ABSTRACT
Biodiversity declines in an unprecedented way, mostly due to land use change. Restoration interventions proved to be one of the most effective tools to halt the decline, especially in ecosystems such as agricultural fields. Evidence-based, locally adapted recommendations on grassland restoration, however, are often missing, so we present a novel approach for such interventions that can be implemented anywhere and that are based on scientific rigor. In a recently started long-term field ecological study, we established 0.5 ha wildflower parcels, using a diverse local seed mixture of 32 insect-visited plant species in Central European agricultural landscapes in 2020. Our focus is on the landscape-scale effects of this ecological intensification on ecosystem services such as crop yield, pollination and pest control, and the long-term monitoring of the successional processes. The aim is to showcase an effective restoration protocol that could serve as a model for future farm management, and provide much-needed evidence for policy on landscape ecological restoration of international relevance.
Introduction
Biodiversity declines rapidly, threatening ecosystem functioning and finally human well-being. A major driver behind the decline is land use change due to agriculture. In Europe, about half of the land is used for agriculture (European Environment Agency Citation2019), much of which has been intensified in the last decades (Emmerson et al. Citation2016). To counter the decline of biological diversity due to field- and landscape-scale intensification, immense efforts have been made to conserve and restore ecosystems and biodiversity (Kleijn et al. Citation2011). The target of the current UN Decade on Ecosystem Restoration is to restore 30% of today’s degraded areas (UNEP/FAO Citation2020), of which grasslands have received the least attention so far (Török et al. Citation2021). Subsequently, Dudley et al. (Citation2020) and Csákvári et al. (Citation2021) identified restoration as a key research field and called for publications and the showcasing of restoration approaches. Effective restoration protocols based on well-established evidence and an increased understanding among stakeholders of how nature, economy and society can benefit, are badly needed (Díaz et al. Citation2019; Farrell et al. Citation2021; Fischer et al. Citation2021). Despite the enormous amount of money spent on ecosystem conservation, results are rather mixed, probably also due to inappropriate practices (Batáry et al. Citation2015; Pe’er et al. Citation2017). That is a major weakness for regions that still hold high biodiversity, such as Central and Eastern Europe (Stoate et al. Citation2009; Vačkář and Báldi Citation2016).
Semi-natural grasslands are a prominent target of restoration interventions because of their high biodiversity value (Habel et al. Citation2013). However, the literature on grassland restoration lacks three major issues: i) landscape-scale studies on ecological intensification (von Holle, Yelenik, and Gornish Citation2020; Lowe, Groves, and Gratton Citation2021), ii) the long-term monitoring of successional processes of restored grassland on former cropland (Lengyel et al. Citation2012; Holland et al. Citation2017; Lowe, Groves, and Gratton Citation2021), and iii) restoration with diverse native and local floral seed mixtures (Haaland, Naisbit, and Bersier Citation2011). Furthermore, research on restored flower-rich grasslands is especially missing from Central and Eastern Europe, and more broadly from the steppe belt stretching from Central Europe to Asia.
The aim of our recently started large-scale and long-term field study is to test the effects of restoring species-rich semi-natural grassland islands in crop fields on biodiversity and ecosystem services, considering landscape context and spatial configuration of restored patches – linked to the single large or several small (SLOSS) dilemma (Fahrig et al. Citation2022). In principle, we focus on pollinators and pest control agents as important ecosystem service providers to crops and other plants (Kovács-Hostyánszki et al. Citation2017). We monitor the successional development and the restoration success of such wildflower parcels and intend to feed research evidence into agricultural policy developments.
Experimental setup
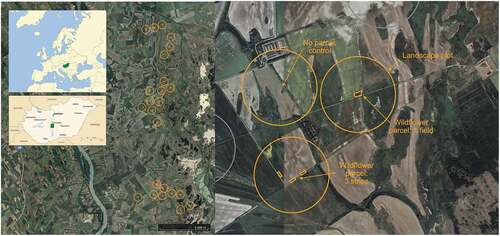
We selected 24 circular landscape plots of 500 m radius with either homogeneous (<10% of semi-natural habitats) or heterogeneous (40-60% of semi-natural habitats) landscapes in Central Hungary (). Within 16 landscape plots, we sowed 0.5 ha with a wildflower seed mixture (Appendix 1) in February 2020. These sown parcels either consist of a single large field or three smaller, spatially associated strips about 100–150 m apart (see also here), yet of consistently 0.5 ha in total area. Further eight landscape plots without sown treatments serve as control sites. With this setup, we not only study the sown parcels, but also their landscape-scale effects. The setup of compact and diverse islands differs from the commonly used setup of long, narrow strips at the edge of agricultural fields with few species (Haaland, Naisbit, and Bersier Citation2011; Ouvrard, Transon, and Jacquemart Citation2018; Schmidt et al. Citation2022). Well-composed, high diversity seed mixtures have been shown to support higher habitat quality for pollinators (Williams et al. Citation2015; Meissen et al. Citation2019; Hyvönen et al. Citation2021) and enhance the reestablishment success of the target vegetation (Török et al. Citation2021). Yet they are often compiled irrespective of their nativeness and are questionable in terms of their sufficiency for potential pollinators and the risk of plant invasion. Therefore, our seed mixture consisted of 32 local insect-visited plant species flowering additively during the whole vegetation period (Appendix 1) and providing morphologically manifold flowers to offer food resources to diverse pollinator guilds (see also Nichols, Goulson, and Holland Citation2019). Grasses were omitted to avoid their swift dominance due to their higher germination and quick colonization rate (Meissen et al. Citation2019). We apply adaptive management to the parcels, continuously monitoring the development of the vegetation, and applying necessary mowing (e.g., against weedy or invasive plants) or reseeding if its growth is hindered (e.g., extreme drought in 2020; see also Meissen et al. Citation2019).
Figure 1. The study design consists of 24 circular landscape plots in Central Hungary (left). They are grouped into trios with a sown wildflower field, a triplet of sown wildflower strips and a control (right). Based on images from Google Earth (Citation2021).
Monitoring protocol
The successional development of the sown vegetation is being monitored with biannual botanical surveys. Species richness and coverage, vegetation cover and height, litter and bare soil cover are recorded in twelve 1 × 1 m permanent quadrats per field and strip triplet () in early June and late September.
Figure 2. Schematic representation of the landscape plot (left) with a wildflower parcel (rectangle), the distribution of the pan traps (dots) and trap nests (crosses). The sampling methods in the parcels (right) consist of botanical surveys (squares), pollinator transects (solid line) and malaise traps (triangle).
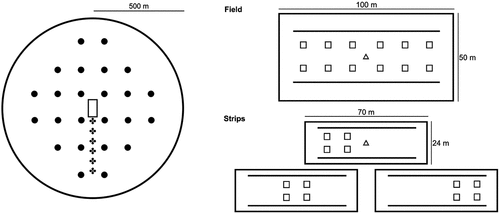
Pollinators and other flying insects are sampled with a wide array of methods in the sown parcels (transect sampling and malaise traps in late May and late July) and at the landscape scale (trapnests throughout the season, pan traps in July) (). Along two parallel transects, we record bees and wasps (Aculeata), hoverflies (Syrphidae) and diurnal butterflies (Macrolepidoptera), their activity and visited flower species. We catch observed wild bees and hoverflies with sweep nets for identification in the lab. Along the pollinator transects, the floral resources (i.e., insect-pollinated flowering species and their number of flower units) are recorded in 1 × 1 m quadrats. We establish a malaise trap in the center of each field (, right) and one of the strips twice a year for a week each (, right). These non-attractant mesh tents target most flying insects and represent the majority of present insect guilds (Matthews and Matthews Citation1971). Trapnests are set up at increasing distance from the center of the landscape plot (every 80 m, up to 400 m) (, left) to monitor the landscape-scale effect of the wildflower parcels during the whole vegetation season. At each distance, two trapnests are mounted on a pole and covered by a simple roof (, left). Trapnests consist of a bunch of reed stems which provide nesting opportunities for cavity-nesting bees and wasps (, right) (Staab et al. Citation2018). For the pan trap survey, 24 equally distributed sampling points are established per landscape plot in late July (576 in total, , left). At each location, a painted yellow plastic pan filled with water and a drop of detergent is mounted on top of a pole and left standing for 48 hours (). The bright color attracts flower-visiting insects such as Diptera, Hymenoptera and Coleoptera, among others (Taylor and Palmer Citation1972; Templ et al. Citation2019). The floral resource abundances and richness, habitat type (including crop type) and vegetation attributes are recorded during the establishment of the pan traps as local environmental factors.
Figure 3. The overview of a wildflower strip in a homogeneous landscape (left) shows its island-like setting. A malaise trap is set out for a week in one of the wildflower parcels and targets most flying insects (right). Photographer: Viktor Szigeti (left), Zoltán Soltész (right).

Figure 4. Occupied reed stems in a trap nest are sealed with a mud plug (left). They can contain several brood cells of bees or wasps (Aculeata) that consist of a developing offspring with a pollen package or prey (e.g., spiders) as food resource (right). Photographer: András Báldi (left), Áron Bihaly (right).

Perspectives
Two years after setting up the wildflower parcels, the vegetation is well established () and buzzing with insects (). In the coming years, our wildflower parcels are expected to further develop into biodiverse islands that serve as refuges in the agricultural landscape for an increasing number of species (Lowe, Groves, and Gratton Citation2021). The spatial arrangement and design of the experiment provides a firm basis to test hypotheses, for example, regarding the direct and interactive effects of spatial heterogeneity and effects of landscape context on biodiversity. The success of the restoration intervention will be measured along time with special attention to pollinator richness and abundance, pest control effectiveness and stable or increasing yield. The experiment further brings future opportunities for scrutinizing exciting questions from individual behavior (e.g., butterfly movement) to key ecosystem services (e.g., pollination, pest control, soil decomposition).
Figure 6. Close-ups of the wildflower parcel show the successional development from the first year (left) and the second year (right). In the first year, the vegetation was dominated by fast-growing agricultural weeds, which were largely outcompeted in the second year by the sown plant species. Photographer: Viktor Szigeti.

Figure 7. A large pollinator community visits the flowers of the wildflower parcels and will find suitable permanent habitat in the agricultural landscape, among them Halictus eurygnathus on Centaurea cyanus (left) and Lycaena thersamon on Matricaria chamomilla (right). Photographer: Raoul Pellaton (left), Viktor Szigeti (right).

We intend to demonstrate an effective restoration practice to farmers with benefits beyond crop area reduction (e.g., pollination, pest control, wildlife management). Furthermore, we strive to provide evidence to policymakers that such comparably small parcels have a positive effect on the landscape scale, and they can be worth integrating into farm management or rural and urban development policies. Diversified agricultural ecosystems can support food production while harboring rich biodiversity (Emmerson et al. Citation2016), a win-win strategy that this experiment can demonstrate.
Conclusion
The long-term experiment we started will support sustainable farming as it demonstrates the viability of ecological intensification, where both yield and biodiversity can flourish. The continuous monitoring of the effects of a local wildflower seed mixture on the landscape scale will provide evidence on grassland restoration of international relevance. Beyond that, it may serve as a starting point for the establishment of other similar experiments in other farming systems across Europe and globally, contributing to the goals of the EU Green Deal and the UN Decade on Ecosystem Restoration.
Supplemental Material
Download MS Word (19.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/20964129.2022.2090449
Additional information
Funding
References
- Batáry, P., L. V. Dicks, D. Kleijn, and W. J. Sutherland. 2015. “The Role of agri-environment Schemes in Conservation and Environmental Management.” Conservation Biology 29 (4): 1006–7. doi:10.1111/cobi.12536.
- Csákvári, E., V. Fabók, S. Bartha, Z. Barta, P. Batáry, G. Borics, Z. Botta-Dukát, et al. 2021. “Conservation Biology Research Priorities for 2050: A Central-Eastern European Perspective.” Biological Conservation 264:109396. doi:10.1016/j.biocon.2021.109396.
- Díaz, S., J. Settele, E. S. Brondízio, H. T. Ngo, J. Agard, A. Arneth, P. Balvanera, et al. 2019. “Pervasive human-driven Decline of Life on Earth Points to the Need for Transformative Change.” Science 366 (6471): 1327. doi:10.1126/science.aaw3100.
- Dudley, N., L. Eufemia, M. Fleckenstein, M. E. Periago, I. Petersen, and J. F. Timmers. 2020. “Grasslands and Savannahs in the UN Decade on Ecosystem Restoration.” Restoration Ecology 28 (6): 1313–1317. doi:10.1111/rec.13272.
- Emmerson, M., M. B. Morales, J. J. Oñate, P. Batáry, F. Berendse, J. Liira, T. Aavik, and I. Guerrero. 2016. “How Agricultural Intensification Affects Biodiversity and Ecosystem Services”. In Advances in Ecological Research 55, A. J. Dumbrell, R. L. Kordas, G. Woodward eds., 43–97. Cambridge, MA, USA: Academic Press. doi: 10.1016/bs.aecr.2016.08.005.
- European Environment Agency. 2019. “The European environment-state and Outlook 2020. Knowledge for Transition to a Sustainable Europe.” Luxembourg. doi:10.2800/96749.
- Fahrig, L., J. I. Watling, C. A. Arnillas, V. Arroyo-Rodríguez, T. Jörger-Hickfang, J. Müller, H. M. Pereira, et al. 2022. “Resolving the SLOSS Dilemma for Biodiversity Conservation: A Research Agenda.” Biological Reviews 97 (1): 99–114. doi:10.1111/brv.12792.
- Farrell, C. A., J. Aronson, G. C. Daily, L. Hein, C. Obst, P. Woodworth, and J. C. Stout. 2021. “Natural Capital Approaches: Shifting the UN Decade on Ecosystem Restoration from Aspiration to Reality.” Restoration Ecology e13613. doi:10.1111/rec.13613.
- Fischer, J., M. Riechers, J. Loos, B. Martin-Lopez, and V. M. Temperton. 2021. “Making the UN Decade on Ecosystem Restoration a Social-Ecological Endeavour.” Trends in Ecology & Evolution 36 (1): 20–28. doi:10.1016/j.tree.2020.08.018.
- Google Earth. (2021). [Hungary]. Retrieved 21 October 2021, from https://earth.google.com
- Haaland, C., R. E. Naisbit, and L.-F. Bersier. 2011. “Sown Wildflower Strips for Insect Conservation: A Review.” Insect Conservation and Diversity 4 (1): 60–80. doi:10.1111/j.1752-4598.2010.00098.x.
- Habel, J. C., J. Dengler, M. Janišová, P. Török, C. Wellstein, and M. Wiezik. 2013. “European Grassland Ecosystems: Threatened Hotspots of Biodiversity.” Biodiversity and Conservation 22 (10): 2131–2138. doi:10.1007/s10531-013-0537-x.
- Holland, J. M., J. C. Douma, L. Crowley, L. James, L. Kor, D. R. W. Stevenson, and B. M. Smith. 2017. “Semi-natural Habitats Support Biological Control, Pollination and Soil Conservation in Europe. A Review.” Agronomy for Sustainable Development 37 (4): 31. doi:10.1007/s13593-017-0434-x.
- Hyvönen, T., E. Huusela, M. Kuussaari, M. Niemi, R. Uusitalo, and V. Nuutinen. 2021. “Aboveground and Belowground Biodiversity Responses to Seed Mixtures and Mowing in a long-term set-aside Experiment.” Agriculture, Ecosystems & Environment 322. doi:10.1016/j.agee.2021.107656.
- Kleijn, D., M. Rundlöf, J. Scheper, H. G. Smith, and T. Tscharntke. 2011. “Does Conservation on Farmland Contribute to Halting the Biodiversity Decline?” Trends in Ecology & Evolution 26 (9): 474–481. doi:10.1016/j.tree.2011.05.00.
- Kovács-Hostyánszki, A., A. Espíndola, A. J. Vanbergen, J. Settele, C. Kremen, and L. V. Dicks. 2017. “Ecological Intensification to Mitigate Impacts of Conventional Intensive Land Use on Pollinators and Pollination.” Ecology Letters 20 (5): 673–689. doi:10.1111/ele.12762.
- Lengyel, S., K. Varga, B. Kosztyi, L. Lontay, E. Déri, P. Török, and B. Tóthmérész. 2012. “Grassland Restoration to Conserve landscape-level Biodiversity: A Synthesis of Early Results from A large-scale Project.” Applied Vegetation Science 15 (2): 264–276. doi:10.1111/j.1654-109X.2011.01179.x.
- Lowe, E. B., R. Groves, and C. Gratton. 2021. “Impacts of field-edge Flower Plantings on Pollinator Conservation and Ecosystem Service Delivery – A meta-analysis.” Agriculture, Ecosystems & Environment 310: 107290. doi:10.1016/j.agee.2020.107290.
- Matthews, R. W., and J. R. Matthews. 1971. “The Malaise Trap: Its Utility and Potential for Sampling Insect Populations.” The Great Lakes Entomologist 4 (4): 117–122.
- Meissen, J. C., A. J. Glidden, M. E. Sherrard, K. J. Elgersma, and L. L. Jackson. 2019. “Seed Mix Design and First Year Management Influence Multifunctionality and cost-effectiveness in Prairie Reconstruction.” Restoration Ecology 28 (4): 807–816. doi:10.1111/rec.13013.
- Nichols, R. N., D. Goulson, and J. M. Holland. 2019. “The Best Wildflowers for Wild Bees.” Journal of Insect Conservation 23 (5–6): 819–830. doi:10.1007/s10841-019-00180-8.
- Ouvrard, P., J. Transon, and A. L. Jacquemart. 2018. “Flower-strip agri-environment Schemes Provide Diverse and Valuable Summer Flower Resources for Pollinating Insects.” Biodiversity and Conservation 27 (9): 2193–2216. doi:10.1007/s10531-018-1531-0.
- Pe’er, G., Y. Zinngrebe, J. Hauck, S. Schindler, A. Dittrich, S. Zingg, T. Tscharntke, et al. 2017. “Adding Some Green to the Greening: Improving the EU’s Ecological Focus Areas for Biodiversity and Farmers.” Conservation Letters 10 (5): 517–530. doi:10.1111/conl.12333.
- Schmidt, A., A. Kirmer, N. Hellwig, K. Kiehl, and S. Tischew. 2022. “Evaluating CAP Wildflower Strips: High-quality Seed Mixtures Significantly Improve Plant Diversity and Related Pollen and Nectar Resources.” Journal of Applied Ecology 59 (3): 860–871. doi:10.1111/1365-2664.14102.
- Staab, M., G. Pufal, T. Tscharntke, and A. M. Klein. 2018. “Trap Nests for Bees and Wasps to Analyse Trophic Interactions in Changing environments—A Systematic Overview and User Guide.” Methods in Ecology and Evolution 9 (11): 2226–2239. doi:10.1111/2041-210X.13070.
- Stoate, C., A. Báldi, P. Beja, N. D. Boatman, I. Herzon, A. van Doorn, G. R. de Snoo, L. Rakosy, and C. Ramwell. 2009. “Ecological Impacts of Early 21st Century Agricultural Change in Europe - A Review.” Journal of Environmental Management 91 (1): 22–46. doi:10.1016/j.jenvman.2009.07.005.
- Taylor, L. R., and J. M. P. Palmer. 1972. “Aerial Sampling.” In Aphid Technology, edited by H. F. van Emden, 189–234. New York: Academic Press.
- Templ, B., E. Mózes, M. Templ, R. Földesi, Á. Szirák, A. Báldi, and A. Kovács-Hostyánszki. 2019. “Habitat-dependency of Transect Walk and Pan Trap Methods for Bee Sampling in Farmlands.” Journal of Apicultural Science 63 (1): 93–115. doi:10.2478/jas-2019-0014.
- Török, P., L. A. Brudvig, J. Kollmann, N. Price, J, and B. Tóthmérész. 2021. “The Present and Future of Grassland Restoration.” Restoration Ecology 29 (S1): e13378. doi:10.1111/rec.13378.
- UNEP/FAO (2020). “The UN Decade on Ecosystem Restoration 2021-2030 “Prevent, Halt and Reverse the Degradation of Ecosystems Worldwide.” https://www.decadeonrestoration.org/
- Vačkář, D., and A. Báldi. 2016. “Ecosystem Management in Transition in Central and Eastern Europe: The Need for a Vision.” Ecosystem Health and Sustainability 2 (8): e01231. doi:10.1002/ehs2.1231.
- von Holle, B., S. Yelenik, and E. S. Gornish. 2020. “Restoration at the Landscape Scale as a Means of Mitigation and Adaptation to Climate Change.” Current Landscape Ecology Reports 5 (3): 85–97. doi:10.1007/s40823-020-00056-7.
- Williams, N. M., K. L. Ward, N. Pope, R. Isaacs, J. Wilson, E. A. May, J. Ellis, et al. 2015. “Native Wildflower Plantings Support Wild Bee Abundance and Diversity in Agricultural Landscapes across the United States.” Ecological Applications 25 (8): 2119–2131. doi:10.1890/14-1748.1.
Appendix 1.
Sown plant species and their primary (dark green) and secondary (light green) flowering season
Table

