhance the contribution to soil microbial diversity and organic matter by mulching crop residue and green fertilizer.
Lower pesticide usage
There is ample evidence that uncontrolled pests can have a serious impact on the yield of ratoon cotton. It is generally believed that monoculture or extended continuous culture of a crop may lead to the accumulation of harmful pests and diseases, and in some cases, perennial crops are condemned for these problems. However, as early as the 1960s in Israel and Australia, pests were not reported to be a main problem. The same results from ratoon cotton were subsequently reported in Pakistan (Khan and Shabbir Citation1974), Brazil (Luttrell et al. Citation1994) and India (Khader and Prakash Citation2014). Since 2002, insect pests have ceased to be an important issue in India due to the large-scale cultivation of genetically modified Bt cotton, and the overall use of pesticides in ratoon cotton is less than that of sown cotton.
Surprisingly, although ratoon cultivated semi-wild cotton landraces are attacked by pests earlier than sown cotton (Macharia Citation2013) and the pesticide applications used in semi-wild cotton are much lower than those used in sown cotton. Under the supervision of agronomists and entomologists, in Brazil’s sown cotton mechanized production areas, pesticides are applied 5 to 6 times per season, while other areas usually apply 8–10 times. However, only one or two applications are used in the ratoon cotton production system to control cotton leafworm (Alabama argillacea) in northern Brazil (Luttrell et al. Citation1994). There are four main reasons for this abnormality: First, ratoon plants are often protected from pests by the quantitative defense of secondary metabolites, such as tannins and gossypol. Second, the success rate of introducing and establishing exotic natural enemies of pests in the ratoon cotton system, with less disturbance to cultivation operations is higher than that in the sown cotton system, i.e., recurrent disturbance caused by tillage operations in the annual cropping system may hinder natural enemies (Risch, Andow, and Altieri Citation1983; Perdikis, Fantinou, and Lykouressis Citation2011). Third, pests in sown cotton are multivoltine, increasing their inherent potential to be resistant to pesticides (Perdikis, Fantinou, and Lykouressis Citation2011). Fourth, sown cotton needs more fertilizer than ratoon cotton, so it grows faster and has tender tissues, leading to a significant increase in natural infection by pests such as cotton stainers (Macharia Citation2013).
Lower weed threat
In the perennial cultivation of ratoon cotton, studies have not reported serious threats to weed control. The rapid growth of ratoon cotton is of great help to the early establishment of a dense canopy. In addition, dormant ratoon plants have strong resistance to herbicide exposure before regrowth, so controlling weeds become easier. Dormant perennial cotton is highly tolerant to herbicides that are toxic to the seedling stage, which may be due to its deep and mature root system (Plucknett, Evenson, and Sanford Citation1970). Currently, glyphosate is the main herbicide used in cotton cultivation. With the introduction of glyphosate-resistant cotton in Australia in 1996, weed control in cotton fields has been further enhanced (Werth et al. Citation2013).
Application in circular agriculture
In Brazil, the perennial cultivation of ratoon cotton is integrated with the local cattle industry to create sustainable circular agriculture. Interestingly, it is noteworthy that ratoon cotton occurs in a cyclic ecosystem known as the cotton-subsistence-cattle triad in northeast Brazil (Sampaio et al. Citation2017). The cotton fields are open to cattle after harvesting, and the residues of plants (including weeds) are fed to cattle, while the activity of cattle can effectively control the boll weevil (Anthonomus grandis) and pink bollworm, and manure nourishes the soil and aids plant regrowth for the following year (Ramalho Citation1994; Fontes et al. Citation2006). In addition, chemical fertilizers and herbicides are not used in this ecosystem (Fontes et al. Citation2006). However, soil erosion occurs (especially in slope areas) and some cotton plants die as a result of cattle activity, which eventually leads to the yield of ratoon cotton peaking between the 5th and 10th year at less than 300 kg/ha (Sampaio et al. Citation2017).
Status of research involving ratoon cotton
Status of research involving ratoon cultivation of annual cotton
At present, there are some reports on the ratoon cultivation of annual cotton. Ratoon cotton can produce a significantly higher yield of seed cotton than sown cotton, because the fruiting period of ratoon cotton is approximately 4–6 weeks earlier than sown cotton; the fiber quality does not significantly change (Bergman et al. Citation1983; Zhang et al. Citation2015a), but the size and weight of bolls tend to decrease with the growth cycle of cotton plants (Evenson Citation1970; Zhang et al. Citation2015a). Some techniques have been proposed for cultivating ratoon cotton including pruning, intercropping, and grafting.
In the tropics, the proper pruning time can be as soon as after the third cotton harvest, and in the near tropics, pruning may be performed 1–3 days before the rainy season or irrigation in the spring of the following year (Fontes et al. Citation2006; Zhang et al. Citation2015a). Macharia (Citation2013) reported the effect that different cutting heights (5 cm, 10 cm and 15 cm) above the ground level have on sown cotton after harvest, and showed that more sprouts and a higher lint yield resulted from a higher cut height. Additionally, in Brazil, perennial mocó cotton is pruned 20 cm above the soil to stimulate regrowth (Fontes et al. Citation2006). Considering that a deteriorating stub is lost each year, the proper cut height of the pruned stalk might be 40–50 cm above the soil in the first year (Evenson Citation1970; Zhang et al. Citation2015a). The cut height should decrease each following year to make the ratoon cotton have a long service life with high and stable yield, and to avoid the serious protuberance at the plant base caused by deep pruning.
Pest attacks and negative plant-soil feedback are important issues in ratoon cotton cultivation, and intercropping may reduce pest attacks and improve soil quality and yield, while making full use of the land, light energy, and growing seasons. A field experiment involving intercropping perennial upland cotton with maize carried out from 1994 to 1996 in Brazil showed that the yield of ratoon cotton increased alongside its test density and decreased as maize density increased, and the best record for the land equivalent ratio for ratoon cotton came from its test population at 10,000 plants/ha with maize at 5,000 plants/ha (Azevedo et al. Citation2000). Another field experiment in China involving winter intercropped ratoon cotton and short-season vegetables (pea and pakchoi) from 2006 to 2011 showed that the presence of the two vegetables that require irrigation reduced the overwintering rate of ratoon cotton by approximately 22%, and the overall economic benefit of two intercropping modes was less than that of non-intercropped cotton (Zhang et al. Citation2015a). Approximately 30% of the perennial mocó cotton (arboreal cotton) in Brazil was planted together with beans or maize for 2–3 years until the treelike cottons were too large to intercrop (Fontes et al. Citation2006; Sampaio et al. Citation2017).
The overwintering of ratoon cotton is an important problem in perennial cultivation. A method of grafting some annual cultivars that cannot survive naturally over the winter onto perennial species for perennial growth was developed in the near tropics (Zhang et al. Citation2013; Zhou Citation2016). The ratoon cultivation area of cotton was extended from the tropical region to some subtropical regions using this method which can also be used to fix the heterosis of hybrids, and the yield of grafted cotton greatly increased due to the prolonged growth period (Zhang et al. Citation2013).
Physiological mechanisms of ratoon cotton
Perennial species and annual species of ratoon cotton have great differences in physiological and biochemical characteristics. Annual cottons have lost most of their drought resistance likely due to root penetration depth being shallower than that of perennial species (Hearn Citation1980). In Brazil, a study found that the starch content in the roots of perennial cotton species was higher than that in the roots of annual cotton. High starch content is related to high root/shoot ratio and drought resistance, and dry matter preferentially accumulated in roots under drought conditions; which may be an effective survival mechanism suited for the water cycle in northeastern Brazil (De Souza and Da Silv Citation1987). When the starch content in the roots of annual cottons decreased continuously for 3 months, the starch content in the roots of perennial species continued to increase; the drought resistance of perennial species was apparently attributed to the allocation of assimilates to the root system and storage in the form of starch. It is thought that the ratoon cotton roots must reserve a sufficient amount of carbohydrates to start a new vegetative growth cycle when conditions are suitable, or to cope with a period of stress (Sadras Citation1996; Wells Citation2002). This could be considered to be a conservative allocation strategy to better ensure the survival of ratoon cotton after cutting. Moreover, competition for additional carbohydrates with fruits is conducive to the lignification of some primary cell walls, and their function in pith cells may provide additional mechanical compressive strength to annual cotton plants that can grow into relatively large perennial shrubs/trees (Macmillan et al. Citation2013).
The bolls and leaves of ratoon cotton are smaller than those of sown cotton. According to a report regarding their photosynthetic physiology from Khader and Prakash (Citation2014), the nitrate reductase activity, the photosynthetic rate, and chlorophyll content in the leaves of ratoon cotton sown for biannual cropping in the same season were slightly lower than those for normally sown cotton, and spraying compound nutrients significantly helped such variables and the yield of both normal and ratoon cotton.
To investigate the overwintering survival rate and physiological and biochemical characteristics of cotton, the winter survival rate and the NPK content in leaves and stems were studied in 1-, 2- and 3-year-old cultivars of four upland cottons. The results showed that the cultivar and sowing date had a significant impact on the overwintering survival rate, which was significantly higher under early sowing rather than late sowing. Planting density and winter fertilization had no significant impact on the overwintering survival rate (Chen et al. Citation2010a). Compared to sown cotton, the NPK content in the leaves of the ratoon plants did not change significantly; however, N fertilizer should be added in the middle and late stages of ratoon cultivation to promote vegetative growth and delay aging (Chen et al. Citation2010b). In addition, hormone regulation can be used to improve the winter hardiness of ratoon cotton.
Ratoon cotton in genetics and breeding research
Ratoon cotton is of great importance in genetics and breeding research, such as preserving sterility, reproducing wild germplasms, decreasing the cost of producing F1 hybrid cottonseeds, fixing heterosis, establishing permanent populations of isolate generations such as F2 for genetic research, and extending the effective breeding time of germplasms for more than one year (Zhang et al. Citation2013; D’Eeckenbrugge and Lacape Citation2014; Muhammad, Rauf, and Naz Citation2015; Zhou Citation2016; Komala, Kumar, and Ganesan Citation2019).
Recently, the production of hybrid seeds from ratoon cotton in the tropics has attracted more attention from breeders. In India and China, the advantages of hybrid F1 cotton have indicated its great production value, but its high price limits wide application (Zhang et al. Citation2013; Komala, Ganesan, and Kumar Citation2018). The cost savings associated with using ratoon cotton or even ratooned male sterile lines to produce F1 seeds will be considerable (Plucknett, Evenson, and Sanford Citation1970; Zhang et al. Citation2015a; Zhou Citation2016; Majjiga, Ganesan, and Kumar Citation2018; Komala, Kumar, and Ganesan Citation2019).
Future studies on ratoon cotton
Exploiting the perennial and indeterminate growth habits of cotton for two or more fruiting cycles is a potential way to enhance the productivity and sustainability of ratoon cotton cropping under the present conditions of limited arable land to increase the acreage of cotton (Khader and Prakash Citation2014). Compared to sown cotton, ratoon cotton can provide a higher net income per unit area and the cost of ratoon cultivation is also lower (Bergman Citation1985). Although there have been many reports regarding ratoon cotton, further enhancing the productivity and sustainability of ratoon cotton will require solving some problems that still exist in ratoon cotton cultivation.
Solving the problems associated with the cultivation of ratoon cotton
The main challenge to overcome in ratoon cotton development is pests and diseases control, which under some current conditions, severely impact yield. Currently, unsatisfactory pest management is the most worrying problem, because it has limited the cultivation of ratoon cotton. For example, ratoon cotton was once banned when increasing populations of the cotton boll weevil (CBW) became uncontrollable in Arizona, USA (Bergman Citation1985; Showler Citation2009). Since the mid-1990s, to control various pests such as the cotton bollworm (H. armigera), several insect-resistant genetically modified (GM) cottons have been commercially used around the world (Macedo et al. Citation2017). However, none of the commercially available GM cotton cultivars are fully effective against the CBW, which urgently needs to be controlled via new cultivars or new methods. Genetic silencing via RNAi is a potential tool for controlling the CBW (Macedo et al. Citation2017), and Trichogramma chilonis (Hymenoptera: Trichogrammatidae) is a good choice for controlling pests in cotton fields (Khan Citation2019). In addition, ratoon cultivation is prone to productivity decline each year due to negative plant-soil feedback, which may involve complex physical, chemical, and biological effects mainly caused by the following aspects: (1) roots of ratoon plants perennially absorb minerals from deep soil, which are difficult to replenish by fertilization, leading to the gradual depletion of nutrients; (2) perennial accumulation of soil pathogens and underground pests can cause significant damage to plants; and (3) root exudates (such as phenols) that accumulate over the years can alter the physical and chemical properties of rhizosphere soil, which is detrimental to the continued growth of plants (Bonanomi et al. Citation2005; Liu et al. Citation2008; Huang et al. Citation2013; Vukicevich et al. Citation2016). Therefore, it is necessary to study how to reduce pests and pathogens in ratoon cotton with biological control, which may be a good alternative to environmentally harmful treatments such as soil fumigation.
Under labor shortages and high labor costs, it is necessary to develop a suitable light and simplified cultivation (LSC) system for the sustainable production of ratoon cotton in the tropics (Dai et al. Citation2017). In addition, research on the physiology of ratoon cotton is currently inadequate. Particularly, the combined effects of various hormones, chemicals, and nutrients on the metabolic activity of ratoon cotton deserve more attention to create methods that will produce stronger sprouts leading to fruiting branches for perennial cycles.
Expanding the ratoon cultivation of island cotton
Compared to upland cotton, island cotton generally has higher-quality fibers, i.e., longer and thinner fibers with higher strength. However, island cotton is only planted on a small scale, accounting for <2% cotton production globally (Zhang et al. Citation2005); for instance, in China, it is only cultivated on a large scale in Xinjiang. Island cotton has the advantages of tolerance to cold, drought, and some pests and diseases. Island cotton and the interspecific hybrid between upland cotton and island cotton are long-staple cottons, and their yield can be greatly increased by ratoon cultivation, as mentioned above (Majjiga, Ganesan, and Kumar Citation2018; Komala, Kumar, and Ganesan Citation2019). Therefore, the development of island cotton in the tropics has good prospects (Khader and Prakash Citation2014), and can open up new avenues for long-staple cotton production to meet the growing demand of the textile industry for premium long-staple cotton.
Conclusions
This review of ratoon cotton identifies immediate and long-term research requirements. The challenges of increased disease and pest incidence in ratoon cotton need to be addressed with urgency. We were not able to identify whether H. armigera contributed to the abandonment of arboreal cotton ratoon cultivation in the mid-20th century in southern China. Some studies from tropical India that we reviewed suggest that Bt cotton, which is resistant to insect pests, shows higher production than non-Bt cotton. More studies are necessary to evaluate the net benefits between ratooned and sown cotton cropping to provide flexible risk management strategies. Such actions may stimulate greater support for the sustainable and innovative development of ratoon cotton cultivation. During periods of persistent labor shortages in most villages, ratoon cultivation offers farmers a way to increase their income with minimal added effort. On-farm studies are particularly important to determine the optimum planting density under local conditions. It is worth mentioning that ratooning is particularly compelling in arid and semi-arid ecosystems as a soil and water management strategy. Moreover, ratoon cotton may be a good option for rehabilitating land contaminated by pesticides or heavy metals that are not suitable for planting food crops.
Base on the three methods for ratooning cotton that were previously discussed, perennial ratoon cropping of semi-wild cotton has the best ecological benefits, perennial ratoon cropping of annual cultivars has comprehensive eco-economic benefits, whereas biannual ratoon cropping of annual cultivars presents the highest economic benefits. Ratooning sown cotton for the second fruiting cycle can provide an additional yield of 60%–70% in the same season, avoid closed-season legislation, and has great potential and prospects for cotton production. However, there has been a sharp global decline in rural populations and urbanization development has created areas with idle arable land. Moreover, even if ratoon cotton was to expand, it would not develop natural and semi-natural environments into arable land since cotton is a labor-intensive crop and mechanized harvest is still difficult. Consequently, these methods require multi-year testing in large-scale agricultural settings, and linking farmers with emerging markets is the next step. Therefore, related services should be provided to meet farmers’ interest in ratoon cultivation of cotton through participatory field trials and demonstrations. Finally, studies can help to overcome the constraints that companies and farmers face with respect to the broader cultivation of ratoon cotton and improve our understanding of its economic and ecological significance.
Acknowledgments
This project is partially supported by the National Key R&D Program of China (2016YFD0101413) and the National Natural Science Foundation of China (31571600). The funder had no role in structure design, data collection, decision to publish, or preparation of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdel-Salam, M. E., M. A. M. E. Negm, and C. S. Ardabb. 2009. The Egyptian Cotton, Current Constraints and Future Opportunities. Alexandria, 1–13. Egypt: Textile Industries Holding Co., Modern Press.
- Abdurakhmonov, I. Y. 2007. “Exploiting Genetic Diversity.” Proceedings of World Cotton Research Conference-4, Lubbock, TX. doi:10.1094/PDIS-91-4-0467B.
- Abdurakhmonov, I. Y., Z. T. Buriev, S. E. Shermatov, A. A. Abdullaev, K. Urmonov, F. Kushanov, S. S. Egamberdiev, et al. 2012. “Genetic Diversity in Gossypium Genus.” In Genetic Diversity in Plants, edited by M. Caliskan, 313–338. Rijeka: InTech. doi:10.5772/35384.
- Alkhafaji, I. 2016. Toxicological Effects of Cotton Seeds on Physiological, Histological and Hematological Parameters in Male, 1–2. Saarbrücken: LAP LAMBERT Academic Publishing.
- Azevedo, D. M. P. D., J. W. Dos Santos, D. J. Vieira, N. E. D. M. Beltrão, L. B. Da Nóbrega, and J. R. Pereira. 2000. “Plant Population in Perennial Cotton/maize Intercrop, Yield Components and Agronomic Efficiency.” Revista de Oleaginosas e Fibrosas 4 (2): 75–85.
- Baker, H. G. 1962. “Comments on the Thesis that There Was a Major Centre of Plant Domestication near the Headwaters of the River Niger.” The Journal of African History 3 (2): 229–233. doi:10.1017/S0021853700003054.
- Bergman, D. K. 1985. Boll Weevil (Coleoptera: Curculionidae) Overwintering in Arizona. Tucson: University of Arizona.
- Bergman, D. K., T. J. Henneberry, L. A. Bariola, and J. M. Gillespie. 1983. Studies of Pest and Beneficial Insects in Arizona Stub and Planted Cotton, 1–2. Oakland: USDA.
- Bonanomi, G., F. Giannino, S. Mazzoleni, and H. Setälä. 2005. “Negative Plant-Soil Feedback and Species Coexistence.” Oikos 111 (2): 311–321. doi:10.1111/j.0030-1299.2005.13975.x.
- Boopathi, N. M., S. Sathish, P. Dachinamoorthy, P. Kavitha, and R. Ravikesav. 2014. “Usefulness and Utilization of Indian Cotton Germplasm.” In World Cotton Germplasm Resources, edited by I. Y. Abdurakhmonov, 315–323. Rijeka: InTech. doi:10.5772/58619.
- Bourgou, L., M. Sawadogo, D. Sanfo, and J. Lacape. 2017. “SSR-based Genetic Diversity of Traditional and Perennial Cotton (Gossypium spp.) Populations Collected in Burkina Faso.” Genetic Resources and Crop Evolution 64 (7): 1743–1759. doi:10.1007/s10722-016-0470-4.
- Bullen, C. W., M. A. Granger, and H. B. Persaud 1982. “Acid Soils of the Intermediate Savannahs of Guyana.” In Proceedings of the joint workshop on the management of low fertility acid soils of the American humid tropics, edited by J. Wienk and H. Wit, 49–84. San Jose (Costa Rica): Ministry of Agriculture, Animal Husbandry, Fisheries and Forestry. Paramaribo (Surinam); Suriname Univ., Paramaribo.
- Bunzel, K., M. Kattwinkel, M. Schauf, and D. Thrän. 2014. “Energy Crops and Pesticide Contamination: Lessons Learnt from the Development of Energy Crop Cultivation in Germany.” Biomass and Bioenergy 70: 416–428. doi:10.1016/j.biombioe.2014.08.016.
- Castro, S. G. Q. D., J. Rossi Neto, O. T. Kölln, B. M. M. N. Borges, and H. C. J. Franco. 2019. “Decision-making on the Optimum Timing for Nitrogen Fertilization on Sugarcane Ratoon.” Scientia Agricola 76 (3): 237–242. doi:10.1590/1678-992x-2017-0365.
- Chen, G. P., X. Zhang, R. Y. Zhou, and H. T. Zhao. 2008. “Study on Economic Characteristics of Biennial and Annual Upland Cotton.” Guihaia 28 (5): 636–639. doi:10.3969/j.issn.1000-3142.2008.05.017.
- Chen, G. P., X. Zhang, R. Y. Zhou, and H. T. Zhao. 2010a. “The Effect of Varieties, Sowing Date and Perennial Cultivation on Survival Rate of Upland Cotton after Winter.” Acta Agriculturae Boreali-Occidentalis Sinica 18 (32): 9784–9788. doi:10.3969/j.issn.1004-1389.2010.12.011.
- Chen, G. P., X. Zhang, R. Y. Zhou, and H. T. Zhao. 2010b. “A Study on the Contents of NPK and Soluble Sugar in Leaves and Stems for Perennial Upland Cotton.” Chinese Agricultural Science Bulletin 26 (7): 72–77.
- Chen, Z., C. E. Grover, P. Li, Y. Wang, H. Nie, Y. Zhao, M. Wang, et al. 2017. “Molecular Evolution of the Plastid Genome during Diversification of the Cotton Genus.” Molecular Phylogenetics and Evolution 112: 268–276. doi:10.1016/j.ympev.2017.04.014.
- Chitakira, M., E. Torquebiau, and W. Ferguson. 2012. “Unique Combinations of Stakeholders in a Transfrontier Conservation Area Promote Biodiversity-agriculture Integration.” Journal of Sustainable Agriculture 36 (3): 275–295. doi:10.1080/10440046.2011.611584.
- Cox, T. S., M. Bender, C. Picone, D. L. V. Tassel, J. B. Holland, E. C. Brummer, B. E. Zoeller, et al. 2002. “Breeding Perennial Grain Crops.” Critical Reviews in Plant Sciences 21 (2): 59–91. doi:10.1080/0735-260291044188.
- Crews, T., and D. Cattani. 2018. “Strategies, Advances, and Challenges in Breeding Perennial Grain Crops.” Sustainability 10 (7): 2192. doi:10.3390/su10072192.
- D’Eeckenbrugge, G. C., and J. M. Lacape. 2014. “Distribution and Differentiation of Wild, Feral, and Cultivated Populations of Perennial Upland Cotton (Gossypium hirsutum L.) In Mesoamerica and the Caribbean.” PloS One 9 (9): e107458. doi:10.1371/journal.pone.0107458.
- Dai, J., X. Kong, D. Zhang, W. Li, and H. Dong. 2017. “Technologies and Theoretical Basis of Light and Simplified Cotton Cultivation in China.” Field Crops Research 214: 142–148. doi:10.1016/j.fcr.2017.09.005.
- De Souza, J. G., and J. V. Da Silv. 1987. “Partitioning of Carbohydrates in Annual and Perennial Cotton (Gossypium hirsutum L.).” Journal of Experimental Botany 38 (7): 1211–1218. doi:10.1093/jxb/38.7.1211.
- De Souza, N. A., and J. S. De Holanda. 1993. “Environmental Adaptability of Perennial Cotton in the Seridó.” Pesquisa Agropecuaria Brasileira 28 (7): 797–801.
- DeHaan, L. R., D. L. Van Tassel, and T. S. Cox. 2005. “Perennial Grain Crops: A Synthesis of Ecology and Plant Breeding.” Renewable Agriculture and Food Systems 20 (1): 5–14. doi:10.1079/RAF200496.
- Evenson, J. P. 1970. “Ratooning of Cotton: A Review.” Cotton Growing Review 47 (1): 1–7.
- Fontes, E. M., F. D. S. Ramalho, E. Underwood, P. Bazrroso, M. F. Simon, E. R. Sujii, C. S. S. Pires, N. Beltrão, W. A. Lucena, and E. C. Freire. 2006. “The Cotton Agricultural Context in Brazil.” Environmental Risk Assessment of Genetically Modified Organisms 2: 21–66. doi:10.1079/9781845930004.0021.
- Geng, X. 2015. Serving China through Agricultural Science: American-trained Chinese Scholars and “Scientific Nationalism” in Decentralized China (1911–1945). Twin Cities: University of Minnesota.
- Gotmare, V., P. Singh, C. D. Mayee, V. Deshpande, and C. Bhagat. 2004. “Genetic Variability for Seed Oil Content and Seed Index in Some Wild Species and Perennial Races of Cotton.” Plant Breeding 123 (2): 207–208. doi:10.1046/j.1439-0523.2003.00914.x.
- Grabowski, P., L. S. Olabisi, J. Adebiyi, K. Waldman, R. Richardson, L. Rusinamhodzi, S. Snapp, et al. 2019. “Assessing Adoption Potential in a Risky Environment: The Case of Perennial Pigeonpea.” Agricultural Systems 171: 89–99. doi:10.1016/j.agsy.2019.01.001.
- Gumber, R. K., M. S. Gill, P. Rathore, D. Pathak, J. S. Gill, and P. Singh. 2016. “History and Status of Cotton.” In Cotton Research, edited by I. Y. Abdurakhmonov, et al., 1–10. Rijeka: InTech.
- Harland, S. C. 1937. “The Genetics of cotton-XVII. Increased Mutability of a Gene in G. Purpurascens as a Consequence of Hybridization with G. hirsutum.” Journal of Genetics 34 (1): 153–169. doi:10.1007/BF02982260.
- Haughton, A. J., A. J. Bond, A. A. Lovett, T. Dockerty, G. Sünnenberg, S. J. Clark, D. A. Bohan, et al. 2009. “A Novel, Integrated Approach to Assessing Social, Economic and Environmental Implications of Changing Rural Land-use: A Case Study of Perennial Biomass Crops.” Journal of Applied Ecology 46 (2): 315–322. doi:10.1111/j.1365-2664.2009.01623.x.
- He, A., W. Wang, G. Jiang, H. Sun, M. Jiang, J. Man, K. Cui, et al. 2019. “Source-sink Regulation and Its Effects on the Regeneration Ability of Ratoon Rice.” Field Crops Research 236: 155–164. doi:10.1016/j.fcr.2019.04.001.
- Hearn, A. B. 1980. “Water Relationships in Cotton.” Outlook Agriculture 10: 159–166. doi:10.1177/003072708001000402.
- Hinze, L. L., D. D. Fang, M. A. Gore, B. E. Scheffler, J. Z. Yu, J. Frelichowski, R. G. Percy, et al. 2015. “Molecular Characterization of the Gossypium Diversity Reference Set of the US National Cotton Germplasm Collection.” Theoretical and Applied Genetics 128 (2): 313–327. doi:10.1007/s00122-014-2431-7.
- Huang, L., L. Song, X. Xia, W. Mao, K. Shi, Y. Zhou, J.-Q. Yu, et al. 2013. “Plant-soil Feedbacks and Soil Sickness: From Mechanisms to Application in Agriculture.” Journal of Chemical Ecology 39 (2): 232–242. doi:10.1007/s10886-013-0244-9.
- Jia, Y. H., J. L. Sun, and X. M. Du. 2014. “Cotton Germplasm Resources in China.” In World Cotton Germplasm Resources, edited by I. Y. Abdurakhmonov, 35–53. Rijeka: InTech.
- Khader, S. E. S. A., and A. H. Prakash. 2014. “Pruning Technique for Second Fruiting Cycle in Cotton Crop.” Cotton Research Journal 6 (1): 46–49.
- Khadi, B. M., V. Santhy, and M. S. Yadav. 2010. “Cotton: An Introduction.” In Cotton: Biotechnological Advances, edited by U. B. Zehr, 1–14. Berlin: Springer Berlin Heidelberg. doi:10.1007/978-3-642-04796-1_1.
- Khan, M. A. 2019. “Integration of Selected Novel Pesticides with Trichogramma Chilonis (Hymenoptera: Trichogrammatidae) for Management of Pests in Cotton.” Journal of Agricultural Science and Technology 21 (4): 873–882.
- Khan, R. A., and G. Shabbir. 1974. “Investigations into the Productivity of the Second Fruiting Cycle in Cotton.” Pakistan Journal of Agricultural Sciences 11 (1–2): 15–20.
- Komala, M., M. Kumar, and N. M. Ganesan. 2019. “Combining Ability Effects for Fibre Quality Traits in First and Ratoon Crops of Cotton Interspecific Hybrids (G. hirsutum× G. barbadense).” Research on Crops 20 (1): 230–235. doi:10.31830/2348-7542.2019.033.
- Komala, M., N. M. Ganesan, and M. Kumar. 2018. “Assessment of Ratooning Ability and Heterotic Effects for Yield and Yield Contributing Traits in Intraspecific Hybrids of Upland Cotton.” Current Agriculture Research Journal 6 (1): 85–94. doi:10.12944/CARJ.6.1.11.
- Lambin, E. F., and H. J. Geist. 2003. “Regional Differences in Tropical Deforestation.” Environment: Science and Policy for Sustainable Development 45 (6): 22–36. doi:10.1080/00139157.2003.10544695.
- Lee, J. A. 1986. “An Early Example of a Viable Hybrid from a Cross of Gossypium barbadense L. And G. davidsonii Kell.” Journal of Heredity 77 (1): 56–57. doi:10.1093/oxfordjournals.jhered.a110170.
- Liu, J., X. Bian, Y. Li, W. Zhang, and S. Li. 2008. “Effects of Long-term Continuous Cropping of Cotton and Returning Cotton Stalk into Field on Soil Biological Activities.” The Journal of Applied Ecology 19 (5): 1027–1032.
- Liu, S., L. Guo, H. Webb, X. Ya, and X. Chang. 2019. “Internet of Things Monitoring System of Modern Eco-agriculture Based on Cloud Computing.” IEEE Access 7: 37050–37058. doi:10.1109/ACCESS.2019.2903720.
- Liu, Y. W. 1998. “Records of Scientific Research Activities of the Central Research Institute of Agriculture.” China Historical Materials Science & Technology 19 (1): 52–61. doi:10.3969/j.issn.1673-1441.1998.01.005.
- Luttrell, R. G., G. P. Fitt, F. S. Ramalho, and E. S. Sugonyaev. 1994. “Cotton Pest Management: Part 1. A Worldwide Perspective.” Annual Review of Entomology 39 (1): 517–526. doi:10.1146/annurev.en.39.010194.002505.
- Macedo, L. L. P., J. D. Antonino De Souza Junior, R. R. Coelho, F. C. A. Fonseca, A. A. P. Firmino, M. C. M. Silva, R. R. Fragoso, et al. 2017. “Knocking down Chitin Synthase 2 by RNAi Is Lethal to the Cotton Boll Weevil.” Biotechnology Research and Innovation 1 (1): 72–86. doi:10.1016/j.biori.2017.04.001.
- Macharia, J. 2013. Effect of Ratooning and Nitrogen Application on Lint Yield and Quality of Cotton Varieties in Central Kenya. Nairobi: University of Nairobi.
- Macmillan, C. P., H. Birke, F. Bedon, and F. A. Pettolino. 2013. “Lignin Deposition in Cotton Cells - Where Is the Lignin?” Journal of Plant Biochemistry & Physiology 1 (2): e106. doi:10.4172/jpbp.1000e106.
- Majjiga, K., N. M. Ganesan, and M. Kumar. 2018. “Combining Ability for Yield and Yield Contributing Traits in Intraspecific Hybrids of Ratoon Upland Cotton.” International Journal of Basic and Applied Agricultural Research 16 (1): 22–28.
- Migicovsky, Z., and S. Myles. 2017. “Exploiting Wild Relatives for Genomics-assisted Breeding of Perennial Crops.” Frontiers in Plant Science 8: 460. doi:10.3389/fpls.2017.00460.
- Mihail, J. D., J. K. Brown, and M. R. Nelson. 1987. The Effects of Cotton Leaf Crumple on Greenhouse-Grown Cotton Incoulated at Five Growth Stages. Tucson, AZ: College of Agriculture, University of Arizona.
- Mubvekeri, W., J. Bare, C. Makaka, and F. Jimu. 2014. “Assessing the Diversity and Intensity of Pesticide Use in Communal Area Cotton Production in Zimbabwe.” Journal of Ecology and the Natural Environment 6 (10): 342–348. doi:10.5897/jene2014.0476.
- Muhammad, A., S. Rauf, and K. Naz. 2015. “Induced Genetic Variability in Selected γ-radiated Cotton Varieties during Second Year Ratooning under Rain Fed Environment.” Asian Journal of Natural & Applied Sciences 4 (1): 70–81.
- Okoduwa, A. I., and A. O. Odigie. 2008. “British Attempt at Developing Cotton as an Export Crop from Esan, Edo State, Nigeria, 1902–1925.” Studies of Tribes & Tribals 6 (1): 29–34. doi:10.1080/0972639X.2008.11886572.
- Percy, R. G., J. E. Frelichowski, M. D. Arnold, T. B. Campbell, J. K. Dever, and D. D. Fang. 2014. “The US National Cotton Germplasm collection–Its Contents, Preservation, Characterization, and Evaluation.” In World Cotton Germplasm Resources, edited by I. Y. Abdurakhmonov, et al., 167–201. Rijeka: InTech.
- Perdikis, D., A. Fantinou, and D. Lykouressis. 2011. “Enhancing Pest Control in Annual Crops by Conservation of Predatory Heteroptera.” Biological Control 59 (1): 13–21. doi:10.1016/j.biocontrol.2011.03.014.
- Pickersgill, B., C. H. B. Spencer, and D. D. Andrade-Lima. 1975. “Wild Cotton in Northeast Brazil.” Biotropica 7 (1): 42–54. doi:10.2307/2989799.
- Pinto De Menezes, I. P., P. A. V. Barroso, L. V. Hoffmann, V. S. Lucena, and M. Giband. 2010. “Genetic Diversity of Mocó Cotton (Gossypium Hirsutum Race Marie-galante) from the Northeast of Brazil: Implications for Conservation.” Botany 88 (8): 765–773. doi:10.1139/B10-045.
- Pkania, K. C., J. Venneman, K. Audenaert, O. Kiplagat, G. Gheysen, and G. Haesaert. 2014. “Present Status of Bacterial Blight in Cotton Genotypes Evaluated at Busia and Siaya Counties of Western Kenya.” European Journal of Plant Pathology 139 (4): 863–874. doi:10.1007/s10658-014-0440-7.
- Plucknett, D. L., J. P. Evenson, and W. G. Sanford. 1970. “Ratoon Cropping.” Advances in Agronomy 22: 285–330. doi:10.1016/S0065-2113(08)60271-0.
- Potts, D. T. 2012. “A Companion to the Archaeology of the Ancient near East.” Lithic Industries during the Holocene Period 21: 236–260. doi:10.1002/9781444360790.
- Rajendran, T. P., A. Birah, and P. S. Burange. 2018. “Insect Pests of Cotton.” In Pests and Their Management, 361–411. Singapore: Springer.
- Ramalho, F. S. 1994. “Cotton Pest Management: Part 4. A Brazilian Perspective.” Annual Review of Entomology 39 (1): 563–578. doi:10.1146/annurev.en.39.010194.003023.
- Risch, S. J., D. Andow, and M. A. Altieri. 1983. “Agroecosystem Diversity and Pest Control: Data, Tentative Conclusions, and New Research Directions.” Environmental Entomology 12 (3): 625–629. doi:10.1093/ee/12.3.625.
- Sachs, M. H., and S. Zilkah. 1985. “Characterization of Climatic Factors Affecting Chilling Injury in Field-grown Ratoon Cotton.” Journal of Agricultural Science 105 (2): 475–478. doi:10.1017/S0021859600056525.
- Sadras, V. O. 1996. “Cotton Compensatory Growth after Loss of Reproductive Organs as Affected by Availability of Resources and Duration of Recovery Period.” Oecologia 106 (4): 432–439. doi:10.1007/BF00329698.
- Sampaio, E. V. D. S., R. S. C. Menezes, Y. D. S. B. Sampaio, and A. D. S. de Freitas. 2017. “Sustainable Agricultural Uses in the Caatinga.” In Caatinga: The Largest Tropical Dry Forest Region in South America, edited by J. M. C. D. Silva, I. R. Leal, and M. Tabarelli, 413–428. Cham: Springer. doi:10.1007/978-3-319-68339-3_16.
- Sarwar, M. 2017. “Pink Bollworm Pectinophora Gossypiella (Saundera)[lepidoptera: Gelechiidae] and Practices of Its Integrated Management in Cotton.” International Journal of Plant Science and Ecology 3 (1): 1–6.
- Showler, A. T. 2009. “Roles of Host Plants in Boll Weevil Range Expansion beyond Tropical Mesoamerica.” American Entomologist 55 (4): 234–242. doi:10.1093/ae/55.4.234.
- Silva, F. P. D., A. P. L. Bezerra, and A. F. D. Silva. 2008. “Boll Weevil (Anthonomus Grandis Boheman) Oviposition and Feed in Ratoon Cotton of Mutants Lines of Upland Cotton.” Revista Ciencia Agronomica 39 (1): 85–89. doi:10.1590/S0100-204X2008000100016.
- Singh, A. K. 2017. “Fiber Crops.” In Wild Relatives of Cultivated Plants in India, 69–76. Singapore: Springer. doi:10.1007/978-981-10-5116-6.
- Smaje, C. 2015. “The Strong Perennial Vision: A Critical Review.” Agroecology and Sustainable Food Systems 39 (5): 471–499. doi:10.1080/21683565.2015.1007200.
- Snapp, S., P. Roge, P. Okori, R. Chikowo, B. Peter, and J. Messina. 2019. “Perennial Grains for Africa: Possibility or Pipedream?” Experimental Agriculture 55 (2): 251–272. doi:10.1017/S0014479718000066.
- Stewart, J. 1994. “Potential for Crop Improvement with Exotic Germplasm and Genetic Engineering.” Challenging the future: Proceeding of the world cotton research conference-I, Brishbane, Australia, February 14–17, Melbourne, 1995, 313–327.
- Templeton, J. 1925. “Ratoon cotton in Egypt: A preliminary note”. Cairo: Government Press.
- Viot, C. 2019. “Domestication and Varietal Diversification of Old World Cultivated Cottons (Gossypium sp.) In the Antiquity.” Revue d’ethnoécologie 15: 1–26. doi:10.4000/ethnoecologie.4404.
- Vukicevich, E., T. Lowery, P. Bowen, J. R. Úrbez-Torres, and M. Hart. 2016. “Cover Crops to Increase Soil Microbial Diversity and Mitigate Decline in Perennial Agriculture. A Review.” Agronomy for Sustainable Development 36 (3): 1–14. doi:10.1007/s13593-016-0385-7.
- Wang, R., T. Zhou, D. Hu, F. Li, and J. Liu. 2011. “Cultivating Eco-sustainability: Social–economic–natural Complex Ecosystem Case Studies in China.” Ecological Complexity 8 (4): 273–283. doi:10.1016/j.ecocom.2011.03.003.
- Wells, R. 2002. “Stem and Root Carbohydrate Dynamics of Two Cotton Cultivars Bred Fifty Years Apart.” Agronomy Journal 94 (4): 876–882. doi:10.2134/agronj2002.8760.
- Wendel, J. F., and C. E. Grover. 2015. “Taxonomy and Evolution of the Cotton Genus, Gossypium.” In Cotton, Agronomy Monograph. 2nd. edited by D. D. Fang and R. G. Percy, 25–44. Madison:American Society of Agronomy, Inc., Crop Science Society of America, Inc., and Soil Science Society of America. doi:10.2134/agronmonogr57.2013.0020.
- Wendel, J. F., C. L. Brubaker, and A. E. Percival. 1992. “Genetic Diversity in Gossypium Hirsutum and the Origin of Upland Cotton.” American Journal of Botany 79 (11): 1291. doi:10.2307/2445058.
- Werth, J., L. Boucher, D. Thornby, S. Walker, and G. Charles. 2013. “Changes in Weed Species since the Introduction of Glyphosate-resistant Cotton.” Crop and Pasture Science 64 (8): 791–798. doi:10.1071/CP13167.
- Wilson, L., S. Heimoana, T. Farrell, and T. Smith. 2008. “Setting Thresholds for Aphids in Cotton.” Australian Cottongrower, The 29 (6): 12–14, 16.
- Xu, Q., G. Xiong, P. Li, F. He, Y. Huang, K. Wang, Z. Li, et al. 2012. “Analysis of Complete Nucleotide Sequences of 12 Gossypium Chloroplast Genomes: Origin and Evolution of Allotetraploids.” PloS One 7 (8): e37128. doi:10.1371/journal.pone.0037128.
- Yang, J., R. Cai, and Y. Deng. 1990. “Studies on Characteristics of 10 Semi-wild Typies of G. hirsutum and Their Genetic Relationships.” Cotton Science 2 (2): 24–30.
- Zeng, Y. S. 2012. “Cotton Science Research of Feng Zefang and His Main Contribution.” Agricultural History of China 32 (4): 18–27.
- Zeng, Y. S., and S. M. Wang. 2013. “Feng Ze-fang and the Cotton Output Improvement in the Period of Anti-Japanese War.” Historical Research in Anhui (2): 37–43. doi:10.3969/j.issn.1005-605X.2013.02.005.
- Zhang, X., Q. Zhou, R. Y. Zhou, and G. P. Chen. 2012b. “Current Research on the Utilization of Perennial Cotton.” Guangdong Agricultural Sciences 39 (6): 37–40. doi:10.3969/j.issn.1004-874X.2012.06.013.
- Zhang, X., R. Zhou, and X. Lou. 2008. “Investigation on Overwintering of Cotton Germplasm Resources in 2008 in Nanning of Guangxi.” Crops 26 (6): 74–76.
- Zhang, X., Z. Zhang, Q. Wang, P. Chen, G. Chen, and R. Zhou. 2013. “Effects of Rootstocks on Cryotolerance and Overwintering Survivorship of Genic Male Sterile Lines in Upland Cotton (Gossypium hirsutum L.).” PloS One 8 (5): e63534. doi:10.1371/journal.pone.0063534.
- Zhang, X. J., F. L. Yue, X. H. Zhang, R. Hou, X. Q. Zhang, and W. J. Li. 2015b. “Technical System of Hybrid Seed Production with Perennial Plants of Cotton Sterile Lines.” Acta Agronomica Sinica 41 (12): 1836–1843. doi:10.3724/SP.J.1006.2015.01836.
- Zhang, X. J., F. L. Yue, X. H. Zhang, W. J. Li, and X. Q. Zhang. 2015a. “Research on Root Retention Reproduction Technique of Genetic Male-sterile Line in Cotton for Seed Production.” Seed Science and Technology 43 (2): 187–196. doi:10.15258/sst.2015.43.2.01.
- Zhang, Y. M., C. Tian, L. M. Jiang, Y. P. Li, Z. M. Xiao, and J. L. Li. 2012a. “Advantages of Perennial Crop on Conservation of Agroecological Environment.” Advanced Materials Research 518–523: 5213–5216. doi:10.4028/www.scientific.net/AMR.518-523.5213.
- Zhang, Z. S., Y. H. Xiao, M. Luo, X. B. Li, X. Y. Luo, L. Hou, D.-M. Li, et al. 2005. “Construction of a Genetic Linkage Map and QTL Analysis of Fiber-related Traits in Upland Cotton (Gossypium hirsutum L.).” Euphytica 144 (1): 91–99. doi:10.1007/s10681-005-4629-x.
- Zhou, R. Y. 2016. Method of Producing Hybrid Seeds for Annual Cotton by Cultivating Perennially. US Patent 9,265,206 B2.
