?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We studied the feeding ecology of the Black Wood Pigeon (Columba janthina), a species endemic to the East Asian Pacific Islands, and determined the species’ dietary composition and food preferences on Ulleung Island, South Korea, through field observations. From March 2016 to February 2018, the diversity of food items consumed was low, with the 10 most common plant species (from a total of 33) accounting for over 80%. Food availability varied depending on plant abundance, but this influence was minimized because of factors such as delayed fruit ripening. Drupes were the most favored food items (60–68%) but were replaced by nuts and weed seeds during the spring and winter months. Dietary composition and food preferences were related to the abundance of food items based on their phenology, but a steady, high preference for Aphananthe aspera was observed throughout the study period. The abundance of Prunus takesimensis in June was correlated with changes in the Black Wood Pigeon population. This study is the first to assess the feeding ecology of Black Wood Pigeons using field observations and suggests that the availability of different food items affects population changes on the East Asian Pacific islands.
Introduction
Isolated habitat studies, like the Galápagos Islands, are important because rapidly changing ecosystem studies have the potential to predict the future (Baldacchino and Niles Citation2011). The acquisition of food fundamentally influences the behavior of birds, their habitat use, and their reproductive success (Hanspach et al. Citation2015; Lack Citation1968). Changes in the diet of birds in resource restricted habitats, such as islands, may have direct and steady effects on population retention (Crome Citation1975; Frith, Crome, and Wolfe Citation1976; Powlesland et al. Citation1997; Oliveira, Marrero, and Nogales Citation2002; McConkey, Meehan, and Drake Citation2005; Emeny et al. Citation2009). Information on dietary composition is required to assess whether changes in food availability affect population dynamics (Newton Citation1980).
The Black Wood Pigeon Columba janthina is endemic to the East Asian Pacific Islands and main habitats are Japanese islands and the southern islands of the Korean Peninsula (Seki Citation2005; Brazil Citation2009). Globally, 81% of the endangered species in the order of Columbiformes inhabit island environments (Walker Citation2007). The Black Wood Pigeon is an internationally protected species categorized as Near Threatened (NT) by the International Union for the Conservation of Nature, according to its Red List criteria (IUCN Citation2017). Anthropogenic disturbance and natural disasters in island ecosystems can alter the breeding and feeding ecology of birds, on a medium- to long-term basis (Williams and Karl Citation1996). Among other factors, this phenomenon may also be related to geographic differences in the types and availability of food items (Murton, Westwood, and Isaacson Citation1964; Luniak Citation2004). Therefore, information on the feeding ecology of the Black Wood Pigeon is an important factor in evaluating whether food availability has an effect on the population of this species. Understanding the relationship between feeding habits and habitat is especially important for rare or reintroduced birds where the relationship between the environment and species is often poorly understood (Austin, Hayes, and Barzen Citation2019).
Common methods of studying avian diets include examining food in the esophagus, or in fecal or pellet samples (Ando et al. Citation2016; Barzen et al. Citation2018). However, only field observation is a method to analyze the relation between food availability and the environmental factor and behavioral characteristics (Rosenberg and Cooper Citation1990). Field observations offer the advantageous potential to study diet shifts and other ecological relationships without capturing or sacrificing animals (Barzen et al. Citation2018). Recently, photographs and videos have been used to record diets (Greer Citation2010), molting, sexual dimorphism, and the use of wintering sites (Vieira, Furness, and Nager Citation2016) and to study the age and gender migration strategies of raptors (Choi et al. Citation2017).
Although the Black Wood Pigeon is an endemic species with a dispersed habitat range, research on the ecology of this avian species is limited, e.g., a DNA analysis through feces of a few subspecies in Japan (Ando et al. Citation2016). The size of Black Wood Pigeon populations varies by season and habitat type; however, the cause of which is unknown. Changes in the population size are proposed to be related to breeding success in response to food availability (Martin Citation1987). However, if the feeding ecological information about a species is unknown, e.g., Black Wood Pigeons, it is impossible to evaluate the relationship between populations and food items (Inglis et al. Citation1990).
The Black Wood Pigeons visited Ulleungdo for breeding, and changes in population size of 200 to 300 animals were recorded (Choi et al. Citation2019a). The purpose of this study is to precisely understand the composition and seasonal diversity of the diet of Black Wood Pigeon (C. j. janthina) on Ulleung Island, South Korea, and to investigate how the availability of food items affects the population. The study objectives were: 1) to describe the dietary composition and food choices of Black Wood Pigeons; 2) evaluate whether these preferences affect the population through seasonal changes in food availability; and 3) identify important food-bearing plant species to assist in the conservation of the Black Wood Pigeon.
Materials and methods
Study location
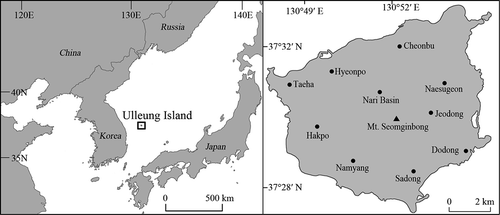
Ulleung Island is an East Asian Pacific Island located between Japan and the Korean peninsula, approximately 130 km offshore of the east coast of the Korean peninsula and covering a total area of about 7255.9 ha (130° 47′ 37″ – 130° 56′ 20″ E and 37° 27′ 2″ – 37° 33′ 01″ N) (). Because of its warm and humid maritime climate, it has a greater distribution of subtropical plants compared with inland areas at the same latitude (Yang et al. Citation2015). The main vegetations of Ulleung Island are Acre okamotoanum, Pinus thunbergii, Zelkova Serrata, Sorbus commixta, Machilus thunbergii, and Fagus multinervis (Choi et al. Citation2019a).
Phenology
This study was conducted from March 2016 to February 2018, during which the timing and duration of the leaf growth, and the ripeness of the fruits of selected forest plants were recorded to determine seasonal variation in potential food supply for Black Wood Pigeons. We designated and collected data of 50 × 50 m plots in 10 areas that contained various food plants. We selected 2–3 high covered diet plants from each food-bearing species in each plot to monitor. The monitored plants were photographed after visual observation of mature fruits that would be edible to Black Wood Pigeons, to determine the identification, fruit status, and other information about the food plant. This information included the monthly abundance (calculated by comparing the fruit size and degree of ripeness), the leaf size and color, and changes in these monitored traits over 1 year. We standardized the comparison score of each food item to be 5 points at maximum and gave the food item an abundance score of 0 to 5 (Emeny et al. Citation2009).
Diet composition survey
We followed a focal animal sampling method (Martin and Bateson Citation1993) to investigate the diet of Black Wood Pigeons from March 2016 to February 2018. Feeding activities were observed through binoculars (Nikon 10 × 25) and a telescope (20–60X) and we recorded videos and images using a digital SLR camera (Nikon D7200, 200–500 mm F5.6 lens) to record foraging behavior and to identify food items. High-resolution (6,000 × 4,000 pixels) photographs were used to identify the food items. In order to avoid duplication of data, 40 points were selected in the survey area and examined for food items. Food items surveys were conducted using the point census method (Howe et al. Citation1997; Huff et al. Citation2000; Gregory, Baillie, and Bashford Citation2004) and we stayed about 15 minutes per point. The food items were divided into drupes (berries), nuts (pine nuts), cereals, weed seeds, and leaves.
Food preferences
We calculated a monthly food preference index by analyzing the seasonal phenology of the plants and the Black Wood Pigeon food items data. The food preference index was based on the Manly-Chesson Index (Manly, McDonald, and Thomas Citation1993). Food preferences were calculated separately for the years 2016 and 2017. The ratio of a particular food item in the diet (di) was divided by the availability of that food item. The formula applied is as follows:
Pi = Black Wood Pigeons preference values for food type i when foraging in a habitat with k food types available; di = the proportion of food of type i in the diet; and Ni = the proportion of food of type i available in the habitat. A preference value above 1/k indicates a preferred food; values below 1/k indicate avoidance (Manly, McDonald, and Thomas Citation1993).
Data analysis
Data analysis was divided into two periods of 24 months according to the seasons (March 2016–February 2017, March 2017–February 2018). Analysis of variations in monthly food items was performed with Pearson’s chi-square tests using the availability of each type of food and the frequency of it in the diet. The analysis of diet similarities were tested by nonmetric multidimensional scaling (NMDS) using the select/frequency data of each food items. Correlation analysis between food items and population was tested using Linear Regression. We analyzed the correlation between the population change of monthly Black Wood Pigeon by Choi et al. (Citation2019a) and food items. All the analyses were performed using SPSS version 20.0 (IBM. USA) software.
Results
Phenology
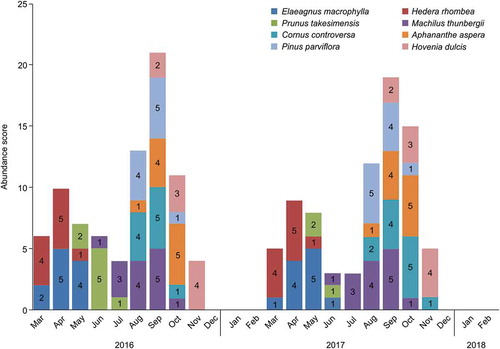
There was snow on Ulleung Island until March in both years of the study period. Leaves began to form in April, the trees were almost completely bare (no leaves or fruits) in November, and snow fell heavily in December. Changes in the abundance of Black Wood Pigeon feeding plants were similar in 2016 and 2017 (), with the highest abundance recorded from August to October and the lowest abundance recorded in June, July, and November (). The drupes of Machilus thunbergii, a known major food source for Black Wood Pigeons, became available in July (abundance score = 3) and the ripe fruit was most plentiful in September (abundance score = 5). This food item was available for the longest time, from June to October (). Elaeagnus macrophylla and Hedera rhombea produced the best ripe fruit from April to May. However, fruits of various conditions (abundance score = 2 or 4) were available to eat in March, when food was scarce (). Prunus takesimensis showed significant differences in abundance during the survey period. There was high abundance in June 2016 (abundance score = 5), but there was almost no fruit in 2017 (abundance score = 1–2).
Monthly variation in diet composition
Black Wood Pigeons were observed feeding a total of 1966 times on Ulleung Island during the study period, and in 1772 instances the diet plants could be identified: 975 instances in 2016 and 797 in 2017. We identified 33 species of diet plants during the entire study period (). The mean monthly dietary diversity during the study period was 8.78 ± 3.27 species (SD, N = 20 range 2–14). Analysis of the Black Wood Pigeons food items showed that the top 10 species: M. thunbergii, Cornus controversa, Aphananthe aspera, Pinus parviflora, P. takesimensis, Morus bombycis, E. macrophylla, Cornus macrophylla, Camellia japonica, and H. rhombea, accounted for 80% (N = 1475) of the total identified food items.
Table 1. List of the number and percentage of feeding observations for each food-bearing species observed on Ulleung Island, March 2016 – February 2018.
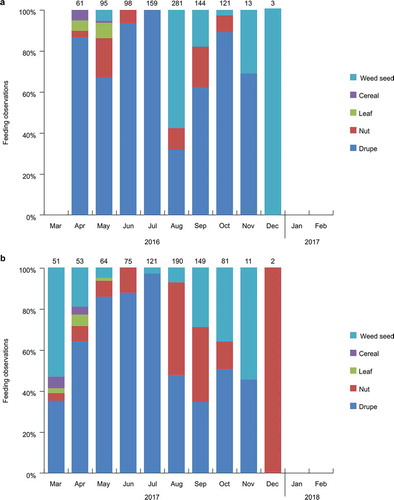
Significant monthly differences in feeding frequency were observed for drupes, nuts, cereals, and weed seeds (χ2 = 1024.08, df = 64, P = 0.0001). The results of the analysis of food type showed that Black Wood Pigeons consumed, in order of frequency, drupes (68.62%, N = 664), weed seeds (20.51%, N = 202), nuts (10.46%, N = 95), and cereals (0.41%, N = 4) from March 2016 to February 2017; and drupes (60.38%, N = 481), nuts (22.21%, N = 177), weed seeds (16.81%, N = 134), and cereals (0.63%, N = 5) from March 2017 to February 2018 (; ). The occurrence of eating drupes was very high (64.90%) throughout the survey period. Nuts, cereals, and weed seeds were mainly consumed in October to February, while leaf-eating was mainly limited to April to May.
Figure 3. Monthly trends in Black Wood Pigeon dietary composition (percentage) by food type (drupe, nut, leaf, cereal, weed seed) from March 2016 to February 2017 (a) and from March 2017 to February 2018 (b). The frequency of food type of Black Wood Pigeons was slightly different according to the survey period, but drupe showed the highest frequency.
Food preferences
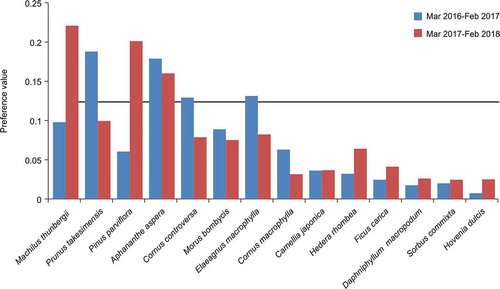
Analysis of the plant preferences revealed that food items from six species were clearly preferred: M. thunbergii, P. takesimensis, P. parviflora, A. aspera, C. controversa, and E. macrophylla (). Specifically, A. aspera was identified as a preferred species in both years. Four of these species, excluding C. controversa and E. macrophylla, were strongly preferred according to food availability ().
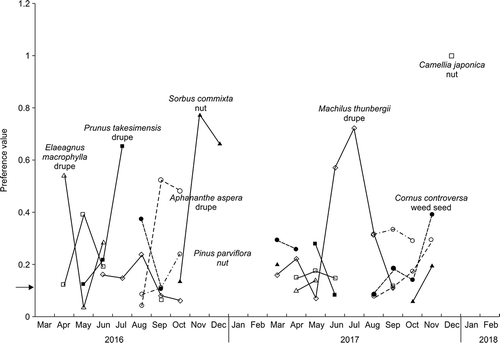
Figure 4. Preference values (Manly-Chesson Index) for twelve foods eaten by Black Wood Pigeons from March 2016 to February 2018. The solid line at 0.125 represents random preference; values above this line reflect a positive preference, while those below reflect a negative preference based on a random encounter rate.
The comparison of the preference values of the top eight species revealed that the preference changed dynamically by month (). Machilus thunbergii was preferred in June and July 2017, but P. takesimensis was more preferred in June and July 2016 (). In particular, P. takesimensis was a highly preferred dietary choice in June 2016, but during the same period in 2017, very low preference was shown for this species. This result is similar to the abundance score ( and ). Elaeagnus macrophylla showed high preference scores in March and April when food items were scarce. Camellia japonica and S. commixta were never preferred () but were more favored in November and December (winter) when other food items were scarce (). Monthly changes in food preferences were found to vary with the abundance and availability of food items ( and ).
Figure 5. Monthly preference values (Manly–Chesson Index) for eight food items in Ulleung Island from March 2016 to February 2018. The arrow indicates random preference and values above the arrow indicate foods preferred by Black Wood Pigeons. Breaks in the line for a species indicate months when that food was not available.
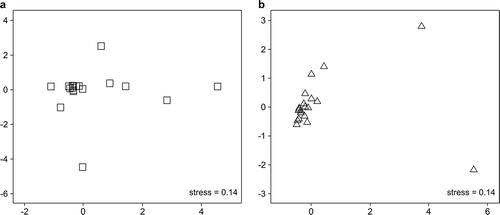
The composition of the available food items for consumption by Black Wood Pigeons was not particularly varied over the total 24-month survey period. However, when the two study periods (each 12 months) were separately compared, the composition of the food items was more varied from March 2016 to February 2017 ().
Figure 6. The results of the similarity of food items analysis with nonmetric multidimensional scaling: March 2016–February 2017 (a) and March 2017–February 2018 (b). Each symbol means a food item, and overlapping indicates a low availability due to the high similarity of food items during the period.
Population variation and relationship with food items
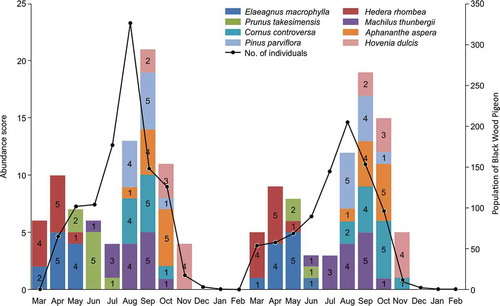
We analyzed the correlation between the population change of monthly Black Wood Pigeon by Choi et al. (Citation2019a) and food items. Population differences were most severe in August when juvenile individuals were observed after breeding season (). To investigate the effect of food availability on the Black Wood Pigeon population, we analyzed the correlation between the abundance scores of the top 10 species identified as supplying important food items and the monthly population size. The food items that were found to be statistically significant were M. thunbergii (β = 0.501, t = 9.428, P = 0.001), P. takesimensis (β = 0.365, t = 8.621, P = 0.001), and A. aspera (β = 0.354, t = 7.915, P = 0.001). In particular, P. takesimensis showed significant differences in abundance scores between 2016 and 2017 which corresponded with the observed changes in population size.
Figure 7. Relationship between plant abundance score and the population size of Black Wood Pigeons in Ulleung Island, South Korea. In 2016, when the abundance of P. takesimensis (green color) was high, the total population was large number in August, but the total population was small number in August 2017 when the abundance of P. takesimensis was also low.
Discussion
Phenology
The food items of the Black Wood Pigeon were found in the highest abundance (abundance score = 4–5) in autumn. On Ulleung Island there are many species of plants some of which, such as M. thunbergii, P. parviflora, E. macrophylla, and H. rhombea, only produce ripe fruit the year following the flower blooms. This delayed ripening helps the survival of the Black Wood Pigeon because it can eat the fruit from the previous year if the flowering rate of the current season is lowered, e.g., of bad weather conditions (Choi et al. Citation2019a). In addition, plants such as the E. macrophylla and H. rhombea, which are abundant in spring, can be an important source of food for the accumulation of the large amount of energy needed prior to breeding (National Institute of Biological Resources Citation2011). The amount and composition of fruit can considerably with season and year (Clout Citation1990; Powlesland et al. Citation2003), and this is likely to affect the dietary ecology and reproduction of the Black Wood Pigeon. Depending on the area, the breeding season of Black Wood Pigeons in South Korea can start in May and continue until August. These breeding season differences are related to the availability of important food items during the breeding season and in the case of Ulleung Island are thought to vary according to the abundance of food items on breeding season. Therefore, the abundance of H. rhombea and E. macrophylla (key food-bearing species in spring) and the abundance of P. takesimensis (a key food-bearing species in summer) are considered to be very important for population maintenance. In addition, Black Wood Pigeon of Ulleung Island is known to migrate to Japan during autumn (Choi et al. Citation2019b). The reason why they migrate to Japan is the availability of food is reduced due to the heavy snow in winter.
Monthly variation in diet composition and food preference
Endemic species that inhabit islands, such as Black Wood Pigeons, are highly vulnerable to environmental changes and ecological disturbances because of narrow habitats, small populations, and lower genetic diversity than mainland-dwelling species (Frankham Citation1997, Citation1998). We assessed the composition and monthly variation in diet and changes in food preferences of the Black Wood Pigeon and found that the composition and diversity of the food items varied according to season and the availability of food. Analysis of the types of food items selected by Black Wood Pigeons indicated that they favored the fast-digesting and energy-efficient drupe all year round and drupe eating frequency was relatively high during the breeding season. The frequency of eating nuts, weed seeds, and cereals increased in the winter season. This may be due to there being few to no food items hanging on the trees, forcing the pigeons to eat food that has fallen to the ground. The difference in the composition of the monthly food items seems to be due to differences in the food plant species that may be available according to the effects of climate, i.e., based on the seasonal phenology of the plants. Food preferences may also vary depending on the nutrient content of the food items. In a study by Ando et al. (Citation2016), a high preference for lipid-rich fruits was shown, but when the preferred food items were lacking at certain times, food preference changed according to the availability of food items; a trend similar to that seen in Black Wood Pigeons on Ulleung Island.
The results of the Manly-Chesson Index showed that the food preferences differed each season. Cereals and nuts showed a very low frequency in the selected food items and were classified as low use food items, similar to those in other studies (Collinge Citation1913; Murton, Westwood, and Isaacson Citation1964; Jiménez, Hódar, and Camacho Citation1994). However, in the monthly food preference analysis, cereals, and nuts were important food types because of their high availability in the winter when food is scarce (Gea-Izquierdo, Cañellas, and Montero Citation2008). Thus, the drupe of M. thunbergii and C. japonica can be eaten during high abundance periods and the remaining nuts with a diameter of 1 cm or more in the winter season can be used all the year round, making them a very important food item. In this study, important seasonal food items of the Black Wood Pigeon were identified as H. rhombea and E. macrophylla in spring, P. takesimensis, and P. parviflora in summer, M. thunbergii and C. controversa in autumn, and H. dulcis and C. japonica in winter. The annual diversity of the food items was not high but the composition differed between years; a result that is similar to that seen in a Japanese study of two different islands (Ando et al. Citation2016). The results of this study suggest that there may be a significant difference in food availability that is dependent on the abundance of plants and based on their phenology, and suggests that the food items available to Black Wood Pigeons are not very diverse on Ulleung Island.
Population variation and relationship with food items
The Black Wood Pigeon breeding season is from May to August on Ulleung Island and at this time the availability of food items is an important factor in the success of reproduction. The food items most likely to affect population fluctuations were found to be M. thunbergii, P. takesimensis, and A. aspera. Machilus thunbergii and A. aspera showed very low variation in abundance during the study, and can be expected to positively affect the population in terms of food availability. However, P. takesimensis offers a relatively low food availability because of a short period of high abundance. P. takesimensis food items are available after May and are fully mature in June, when they reach their highest abundance. In August 2016, the total pigeon population was 326 individuals were large number observed, and the abundance score of P. takesimensis in June of that year was also high. However, in 2017, when the abundance score of P. takesimensis in June was very low, the total pigeon population was 205 individuals in August and fell sharply compared with that in 2016. In general, the abundance of food has the greatest impact on the success rate of reproduction in birds (Martin Citation1987; Brinkhof and Cavé Citation1997). In this study, 33 food-bearing plants species were identified out of the 494 species of flora recorded on Ulleung Island (Yang et al. Citation2015). This low food diversity and a lack of food items could negatively affect the maintenance of the population of Black Wood Pigeons on Ulleung Island.
We confirmed the previously poorly known changes in the monthly dietary composition and food preferences of the Black Wood Pigeon. As Black Wood Pigeons inhabit islands with limited environmental resources, it is suggested that the availability and diversity of food items are crucial to survival and population variability.
Conclusions
This study analyzed the dietary composition, seasonal variation in food availability, and food preferences of the Black Wood Pigeon using field observations. Important food items that were thought to affect population retention were identified. Black Wood Pigeons inhabiting islands with limited environmental resources were not found to have highly diverse diets, and the variation in the diet was partly reflected the seasonal phenology of the plants and the annual differences in food availability. These phenomena may appear to have a significant impact on reproduction and population fluctuations. The interrelationships between inhabiting species are very important to preserve the biodiversity of islands. It is essential for the conservation of the Black Wood Pigeon to study the relationship between the vegetative state of introduced and native species, as well as the environmental changes due to the direct development of the island, which is now frequently used as a tourist destination. In this study, we have shown a link between the diet and population variation of the Black Wood Pigeon. Thus, the conservation of important food plants that satisfy the nutritional and seasonal requirements, as well as the preservation of landscapes that containing patches of various habitat types are necessary for the long-term conservation of Black Wood Pigeon populations.
Acknowledgments
We thank the National Institute of Ecology and the Ulleung County Office for their cooperation. We also thank Prof. H. W. Park, J. M. Park, and C. Y. Choi for valuable advice on this study. This study was included by part of the 5th National Ecosystem Research Project of National Institute of Ecology (NIE-A-2020-01).
Disclosure statement
The authors declare that they have no competing interests. This study did not receive any funding.
References
- Ando, H., S. Setsuko, K. Horikoshi, H. Suzuki, S. Umehara, M. Yamasaki, G. Hanya, M. Inoue-Murayama, and Y. Isagi. 2016. “Seasonal and Inter-island Variation in the Foraging Strategy of the Critically Endangered Red-headed Wood Pigeon Columba Janthina Nitens in Disturbed Island Habitats Derived from High-throughput Sequencing.” Ibis 158: 291–10. doi:10.1111/ibi.12345.
- Austin, J. E., M. A. Hayes, and J. A. Barzen. 2019. “Revisiting the Historic Distribution and Habitats of the Whooping Crane.” In The Biology and Conservation of the Whooping Crane, edited by J. E. Austin, M. A. Hayes, and J. A. Barzen, 25–45. Philadelphia: Elsevier.
- Baldacchino, G., and D. Niles. 2011. Island Futures: Conservation and Development across the Asia-Pacific Region, 183. Tokyo: Springer.
- Barzen, J. A., T. Thousand, J. Welch, M. Fitzpatrick, and T. Tran. 2018. “Determining the Diet of Whooping Cranes (Grus Americana) through Field Measurements.” Waterbirds 41: 22–34. doi:10.1675/063.041.0104.
- Brazil, M. 2009. The Birds of East Asia: China, Taiwan, Korea, Japan and Russia, 528. Princeton: Princeton University Press.
- Brinkhof, M. W., and A. J. Cavé 1997. “Food Supply and Seasonal Variation in Breeding Success: An Experiment in the European Coot.” Proceedings of the Royal Society of London Series B: Biological Sciences 264: 291–296.
- Choi, S. K., S. G. Kang, U. N. Park, and H. W. Park. 2017. “A Study on Morphological Diversity and Migration of Pernis Ptilorhynchus Using A Field Photographic Method.” Journal of Korean Island. 29: 213–223. doi:10.26840/JKI.29.4.213.
- Choi, S. K., S. W. Park, J. W. Kim, and Y. C. Park. 2019a. “Seasonal Distribution and Habitat Selection Characteristics of Japanese Wood Pigeon Columba Janthina in Ulleungdo Island.” Journal of Agriculture and Life Sciences 53: 99–111. doi:10.14397/jals.2019.53.3.99.
- Choi, S. K., Y. C. Park, J. C. Park, G. C. Bing, and W. Y. Kim. 2019b. “Migration by the Japanese Wood Pigeon (Columba Janthina) across the Islands of East Asia - Direct Tracking by Satellite Telemetry.” Pacific Science 73: 225–230. doi:10.2984/73.2.4.
- Clout, M. N. 1990. “The Kereru and Its Forests.” Birds International 2: 10–19.
- Collinge, W. E. 1913. The Food of Some British Wild Birds. A Study in Economic Ornithology, 109. London: Dulau & .
- Crome, F. H. J. 1975. “Breeding, Feeding and Status of the Torres Strait Pigeon at Low Isles, North-Eastern Queensland.” Emu 75: 189–198. doi:10.1080/01584197.1975.11797865.
- Emeny, M. T., R. G. Powlesland, I. M. Henderson, and R. A. Fordham. 2009. “Feeding Ecology of Kererū (Hemiphaga Novaeseelandiae) in Podocarp–hardwood Forest, Whirinaki Forest Park, New Zealand.” New Zealand Journal of Ecology 33: 114–124.
- Frankham, R. 1997. “Do Island Populations Have Lower Genetic Variation than Mainland Populations?” Heredity 78: 311–327. doi:10.1038/hdy.1997.46.
- Frankham, R. 1998. “Inbreeding and Extinction: Island Populations.” Conservation Biology 12: 665–675. doi:10.1046/j.1523-1739.1998.96456.x.
- Frith, H. J., F. H. J. Crome, and T. O. Wolfe. 1976. “Food of Fruit Pigeons in New Guinea.” Emu 76: 49–58. doi:10.1071/MU9760049.
- Gea-Izquierdo, G., I. Cañellas, and G. Montero. 2008. “Acorn Production in Spanish Holm Oak Woodlands.” Forest Systems 15: 339–354.
- Greer, D. M. 2010. “Blue Crab Population Ecology and Use by Foraging Whooping Cranes on the Texas Gulf Coast.” Doctoral dissertation, Texas A&M University. Accessed 14 May 2019. http://oaktrust.library.tamu.edu/handle/1969.1/ETD-TAMU-2010-12-8939
- Gregory, R. D., S. R. Baillie, and R. I. Bashford. 2004. “Monitoring Breeding Birds in the United Kingdom.” Bird Census News 13: 101–112.
- Hanspach, J., J. Loos, I. Dorresteijn, H. von Wehrden, C. I. Moga, and A. David. 2015. “Functional Diversity and Trait Composition of Butterfly and Bird Communities in Farmlands of Central Romania.” Ecosystem Health and Sustainability 1: 1–8. doi:10.1890/EHS15-0027.1.
- Howe, R. W., G. J. Niemi, G. J. Lewis, and D. A. Welsh. 1997. “A Standard Method for Monitoring Songbird Populations in the Great Lakes Region.” Passenger Pigeon 59: 182–194.
- Huff, M. H., K. A. Bettinger, H. L. Ferguson, M. J. Brown, and B. Altman 2000. “A Habitat-based Point-count Protocol for Terrestrial Birds, Emphasizing Washington and Oregon.” Forest Service General Technical Report PNW-GTR-501. United States Department of Agriculture.
- Inglis, I. R., A. J. Isaacson, R. J. P. Thearle, and N. J. Westwood. 1990. “The Effects of Changing Agricultural Practice upon Woodpigeon Columba Palumbus.” Ibis 132: 262–272. doi:10.1111/j.1474-919X.1990.tb01044.x.
- IUCN. IUCN red list of threatened species. 2017. “The IUCN Red List.” International Union for Conservation of Nature. Accessed 14 March 2018. https://www.iucn.org/resources/conservation-tools/iucn-red-list-threatened-species
- Jiménez, R., J. A. Hódar, and I. Camacho. 1994. “Diet of the Woodpigeon (Columba Alumbus) in the South of Spain during Late Summer.” Folia Zoological 43: 163–170.
- Lack, D. 1968. Ecological Adaptations for Breeding in Birds, 409. London: Chapman and Hall.
- Luniak, M. 2004. “Synurbization-adaptation of Animal Wildlife to Urban Development.” W. W. Shaw, L. K. Harris, and L. Vandruff, editors. Proceedings of the 4th International Urban Wildlife Symposium, 50–55. Tucson: University of Arizona.
- Manly, B. F. J., L. L. McDonald, and D. L. Thomas. 1993. Resource Selection by Animals: Statistical Design and Analysis for Field Studies, 222. 1st ed. New York: Chapman and Hall.
- Martin, P. R., and P. P. G. Bateson. 1993. Measuring Behaviour: An Introductory Guide, 186. 3rd ed. Cambridge: Cambridge University Press.
- Martin, T. E. 1987. “Food as a Limit on Breeding Birds: A Life-history Perspective.” Annual Review of Ecology Systematics 18: 453–487. doi:10.1146/annurev.es.18.110187.002321.
- McConkey, K. R., H. J. Meehan, and D. R. Drake. 2005. “Seed Dispersal by Pacific Pigeons (Ducula Pacifica) in Tonga, Western Polynesia.” Emu 104: 369–376. doi:10.1071/MU03060.
- Murton, R. K., N. J. Westwood, and A. J. Isaacson. 1964. “The Feeding Habits of the Woodpigeon Columba Palumbus, Stock Dove C. Oenas and Turtle Dove Streptopelia Turtur.” Ibis 106: 174–188. doi:10.1111/j.1474-919X.1964.tb03694.x.
- National Institute of Biological Resources. 2011. Red Data Book of Endangered Birds in Korea, 272. Seoul: National Institute of Biological Resources.
- Newton, I. 1980. “The Role of Food in Limiting Bird Numbers.” Ardea 68: 11–30.
- Oliveira, P., P. Marrero, and M. Nogales. 2002. “Diet of the Endemic Madeira Laurel Pigeon and Fruit Items Availability: A Study Using Microhistological Analyses.” Condor 104: 811–822. doi:10.1093/condor/104.4.811.
- Powlesland, R. G., P. J. Dilks, I. A. Flux, A. D. Grant, and C. J. Tisdall. 1997. “Impact of Food Abundance, Diet and Food Quality on the Breeding of the Fruit Pigeon, Parea Hemiphaga Novaeseelandiae Chathamensis, on Chatham Island, New Zealand.” Ibis 139: 353–365. doi:10.1111/j.1474-919X.1997.tb04634.x.
- Powlesland, R. G., D. E. Wills, A. C. L. August, and C. K. August. 2003. “Effects of a 1080 Operation on Kaka and Kereru Survival and Nesting Success, Whirinaki Forest Park.” New Zealand Journal of Ecology 27: 125–137.
- Rosenberg, K. V., and R. J. Cooper. 1990. “Approaches to Avian Diet Analysis.” Studies in Avian Biology 13: 80–89.
- Seki, S.-I. 2005. “The Molecular Phylogeny of the Three Subspecies of Japanese Wood Pigeon, Columba Janthina, Inferred from the Mitochondrial Cytochrome B Sequences.” Kyushu Joural For Research 58: 193–194.
- Vieira, B. P., R. W. Furness, and R. G. Nager. 2016. “Using Field Photography to Study Avian Moult.” Ibis 159: 443–448. doi:10.1111/ibi.12445.
- Walker, J. S. 2007. “Geographical Patterns of Threat among Pigeons and Doves (Columbidae).” Oryx 41: 289–299. doi:10.1017/S0030605307001016.
- Williams, P. A., and B. J. Karl. 1996. “Fleshy Fruits of Indigenous and Adventive Plants in the Diet of Birds in Forest Remnants, Nelson.” New Zealand Journal of Ecology 20: 127–145.
- Yang, S. G., H. D. Jang, B. M. Nam, G. Y. Chung, R. Y. Lee, J. H. Lee, and B. U. Oh. 2015. “A Floristic Study of Ulleungdo Island in Korea.” Sigmul Bunryu Hag-hoeji 45: 192–212.