ABSTRACT
Degradation of meadow ecosystems in the largest alpine region of the world, i.e., the Qinghai-Tibetan Plateau (QTP), is a crucial ecological issue that has ardently discussed in recent years. Many factors, such as livestock overgrazing, climate change and overpopulation of small mammals are treated as important factors that cause the degradation of meadow ecosystems in the QTP. However, there are few hypotheses focus on the potential role of plant compensatory growth on meadow degradation. We proposed a compensatory growth-related hypothesis to understand the potential degradation process of meadow ecosystems in the QTP. We discussed that there are two stages of meadow degradation, i.e. the beginning stage of meadow degradation that is triggered by high-strength overcompensation; and the intensification stage of meadow degradation, which are driven by external factors such as climate warming, small mammals and thawing of permafrost.The mechanism of meadow degradation driven by plant compensatory growth is the asynchronism of plant consumption and the availability of soil nutrients. Our hypothesis that plant compensatory growth drives meadow degradation under the overgrazing condition requires re-examination and modification by testing the balance between soil nutrient cycling rates and the strength of plant compensatory growth in alpine regions.
The QTP, also called the third pole, with an area of nearly 2.5 × 106 km2, is the largest alpine region on the Earth. It has generally been reported that the alpine meadow ecosystems are suffering serious degradation in the QTP (Qiu Citation2016; Yao et al. Citation2012). There are numerous studies describing the performances of meadow degradation, such as the reduction of plant cover (Feng et al. Citation2009), decrease in plant reproductive success (Dong et al. Citation2015), shift in plant community structure (Tang et al. Citation2015), and altered ecosystem functions (Wang et al. Citation2015). The degradation of meadow ecosystems is primarily theorized to be the consequence of climate change and long-term livestock overgrazing (Dong et al. Citation2013; Harris Citation2010; Lehnert et al. Citation2016; Wang et al. Citation2016; Zhou et al. Citation2005). It has been shown that repeated heavy grazing can inhibit plant recovery from damage and cause the degradation of the meadow ecosystem (Loeser, Crews, and Sisk Citation2004). However, an important issue remains unanswered: how does plant–soil interaction trigger meadow degradation under the overgrazing condition? The clarification of this issue is helpful to examine the strategies of plants in response to herbivory. The compensatory growth, defined as positive responses of plants to damage (Belsky Citation1986), is an important strategy of plants in response to herbivory (Agrawal Citation2000; Paige and Whitham Citation1987). There are three types of compensatory growth, i.e., under-compensation, no-compensation, and over-compensation (Belsky Citation1986). We mean compensatory growth as over-compensation in this essay. It suggests that plants tend to overcompensate more frequently under unfavorable growth conditions to survive (Hawkes and Sullivan Citation2001). An appropriate compensatory growth is of benefit to maintain or even improve the efficiency of a livestock grazing system (Grime Citation1973; Hilbert et al. Citation1981; McNaughton Citation1983). The interaction between plant compensatory growth and herbivory could be stable if grazing intensity is suitable (Agrawal Citation2000; Sun et al. Citation2019); however, this balance would be disrupted under overgrazing conditions. Overgrazing is easier to happen in the present than the historical period as the increase of livestock number and the shift of grazing regimes, which changed from a nomadic one to an immobilization one, in the QTP (Cao et al. Citation2013; Sun et al. Citation2014). Therefore, we hypothesized a possible process of meadow degradation in the QTP: high-strength compensatory growth under overgrazing in the alpine meadow drives the degradation of the entire ecosystem. To illustrate this hypothesis, several questions have to be considered and addressed:
Does the plant compensatory growth occur in alpine meadow ecosystems under grazing conditions?
How plant compensatory growth drives the degradation of meadow ecosystems in alpine regions? What does the role of other external factors (especially climate warming) play during the process of meadow degradation?
What is the mechanism of the degradation process driven by plant compensatory growth in alpine regions?
Does the plant compensatory growth occur in alpine meadow ecosystems?
Compensatory growth phenomena
Plant compensatory growth usually happens after tissue reduction caused by herbivory (McNaughton Citation1983). Plant functional types (e.g., growth form) and the resources level of the habitat (e.g., light, nutrients, and water) strongly determine the compensatory growth of plant community (Li et al. Citation2011a; Wang and Wesche Citation2016; Wise and Abrahamson Citation2005). The compensatory growth of the same plant functional type varies among different resource levels (Hawkes and Sullivan Citation2001). More details about the mechanism of the compensatory growth could find in previous reviews by McNaughton (Citation1983), Tiffin (Citation2000), etc. We, here, focus on the general conditions on the appearance of compensatory growth and discuss whether the compensatory growth is detected by experimental studies in alpine regions. Generally, the compensatory growth occurs under special conditions, e.g., resource-rich environment (high light, nutrients, and water levels), and low levels of competition (Agrawal Citation2000; Kinsinger and Hopkins Citation1961). Experimental studies suggested that in tropical or temperate grassland ecosystems that are dominated by C4 grasses, there is little evidence for plant compensatory growth (Knapp et al. Citation2012). However, plant compensatory growth under livestock grazing was observed in the Eurasian steppe ecosystems that are dominated by C3 grasses when the growing season was wet (Schönbach et al. Citation2011). In alpine and subalpine regions where are cold and wet, such as the QTP where alpine meadow ecosystems occupy over 4.5 × 105 km2 of the area (Miehe Citation2008), both clipping (Klein, Harte, and Zhao Citation2007) and livestock grazing (Zhu et al. Citation2010; Li et al. Citation2020a) caused plant compensatory growth. A meta-analysis suggests that plant compensatory growth, which is a primary mechanism that causes the enhancement of belowground biomass, is generally detected under moderate grazing intensity on the QTP (Sun et al. Citation2019). In addition, plant compensatory growth has been observed after both clipping and livestock grazing in grassland ecosystems in subalpine regions outside the QTP (Loeser, Crews, and Sisk Citation2004). These results give evidence for the phenomena of plant compensatory growth in alpine and subalpine regions.
Compensatory growth foundation
In alpine regions, livestock grazing could promote the root turnover rate under moderate stocking rates (Pucheta et al. Citation2004). Moreover, fine roots are the primary structure through which plants take up water and nutrients (Bardgett, Denton, and Cook Citation1999). Therefore, the increase in root turnover rate and fine root growth is an enhancement strategy for plant structure, which complies with the definition of compensatory growth. In addition, removal of plant aboveground biomass to some extent, e.g., clipping, could stimulate soil microbial biomass in upland grasslands (Mawdsley and Bardgett Citation1997). The increase in soil microbial biomass could accelerate soil nutrient cycling (Bardgett Citation2005), producing sufficient available nutrients for enhanced plant growth. Moreover, the growing season is about four months (i.e., from May to August) in alpine regions of the QTP because the precipitation is concentrated from May to September (Kato et al. Citation2004), while there is a considerably long time for the mineralization of soil nutrients underground, which could support the compensatory growth of plants.
Therefore, it is clear that plant compensatory growth generally happens in alpine meadow ecosystems in alpine regions such as the QTP.
How does plant compensatory growth drive degradation of meadow ecosystems?
The process of meadow degradation under heavy grazing condition could divide into two stages. The first stage is the beginning of meadow degradation when high-strength compensatory growth undermines the health of meadow ecosystems by causing the loss of nutrients, the reduction of root biomass, and the shortage of available nutrients. The second stage is the intensification of meadow degradation when the health of meadow ecosystems are strongly deteriorated by external factors, such as rainfall, thawing of permafrost, climate warming, and small mammals.
The beginning of meadow degradation
The degradation of a meadow ecosystem will occur when plants could not survive. The survival and growth of plants is largely based on a good ability of root in nutrients absorbing and an adequate supply of soil nutrients. Livestock browses a mass of aboveground biomass and promotes plant compensatory growth, which would undermine the health of meadow ecosystems if grazing intensity was strong (or compensatory growth intensity was strong) (). There are at least three primary processes contribute to the beginning of meadow degradation:
Figure 1. The process of meadow degradation driven by compensatory growth. Two primary processes cause the nonviability of plants and the entire degradation of alpine meadow ecosystems: (1) Livestock grazing stimulates excessive plant compensatory growth and undermines the health of meadow ecosystems. Under an excessive compensatory growth, livestock would take a lot of plant biomass, and fewer photosynthesis products accumulate into the plant root. The reducing of root not only weaken the ability of plant in nutrients absorbing but makes soil erodible and affects the process of matter decomposition in soil, decreasing the supply of soil available nutrients. Consequently, the alpine plants could not survive. (2) External factors (such as climate change and break out of small mammals) accelerate the degradation of meadow ecosystems after the reduction of root biomass and the appearance of bare ground.
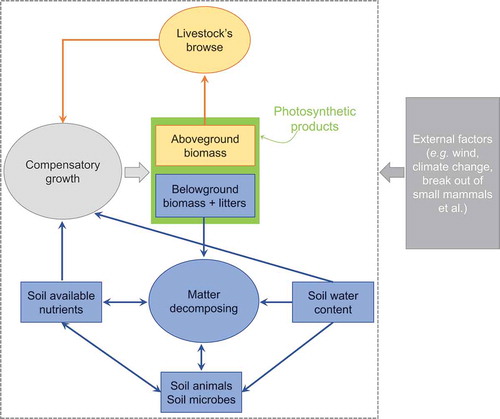
Firstly, a mass of nutrients lose from meadow ecosystems. Livestock could ingest more biomass if there is an attendance of compensatory growth when compared with an absence one. Correspondingly, a lot of soil nutrients and photosynthesis products accumulate into livestock products (e.g., meat and milk) via livestock grazing under the condition of compensatory growth. Most of these livestock products do not return to meadow ecosystems. They are consumed by anthropic economic activities. The return of livestock dung could enhance soil nitrogen supply for the growth of meadow plants in the QTP (Cheng, Cai, and Wang Citation2016). However, as the main living fuel in the pastoral area in the QTP, most of the dung of livestock is collected and burned by local pastoralists, which is beneficial to the regeneration of plants by reducing the amount of dung patches but reduces the return of nutrients to soil at the same time. The processes mentioned above would cause a drastic loss of nutrients from soil, which will negatively affect the sustainability of meadow ecosystems in the long term.
Secondly, the ability of plants in nutrient absorbing would decrease and soil becomes erodible because of the reduction of root biomass. Compensatory growth could lead to the reallocation of products of photosynthesis, resulting in fewer photosynthetic products into the roots. There is a prominent hypothesis that the compensatory growth of aboveground biomass may come at the expense of belowground biomass (Kinsinger and Hopkins Citation1961; Overbeek Citation1966). In fact, evidence from both simulated grazing (i.e., clipping) and mammalian grazing studies suggest that long-term livestock grazing could reduce root biomass (Biondini, Patton, and Nvren Citation1998; Dawson, Grayston, and Paterson Citation2000; Jameson Citation1963). We get some direct evidence to illustrate the process of photosynthetic relocation between shoot biomass and root biomass in a rotational grazing experiment conducted in the QTP, which grazed by yaks with a high stocking rate. In our experiment, the aboveground biomass showed an over compensatory growth ()). Moreover, the aboveground biomass showed an increasing trend but the belowground biomass showed a decreasing trend from 2014 to 2016 (). A mowing experiment conducted in Canada obtained the same results, i.e., the aboveground biomass under mowing treatment trends to be higher than that under control treatment while the belowground biomass showed an opposite pattern (Zhang et al. Citation2018). In a healthy alpine meadow of the QTP, over 95% of plant roots concentrate in 0 ~ 30 cm belowground (Yang et al. Citation2009). The surface 30 cm range is generally called “sod layer,” which plays crucial roles in the conservation of water and soil in QTP (Shang et al. Citation2008). The reduction of root biomass (especially the reduction of living roots) weakens the primary pathway through which plants uptake water and nutrients. Meanwhile, this reduction provides preconditions for eventual soil erosion and the exposure of bare ground.
Figure 2. Aboveground net primary productivity (ANPP) (a), belowground net primary productivity (BNPP) (b) and the contents of available nitrogen (c, d) and available phosphorous (AP) (e) of an alpine meadow ecosystem under a heavy rotational grazing management in the QTP. 2014BG: before grazing in 2014, 2014AG: after grazing in 2014. CK: no-grazing treatment. Cited from Zhang (Citation2016a). The detailed methodology of this field experiment could find in Zhang (Citation2016a). Here, we briefly introduce the methodology of cited results. The grazing intensity was high, i.e. four adult yaks per hm2, in this rotational grazing experiment. The ANPP is estimated by the aboveground weight of air-drying plant tissues that clipped from 1 m × 1 m quadrats. The BNPP is estimated by the belowground weight of air-drying plant tissues that collected from soil cores with a scale of 3.14 × 6.25 cm2 × 15 cm. The contents of soil available nutrients are measured by a flow injection auto-analyzer (AACE, Germany). One-way ANOVA is used to test the variance of parameters between CK and Rotational grazing (the significant level is α = 0.05).
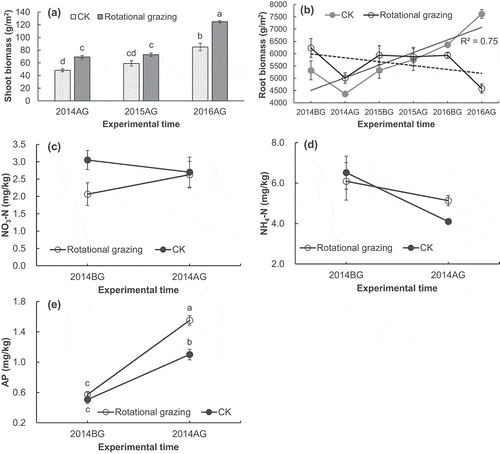
Thirdly, plant growth could be restricted because of the shortage of available nutrients caused by long-term compensatory growth. Soil fauna and microbes, which have mutualistic relationships with plant roots, play irreplaceable roles in soil nutrient transfer and root growth in grassland ecosystems (Bardgett, Denton, and Cook Citation1999). Under an excessive compensatory growth, the decrease of plant roots would change the species richness and community structure of these mutualisms. Moreover, fewer plant litters introduce into soil because of the ingestion of livestock, which could make these soil dwellers hard to survive (Bardgett, Denton, and Cook Citation1999). In addition, low-temperature can restrict the decomposition rate of matter in alpine meadow ecosystems (Xu et al. Citation2013; Li et al. Citation2020b). Consequently, the supply of soil available nutrients would become unsustainable.
The intensification of meadow degradation
Fewer photosynthetic products allocate into belowground (i.e., the reduction of plant roots) under an excessive compensatory growth would make the soil become easier to be eroded by external forces in alpine regions. High root: shoot ratio is typical for a healthy alpine meadow ecosystem (Yang et al. Citation2009). The fixation of vast plant roots is benefits to soil physical structure to resist livestock’s trampling. Soil physical structure will be easily destroyed by livestock’s trampling as the loss of plant roots. Permafrost generally exists in the QTP, but it is melting due to the climate change (Yang et al. Citation2010). It reported that the loss of permafrost could change alpine meadows into steppes (Jin et al. Citation2009). This suggests that erosion caused by external factors (e.g., rainfall and thawing of permafrost) will speed up the degradation process. Moreover, if the plant cover is removed (i.e., barren patches are formed) the variation of radiation among patches would enlarge the scale of bare ground in the QTP (Wei, Lin, and Wang Citation2015).
Climate change could promote the process of plant compensatory growth. The climatic condition tends to be warm and wet in the central of QTP (Ganjurjav et al. Citation2018). Field experiments with open top chambers (OTCs) in the QTP found that warming could change soil physical (especially surface temperature and moisture) and chemical conditions, shift the composition of soil microbial communities (Ganjurjav et al. Citation2018; Zhang et al. Citation2016b) and accelerate the turnover rate of nitrogen (Wang et al. Citation2014). These warming-induced changes could increase the access of soil available nutrients, which improved plant biomass in a short-term period (Ganjurjav et al. Citation2016). The enhancement of plant compensatory growth may speed up the process of meadow degradation if soil available nutrients could not maintain under a warmer and wetter climate condition.
Some small mammals, mainly plateau pika (Ochotona curzoniae), are keystone species in the QTP (Smith and Foggin Citation1999). Activities of these mammals could promote plant growth and maintain hydrological functioning in this region (Wilson and Smith Citation2015; Zhang et al. Citation2016c). However, these mammals can overpopulate in degraded meadow ecosystems and aggravate grassland degradation. In fact, it might be the change in soil surface temperature and soil physical structure caused an increase in pika populations (Li et al. Citation2011b). There are more cracks in degraded areas because of the reduction in plant roots and soil moisture. These cracks make it easier for pikas to burrow and create a drier dwelling (Wang, Zhang, and Wang Citation2006).
The mechanism of meadow degradation driven by plant over compensatory growth
Each biological process has a specific reaction time in an ecosystem. The entire efficiency of an ecosystem is determined by the slowest process, which means that any external factor that forces part of the ecosystem to operate faster than the overall rate will lead to an imbalance (Commoner Citation1971). The maintenance of nutrient’s cycling is the most mentioned issue when the grazing optimization is discussed (De Mazancourt, Loreau, and Abbadie Citation1998). We proposed that the mechanism of meadow degradation driven by plant compensatory growth was the asynchronism of the rate of plant consumption and the availability of soil nutrients. In other words, the nutritional requirement of plant growth is greater than the supply of soil available nutrients in a meadow ecosystem. This asynchronism between plant consumption and soil available nutrient is an endogenous driver that causes the degradation of meadow ecosystems. There is a rebalancing process between plant consumption and the availability of soil nutrients during meadow degradation. This rebalancing process can be separated into three stages.
The first stage is the beginning of compensatory growth. Plant consumption increases with the appearance of compensatory growth. At the beginning of plant compensatory growth, which is similar to the condition of moderate grazing, available nutrients in soil may be promoted by an increased mineralization rate (Qiao et al. Citation2012). Our grazing experiment obtained some evidence as well (). Therefore, both plant consumption and the ability of soil nutrients supply are higher than the normal condition ()).
Figure 3. The mechanism of alpine meadow degradation driven by compensatory growth of meadow plants (a). And a process of healthy compensatory growth in alpine meadow ecosystems (b). The reason why the meadow keeps healthy in (b) is that livestock’s grazing ceases before peaking the compensatory growth of plants. Theoretically, the factors that may affect compensatory growth process are assume to same in (a) and (b). Normal: the plants’ consumption of soil available nutrients is lower than the supply of soil available nutrient when there is no livestock grazing. The “normal” situation meets a condition of “resource-rich environment.” Overcompensation: both the strength of plants’ consumption and soil’s nutrient supply are higher than them in “normal” condition when livestock grazing do not stimulate an excessive compensatory growth. Degradation: the degradation of meadows occurs when the strength of plants’ consumption is higher than but the ability of soil nutrient supply is less than them in “normal” condition. The reduce of the ability of soil nutrients supply happens when livestock grazing stimulate an excessive compensatory growth in the “degradation” period. We propose the time scale of this mechanism is grazing intensity-related. According to our rotational grazing experiment, the time scale could be measured by year when grazing intensity is high. It might be measured by decades if the grazing intensity is low.
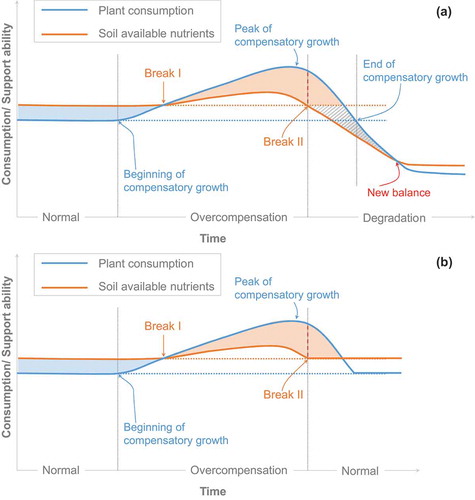
The second stage is the peak of compensatory growth. Plant compensatory growth peaks through the increased supply of soil available nutrients. The peak of plant compensatory growth appears after the peak of the availability of soil nutrients ()). The consumption and supply relationship of the meadow ecosystem might return to its normal condition if livestock grazing is excluded before or near the peak of compensatory growth ()). The process that showed in ) might be the reason why traditional grazing management in the QTP, i.e. the nomadism, was sustainable, while if livestock grazing continues at the peak of compensatory growth, meadow degradation is triggered because the support of soil available nutrients is lower than a normal situation.
The third stage is the degradation of the meadow ecosystem. In this stage, the availability of soil nutrients is lower than it is in a normal condition. Plant consumption continuously decreases until it is less than in the normal condition ()). Then, the grassland ecosystem shifts from a high-level plant–soil balance to a low-level one.
Conclusion and significance
In brief, we discussed a possible hypothesis of meadow degradation in the QTP by demonstrating: 1) high-strength overcompensation is a potential endogenous driver that triggers the degradation of alpine meadow ecosystems under an overgrazing condition in the QTP. 2) Well-known factors, including climate warming, small mammals, and thawing of permafrost are external forces that intensify meadow degradation. And 3) the asynchronism of the rate of plant consumption and the availability of soil nutrients are the fundamental issue of the hypothesis that high-strength compensatory growth drives the degradation of alpine meadow ecosystems. Our hypothesis sheds light on the degradation processes of alpine meadow and would contribute to formulate grassland protection policy for local government.
To test this meadow degradation hypothesis, some issues should be considered in future studies: 1) the reallocation of photosynthetic products between aboveground and belowground and its consequences (including soil fauna, soil microbes and soil nutrient’s cycling, etc.) under the condition of long-term overgrazing. 2) The point at which plant compensatory growth peaks in alpine meadow ecosystems. 3) The ability of the soil system (especially the availability of nutrients) to support the growth of plants at different spatial and temporal scales. 4) The balance between the rate of soil nutrient cycling and the strength of plant compensatory growth under livestock grazing in the QTP. Moreover, the effects of external factors (e.g., climate change) on this balance should be considered.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31901394), the Second Tibetan Plateau Scientific Expedition and Research (2019QZKK0307) and Young Elite Scientists Sponsorship Program by CAST (2019QNRC001). The authors thank the editors and anonymous reviewers for their time and efforts.
Disclosure statement
The authors declare no conflict of interest in relation to the submitted work.
Additional information
Funding
References
- Agrawal, A. A. 2000. “Overcompensation of Plants in Response to Herbivory and the By-product Benefits of Mutualism.” Trends in Plant Science 5: 309–9. doi:10.1016/S1360-1385(00)01679-4.
- Bardgett, R. D. 2005. The Biology of Soil: A Community and Ecosystem Approach. Oxford: Oxford University.
- Bardgett, R. D., C. S. Denton, and R. Cook. 1999. “Below-ground Herbivory Promotes Soil Nutrient Transfer and Root Growth in Grassland.” Ecology Letters 2: 357–360. doi:10.1046/j.1461-0248.1999.00001.x.
- Belsky, A. J. 1986. “Does Herbivory Benefit Plants? A Review of the Evidence.” The American Naturalist 127: 870–892. doi:10.1086/284531.
- Biondini, M. E., B. D. Patton, and P. E. Nvren. 1998. “Grazing Intensity and Ecosystem Processes in a Northern Mixed‐grass Prairie, USA.” Ecological Applications 8: 469–479. doi:10.1890/1051-0761(1998)008[0469:GIAEPI]2.0.CO;2.
- Cao, J. J., E. T. Yeh, N. M. Holden, Y. Y. Yang, and G. Z. Du. 2013. “The Effects of Enclosures and Land-use Contracts on Rangeland Degradation on the Qinghai-Tibetan Plateau.” Journal of Arid Environments 97: 3–8. doi:10.1016/j.jaridenv.2013.05.002.
- Cheng, Y., Y. J. Cai, and S. Q. Wang. 2016. “Yak and Tibetan Sheep Dung Return Enhance Soil N Supply and Retention in Two Alpine Grasslands in the Qinghai-Tibetan Plateau.” Biology and Fertility of Soils 52: 413–422. doi:10.1007/s00374-016-1088-6.
- Commoner, B. 1971. The Closing Circle: Nature, Man, and Technology. New York: Alfred Knopf.
- Dawson, L. A., S. J. Grayston, and E. Paterson. 2000. “Effects of Grazing on the Roots and Rhizosphere of Grasses.” In Grassland Ecophysiology and Grazing Ecology, edited by G. Lemaire, J. Hodgson, A. de Moraes, C. Nabinger, and F. Carvalho, 61–84. Cambridge: CABI Publishing.
- De Mazancourt, C., M. Loreau, and L. Abbadie. 1998. “Grazing Optimization and Nutrient Cycling: When Do Herbivores Enhance Plant Production?” Ecology 79: 2242–2252. doi:10.1890/0012-9658(1998)079[2242:GOANCW]2.0.CO;2.
- Dong, Q. M., X. Q. Zhao, G. L. Wu, J. J. Shi, and G. H. Ren. 2013. “A Review of Formation Mechanism and Restoration Measures of “Black-soil-type” Degraded Grassland in the Qinghai-Tibetan Plateau.” Environmental Earth Sciences 70: 2359–2370. doi:10.1007/s12665-013-2338-7.
- Dong, S. K., X. X. Wang, S. L. Liu, Y. Y. Li, X. K. Su, L. Wen, and L. Zhu. 2015. “Reproductive Responses of Alpine Plants to Grassland Degradation and Artificial Restoration in the Qinghai-Tibetan Plateau.” Grass and Forage Science 70:229–238. doi:10.1111/gfs.12114.
- Feng, Y., Q. Lu, T. Tokola, H. Liu, and X. Wang. 2009. “Assessment of Grassland Degradation in Guinan County, Qinghai Province, China, in the past 30 Years.” Land Degradation & Development 20: 55–68. doi:10.1002/ldr.877.
- Ganjurjav, H., G. Z. Hu, Y. F. Wan, Y. Li, L. B. Danjiu, and Q. Z. Gao. 2018. “Different Responses of Ecosystem Carbon Exchange to Warming in Three Types of Alpine Grassland on the Central Qinghai-Tibetan Plateau.” Ecology and Evolution 8: 1507–1520. doi:10.1002/ece3.3741.
- Ganjurjav, H., Q. Z. Gao, E. S. Gornish, M. W. Schwartz, Y. Liang, X. J. Cao, W. N. Zhang, Y. Zhang, W. H. Li, Y. F. Wan, Y. E. Li, L. B. Danjiu, H. B. Guo, and E. Lin. 2016. “Differential Response of Alpine Steppe and Alpine Meadow to Climate Warming in the Central Qinghai-Tibetan Plateau.” Agricultural and Forest Meteorology 223:233–240. doi:10.1016/j.agrformet.2016.03.017.
- Grime, J. P. 1973. “Competitive Exclusion in Herbaceous Vegetation.” Nature 242: 344–347. doi:10.1038/242344a0.
- Harris, R. B. 2010. “Rangeland Degradation on the Qinghai-Tibetan Plateau: A Review of the Evidence of Its Magnitude and Causes.” Journal of Arid Environments 74: 1–12. doi:10.1016/j.jaridenv.2009.06.014.
- Hawkes, C. V., and J. J. Sullivan. 2001. “The Impact of Herbivory on Plants in Different Resource Conditions: A Meta-analysis.” Ecology 82: 2045–2058. doi:10.1890/0012-9658(2001)082[2045:TIOHOP]2.0.CO;2.
- Hilbert, D. W., D. M. Swift, J. K. Detling, and M. I. Dyer. 1981. “Relative Growth Rates and the Grazing Optimization Hypothesis.” Oecologia 51: 14–18. doi:10.1007/BF00344645.
- Jameson, D. A. 1963. “Responses of Individual Plants to Harvesting.” Botanical Review 29: 532–594.
- Jin, H. J., R. X. He, G. D. Cheng, Q. B. Wu, S. L. Wang, L. Z. Lü, and X. L. Chang. 2009. “Changes in Frozen Ground in the Source Area of the Yellow River on the Qinghai–Tibet Plateau, China, and Their Eco-environmental Impacts.” Environmental Research Letters 4:045206. doi:10.1088/1748-9326/4/4/045206.
- Kato, T., Y. H. Tang, S. Gu, X. Y. Cui, M. Hirota, M. Du, Y. N. Li, X. Q. Zhao, and T. Oikawa. 2004. “Carbon Dioxide Exchange between the Atmosphere and an Alpine Meadow Ecosystem on the Qinghai–Tibetan Plateau, China.” Agricultural and Forest Meteorology 124:121–134. doi:10.1016/j.agrformet.2003.12.008.
- Kinsinger, F. E., and H. H. Hopkins. 1961. “Carbohydrate Content of Underground Parts of Grasses as Affected by Clipping.” Journal of Range Management 14: 9–12. doi:10.2307/3894824.
- Klein, J. A., J. Harte, and X. Q. Zhao. 2007. “Experimental Warming, Not Grazing, Decreases Rangeland Quality on the Tibetan Plateau.” Ecological Applications 17: 541–557. doi:10.1890/05-0685.
- Knapp, A. K., D. L. Hoover, J. M. Blair, G. Buis, D. E. Burkepile, A. Chamberlain, S. L. Collins, R. W. S. Fynn, K. P. Kirkman, M. D. Smith, D. Blake, N. Govender, P. O'Neal, T. Schreck, and A. Zinn. 2012. “A Test of Two Mechanisms Proposed to Optimize Grassland Aboveground Primary Productivity in Response to Grazing.” Journal of Plant Ecology 5:357–365. doi:10.1093/jpe/rts020.
- Lehnert, L. W., K. Wesche, K. Trachte, C. Reudenbach, and J. Bendix. 2016. “Climate Variability Rather than Overstocking Causes Recent Large Scale Cover Changes of Tibetan Pastures.” Scientific Reports 6: 24367. doi:10.1038/srep24367.
- Li, G. Y., Y. Z. Liu, L. E. Frelich, and S. Sun. 2011b. “Experimental Warming Induces Degradation of a Tibetan Alpine Meadow through Trophic Interactions.” Journal of Applied Ecology 48: 659–667. doi:10.1111/j.1365-2664.2011.01965.x.
- Li, J. Z., S. Lin, F. Taube, Q. M. Pan, and K. Dittert. 2011a. “Above and Belowground Net Primary Productivity of Grassland Influenced by Supplemental Water and Nitrogen in Inner Mongolia.” Plant and Soil 340: 253–264. doi:10.1007/s11104-010-0612-y.
- Li, Y., S. K. Dong, Q. Z. Gao, Y. Zhang, S. L. Liu, H. Ganjurjav, G. Z. Hu, X. X. Wang, Y. L. Yan, H. B. Wu, X. X. Gao, S. Li, and J. Zhang. 2020a. “Rotational Grazing Promotes Grassland Aboveground Plant Biomass and Its Temporal Stability under Changing Weather Conditions on the Qinghai-Tibetan Plateau.” Land Degradation & Development. doi:10.1002/ldr.3596.
- Li, Z. L., Z. Q. Zeng, D. S. Tian, J. S. Wang, Z. Fu, F. Y. Zhang, R.Y. Zhang, W. N. Chen, Y. Q. Luo, and S. L. Niu. 2020b. “Global Patterns and Controlling Factors of Soil Nitrification Rate.” Global Change Biology 26 (7): 4147–4157. doi:10.1111/gcb.15119.
- Loeser, M. R., T. E. Crews, and T. D. Sisk. 2004. “Defoliation Increased Above-ground Productivity in a Semi-arid Grassland.” Journal of Range Management 57: 442–447. doi:10.2307/4003972.
- Mawdsley, J. L., and R. R. Bardgett. 1997. “Continuous Defoliation of Perennial Ryegrass (Lolium Perenne) and White Clover (Trifolium Repens) and Associated Changes in the Composition and Activity of the Microbial Population of an Upland Grassland Soil.” Biology and Fertility of Soils 24: 52–58. doi:10.1007/BF01420220.
- McNaughton, S. J. 1983. “Compensatory Plant Growth as a Response to Berbivory.” Oikos 40: 329–336. doi:10.2307/3544305.
- Miehe, G., S. Miehe, K. Kaiser, J. Q. Liu, and X. Q. Zhao. 2008. “Status and Dynamics of the Kobresia Pygmaea Ecosystem on the Tibetan Plateau.” AMBIO: A Journal of the Human Environment 37: 272–279. doi:10.1579/0044-7447(2008)37[272:SADOTK]2.0.CO;2.
- Overbeek, J. V. 1966. “Plant Hormones and Regulators.” Science 3723: 721–731. doi:10.1126/science.152.3723.721.
- Paige, K. N., and T. G. Whitham. 1987. “Overcompensation in Response to Mammalian Herbivory: The Advantage of Being Eaten.” The American Naturalist 129: 407–416. doi:10.1086/284645.
- Pucheta, E., I. Bonamici, M. Cabido, and S. Díaz. 2004. “Below‐ground Biomass and Productivity of a Grazed Site and a Neighbouring Ungrazed Exclosure in a Grassland in Central Argentina.” Austral Ecology 29: 201–208. doi:10.1111/j.1442-9993.2004.01337.x.
- Qiao, C. L., J. H. Wang, S. D. Ge, D. D. Chen, L. Zhao, Y. N. Li, and S. X. Xu. 2012. “Comparison of Soil Properties under Fencing and Grazing in Alpine Meadow on Qinghai-Tibet Plateau.” Pratacultural Science 29:341–345.
- Qiu, J. 2016. “Trouble in Tibet.” Nature 529: 142–145. doi:10.1038/529142a.
- Schönbach, P., H. W. Wan, M. Gierus, Y. F. Bai, K. Müller, L. J. Lin, A. Susenbeth, and F. Taube. 2011. “Grassland Responses to Grazing: Effects of Grazing Intensity and Management System in an Inner Mongolian Steppe Ecosystem.” Plant and Soil 340:103–115. doi:10.1007/s11104-010-0366-6.
- Shang, Z. H., Y. S. Ma, R. J. Long, and L. M. Ding. 2008. “Effect of Fencing, Artificial Seeding and Abandonment on Vegetation Composition and Dynamics of ‘Black Soil Land’ in the Headwaters of the Yangtze and the Yellow Rivers of the Qinghai-Tibetan Plateau.” Land Degradation & Development 19: 554–563. doi:10.1002/ldr.861.
- Smith, A. T., and J. M. Foggin. 1999. “The Plateau Pika (Ochotona Curzoniae) Is a Keystone Species for Biodiversity on the Tibetan Plateau.” Animal Conservation 2: 235–240. doi:10.1111/j.1469-1795.1999.tb00069.x.
- Sun, J., T. Y. Zhan, M. Liu, Z. C. Zhang, Y. Wang, S. L. Liu, G. L. Wu, G. H. Liu, and A. Tsunekawa. 2019. “Verification of the Biomass Transfer Hypothesis under Moderate Grazing across the Tibetan Plateau: A Meta-analysis.” Plan and Soil. doi:10.1007/s11104-019-04380-8.
- Sun, J., X. D. Wang, G. W. Cheng, J. B. Wu, J. T. Hong, and S. L. Niu. 2014. “Effects of Grazing Regimes on Plant Traits and Soil Nutrients in an Alpine Steppe, Northern Tibetan Plateau.” PloS One 9: e108821. doi:10.1371/journal.pone.0108821.
- Tang, L., S. K. Dong, R. Sherman, S. L. Liu, Q. R. Liu, X. X. Wang, X. K. Su, Y. Zhang, Y. Y. Li, Y. Wu, H. D. Zhao, C. Zhao, and X. Y. Wu. 2015. “Changes in Vegetation Composition and Plant Diversity with Rangeland Degradation in the Alpine Region of Qinghai-Tibet Plateau.” Rangeland Journal 37:107–115. doi:10.1071/RJ14077.
- Tiffin, P. 2000. “Mechanisms of Tolerance to Herbivore Damage: What Do We Know?” Evolutionary Ecology 14: 523–536. doi:10.1023/A:1010881317261.
- Wang, J. M., Y. M. Zhang, and D. H. Wang. 2006. “Seasonal Thermogenesis and Body Mass Regulation in Plateau Pikas (Ochotona Curzoniae).” Oecologia 149: 373–382. doi:10.1007/s00442-006-0469-1.
- Wang, X. X., S. K. Dong, Q. Z. Gao, H. K. Zhou, S. L. Liu, X. K. Su, and Y. Y. Li. 2014. “Effects of Short-term and Long-term Warming on Soil Nutrients, Microbial Biomass and Enzyme Activities in an Alpine Meadow on the Qinghai-Tibet Plateau of China.” Soil Biology & Biochemistry 76:140–142. doi:10.1016/j.soilbio.2014.05.014.
- Wang, X. X., S. K. Dong, R. Sherman, Q. R. Liu, S. L. Liu, Y. Y. Li, and Y. Wu. 2015. “A Comparison of Biodiversity–ecosystem Function Relationships in Alpine Grasslands across A Degradation Gradient on the Qinghai–Tibetan Plateau.” Rangeland Journal 37:45–55. doi:10.1071/RJ14081.
- Wang, Y., and K. Wesche. 2016. “Vegetation and Soil Responses to Livestock Grazing in Central Asian Grasslands: A Review of Chinese Literature.” Biodiversity and Conservation 25: 2401–2420. doi:10.1007/s10531-015-1034-1.
- Wang, Z. Q, Y. Z. Zhang, Y. Yang, W. Zhou, C. C. Gang, Y. Zhang, J. L. Li, R. An, K. Wang, I. Odeh, and J. G. Qi. 2016. “Quantitative Assess the Driving Forces on the Grassland Degradation in the Qinghai–Tibet Plateau, in China.” Ecological Informatics 33:32–44. doi:10.1016/j.ecoinf.2016.03.006.
- Wei, M. H., H. L. Lin, and Z. Q. Wang. 2015. “Study on the “Heat Island Effect” of Barren Patch on Degradation Sequences of Alpine Meadow in the Source Region of the Yangtze and Yellow River, Qinghai-Tibetan Plateau, China.” Chinese Journal of Grassland 37: 22–29.
- Wilson, M. C., and A. T. Smith. 2015. “The Pika and the Watershed: The Impact of Small Mammal Poisoning on the Ecohydrology of the Qinghai-Tibetan Plateau.” AMBIO: A Journal of the Human Environment 44: 16–22. doi:10.1007/s13280-014-0568-x.
- Wise, M. J., and W. G. Abrahamson. 2005. “Beyond the Compensatory Continuum: Environmental Resource Levels and Plant Tolerance of Herbivory.” Oikos 109: 417–428. doi:10.1111/j.0030-1299.2005.13878.x.
- Xu, L., S. X. Yu, N. P. He, X. F. Wen, P. L. Shi, Y. J. Zhang, J. Z. Dai, and R. M. Wang. 2013. “Soil C Mineralization and Temperature Sensitivity in Alpine Grasslands of the Qinghai-Xizang Plateau.” Chinese Journal of Plant Ecology 37:988–997. doi:10.3724/SP.J.1258.2013.00102.
- Yang, Y. H., J. Y. Fang, C. J. Ji, and W. X. Han. 2009. “Above- and Belowground Biomass Allocation in Tietan Grasslands.” Journal of Vegetation Science 20: 177–184. doi:10.1111/j.1654-1103.2009.05566.x.
- Yang, Z. P., Y. H. Ou, X. L. Xu, L. Zhao, M. H. Song, and C. P. Zhou. 2010. “Effects of Permafrost Degradation on Ecosystems.” Acta Ecologica Sinca 30: 33–39. doi:10.1016/j.chnaes.2009.12.006.
- Yao, T. D., L. G. Thompson, V. Mosbrugger, F. Zhang, Y. M. Ma, T. X. Luo, B. Q. Xu, X. X. Yang, D. R. Joswiak, W. C. Wang, M. E. Joswiak, L. P. Devkota, S. Tayal, R. Jilani, and R. Fayziev. 2012. “Third Pole Environment (TPE).” Environmental Development 3: 52–64. doi:10.1016/j.envdev.2012.04.002.
- Zhang, R. Y., M. P. Schellenberg, G. D. Han, H. Wang, and J. X. Li. 2018. “Drought Weakens the Positive Effects of Defoliation on Native Rhizomatous Grasses but Enhances the Drought-tolerance Traits of Native Caespitose Grasses.” Ecology and Evolution 8: 12126–12139. doi:10.1002/ece3.4671.
- Zhang, Y. 2016a. “Responses of Alpine Grassland Ecosystems to Climate Change and Grazing on the Qinghai-Tibetan Plateau.” Phd thesis: Beijing Normal University.
- Zhang, Y., S. K. Dong, Q. Z. Gao, S. L. Liu, H. K. Zhou, H. Ganjurjav, and X. X. Wang. 2016b. “Climate Change and Human Activities Altered the Diversity and Composition of Soil Microbial Community in Alpine Grasslands of the Qinghai-Tibetan Plateau.” Science of the Total Environment 562: 353–363. doi:10.1016/j.scitotenv.2016.03.221.
- Zhang, Y., S. K. Dong, Q. Z. Gao, S. L. Liu, Y. Liang, and X. J. Cao. 2016c. “Responses of Alpine Vegetation and Soils to the Disturbance of Plateau Pika (Ochotona Curzoniae) at Burrow Level on the Qinghai–Tibetan Plateau of China.” Ecological Engineering 88: 232–236. doi:10.1016/j.ecoleng.2015.12.034.
- Zhou, H., X. Zhao, Y. Tang, S. Gu, and L. Zhou. 2005. “Alpine Grassland Degradation and Its Control in the Source Region of the Yangtze and Yellow Rivers, China.” Grassland Science 51: 191–203. doi:10.1111/j.1744-697X.2005.00028.x.
- Zhu, Z. H., B. Xi, Y. N. Li, Y. M. Zang, W. J. Wang, J. X. Liu, and H. Guo. 2010. “Compensatory Growth of Carex Scabrirostris in Different Habitats in Alpine Meadow.” Chinese Journal of Plant Ecology 34: 348–358.
