Abstract
This review surveys the chemical and biological literature dealing with the isolation, structural elucidation and bioactivity of hericenones and erinacines from the fruiting body and mycelium of Hericium erinaceus, concentrating on work that has appeared in the literature up to December 2009.
1. Introduction
Nerve growth factor (NGF) has potent biological activities, such as preventing neuronal death and promoting neurite outgrowth, and is essential to maintain and organize neurons functionally (Obara and Nakahata Citation2002). It is assumed that functional deficiency of NGF is related to Alzheimer's disease and plays a part in the etiology of the disease process (Allen and Dawbarn Citation2006). NGF is expected to be applied to the treatment of Alzheimer's disease (Takei et al. Citation1989). However, NGFs are proteins and so are unable to cross the blood–brain barrier; it is also easily metabolized by peptidases. Therefore, its application as a medicine for treatment of neurodegenerative disorders will be difficult. Alternatively, research has been carried out on low-molecular weight compounds that promote NGF biosynthesis, such as catecholamines (Furukawa et al. Citation1986), scabronions (Obara et al. Citation1999), cyrneines (Marcotullio et al. Citation2007), hericenones and erinacines.
Hericium erinaceus is a mushroom belonging to the family Hericiaceae and has been known as Chinese medicine or food in China and Japan without harmful effects. H. erinaceus grows on old or dead broadleaf trees and has been used as a medicine for treatment of gastricism in traditional Chinese medicine for more than 1000 years (Mizuno et al. Citation1999). Recently, the chemical constituents of H. erinaceus have been investigated for its interesting and significant bioactivities. Hericenones and erinacines were isolated from the fruiting body and mycelium of H. erinaceus, respectively, and most of the compounds promote NGF biosynthesis in rodent cultured astrocytes (). These results suggest the value of H. erinaceus for the treatment and prevention of dementia. However, there has been no review article on bioactive compounds isolated from H. erinaceus to date. This report covers the isolation and structural elucidation of hericenones and erinacines from the fruiting body and mycelium, and their biological activity of stimulating NGF biosynthesis. In addition, this report examines the research on erinacines produced by H. erinaceus grown in mycelial culture and the cultural conditions for the fermentation of H. erinaceus.
Table 1. List of hericenones and erinacines in Hericium erinaceus.
2. Hericenones in the fruiting body of H. erinaceum
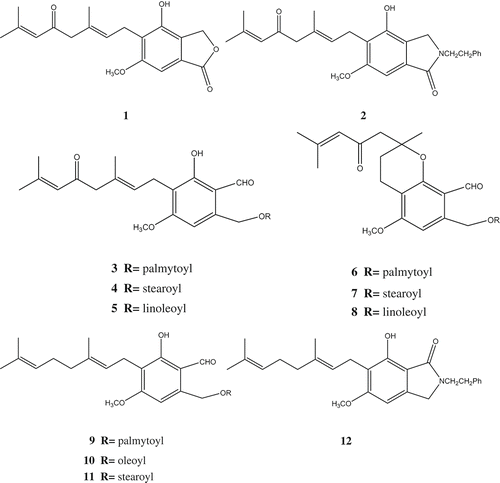
Hericenones are aromatic compounds isolated from the fruiting body of H. erinaceus. Fresh fruiting bodies of the fungus were extracted with acetone. Repeated chromatography of the chloroform-soluble fraction obtained by solvent partitions (chloroform and then ethyl acetate) of the extract with silica gel followed by HPLC with ODS column gave hericenones. Hericenones A (1), B (2) (Kawagishi et al. Citation1990), C (3), D (4), E (5) (Kawagishi et al. Citation1991), F (6), G (7), H (8) (Kawagishi et al. Citation1993), hericenes A–C (9–11) (Alberto et al. Citation1995) and hericerin (12) (Kimura et al. Citation1991) were isolated from the mushroom H. erinaceus. Hericenones C, D and E exhibited stimulating activity for the biosynthesis of NGF in vitro. In the presence of hericenones C, D, E and H at 33 μg/ml, mouse astroglial cells secreted 23.5 ± 1.0, 10.8 ± 0.8, 13.9 ± 2.1 and 45.1 ± 1.1 pg/ml NGF into the culture medium, respectively. The degree of activity for hericenones D was almost at the same level as the potent stimulator, epinephrine. It is of interest that the difference of the activity among those compounds was dependent on the nature of the fatty acid ().
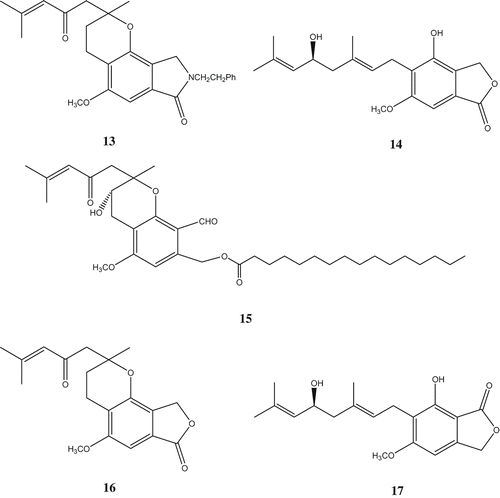
Erinacerin A (13) and B (14) were also isolated from the fruiting bodies of H. erinaceus. It was found that erinacerin A occurred as a racemate (Yaoita et al. Citation2005). 3-Hydroxyhericenone F (15), hericenone I (16) and hericenone J (17) were isolated from the same mushroom. 3-Hydroxyhericenone F showed the protective activity against endoplasmic reticulum stress-dependent Neuro2a cell death (Ueda et al. Citation2008) ().
3. Erinacines in the mycelium of H. erinaceum
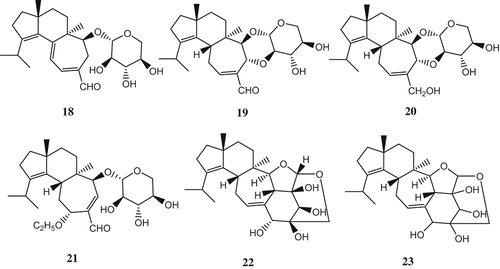
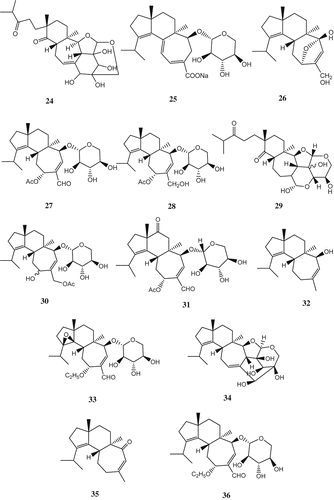
A number of cyathane-type diterpenoids with potent inductive activity for NGF synthesis were isolated from the mushroom, for example scabronines A (Ohta et al. Citation1998), B–F (Kita et al. Citation1998) isolated from the fruiting bodies of Sarcodon scabrosus, and the cyrneines A, B (Marcotullio et al. Citation2006; Obara et al. Citation2007), C, D (Marcotullio et al. Citation2007) isolated from the fruiting bodies of Sarcodon cyrneus. All erinacines possess a cyathane skeleton consisting of angularly condensed five-, six-, and seven-membered rings. Erinacines A (18), B (19), C (20) (Kawagishi et al. Citation1994), D (21) (Kawagishi et al. Citation1996a), E (22), F (23), G (24) (Kawagishi et al. Citation1996b), H (25), I (26) (Lee et al. Citation2000), P (27) (Kenmoku et al. Citation2000), Q (28) (Kenmoku et al. Citation2002), J (29), K (30) (Kawagishi et al. Citation2006), R (31) (Ma et al. Citation2008) and erinacol (32) (Kenmoku et al. Citation2004), isolated from the mycelia of H. erinaceus, show stimulating activity for NGF biosynthesis. The fungus was cultivated by shaking at 30°C for 4 weeks; then the culture was centrifuged and the mycelia were extracted with ethanol. The extract, after concentrating the solvent, was fractionated by solvent partition between ethyl acetate and water. Repeated silica gel chromatography and HPLC of the ethyl acetate extract gave erinacines. Erinacine F was a diastereomer of erinacine E in the sugar part. However, the stereochemistry of the sugar part in erinacine F remained undetermined since NOSY experiments did not give any valuable information. In the bioassay using mouse astroglial cell, the amounts of NGF secreted into the medium in the presence of erinacines A, B, and C at 1.0 mM were 250.1 ± 36.2, 129.7 ± 6.5 and 299.1 ± 59.6 pg/ml, respectively. The amounts of NGF secreted into the medium in the presence of erinacines E and F at 5.0 mM were 105 ± 5.2 and 175 ± 5.2 pg/ml, respectively. These activities were much stronger than that (69.2 ± 17.2 mM) of a known potent stimulator, epinephrine, used as a positive control in the bioassay.
Two erinacine derivatives (33, 34) isolated from the mycelia of H. erinaceus were claimed to induce the biosynthesis of NGF, which were expected to be applicable for the treatment of dementia (Shimada et al. Citation1996). Another two erinacine diterpenoids (35, 36) (Kawagishi et al. Citation1995), isolated from the mycelia of H. erinaceus, were also claimed to induce the production of NGF ().
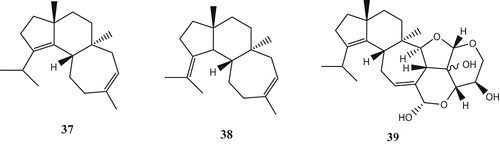
Cyatha-3, 12-diene (37), together with its isomer (38), was isolated from the mycelia of H. erinaceus as a biosynthetic intermediate of cyathane diterpenoids (Kenmoku et al. Citation2001). Biotransformation of erinacine E was examined using 81 microorganisms. One of them, Caladariomyces fumago ATCC 16373, was found to transform erinacine E to a new analog CP-412,065 (39) at a conversion rate of 29% (Saito et al. Citation1998) ().
4. Discussion
Hericenones and erinacines are two natural products isolated from the fruiting body and mycelium of H. erinaceus, respectively, and most compounds exhibit the activity of promoting NGF synthesis. Hericenones and erinacines are low-molecular weight compounds that easily cross the blood–brain barrier. In a bioassay using mouse astroglial cell, the amounts of NGF secreted into the medium in the presence of erinacines were greater than for hericenones. There is debate as to whether hericenones are active components stimulating biosynthesis of NGF and the recent result have shown that hericenone C, D and E did not increase NGF mRNA expression at 10–100 μg/ml in 1321 N1 cells (Mori et al. Citation2008). Therefore, erinacines have potential as medicines for degenerative neuronal disorders such as Alzheimer's disease and peripheral nerve regeneration. It has been reported that oral administration of erinacine A significantly increases the level of NGF in the rat locus coeruleus and hippocampus, but not in the cerebral cortex (Shimbo et al. Citation2005). However, the detailed mechanism by which erinacines induces NGF synthesis remains unknown. It is interesting that hericenones have been only reported in the fruiting bodies of H. erinaceus and erinacines only in the mycelia.
Biosynthesis of natural products is complex and the expression of many of the key synthase genes is affected by a number of factors. Biosynthetic studies on the cyathane skeleton, which does not follow the isoprene rule, was carried out by Ayer and co-workers in the late 1970s (Ayer et al., Citation1978; Kenmoku et al. Citation2001). However, the search for fungal cyathadiene cyclases is still in progress. The structural novelty and significant biological activities displayed by the erinacines have also made members of this family attractive targets for total synthesis. Testimony to this is found in the number and diversity of approaches that have been developed to construct these fascinating natural products (Wright and Whitehead Citation2000; Takano et al. Citation2004; Trost et al. Citation2005), and construction of the 5-6-7 tricyclic core of the erinacines is the key step. However, the low yield, multi-step synthetic methods restrict their commercial application. Currently, fermentation is perhaps the best way to provide erinacines for further exploitation.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (30901957) and Program for Excellent Young Teachers of He'nan Province, China.
References
- Alberto , A , Rosanna , C , Gianluca , N and Orso , VP. 1995 . Secondary mold metabolites: part 46. hericenes A-C and erinapyrone C: new metabolites produced by the fungus Hericium erinaceum . J Nat Prod. , 57 : 602 – 606 .
- Allen , SJ and Dawbarn , D. 2006 . Clinical relevance of the neurotrophins and their receptors . Clin Sci. (Lond.) , 110 : 175 – 191 .
- Ayer , WA , Homast , T , Nakashima , TT and Ward , DE. 1978 . Metabolites of bird's nest fungi. Part 10. Carbon-13 nuclear magnetic resonance studies on the cyathins . Can J Chem. , 56 : 2197 – 2199 .
- Furukawa , Y , Furukawa , S , Ikeda , F and Satoyoshi , K. 1986 . Aliphatic side chain of catecholamine potentiates the stimulatory effect of the catechol part on the synthesis of nerve growth factor . FEBS Lett. , 208 : 258 – 262 .
- Kawagishi , H , Ando , M and Mizuno , T. 1990 . Hericenone A and B as cytotoxic principles from the mushroom Hericium erinaceum . Tetrahedron Lett. , 31 : 373 – 376 .
- Kawagishi , H , Ando , M , Sakamoto , H , Yoshida , S , Ojima , F , Ishiguro , Y , Ukai , N and Furukawa , S. 1991 . Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis from the mushroom Hericium erinaceum . Tetrahadron Lett. , 32 : 4561 – 4564 .
- Kawagishi , H , Ando , M , Shinba , K , Sakamoto , H , Yoshida , S , Ojima , F , Ishiguro , Y , Ukai , N and Furukawa , S. 1993 . Chromans, hericenones F, G and H from the mushroom Hericium erinaceum . Phytochemistry , 32 : 175 – 178 .
- Kawagishi , H , Shimada , A , Shirai , R , Okamoto , K , Ojima , F , Sakamoto , H , Ishiguro , Y and Furukawa , S. 1994 . Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum . Tetrahedron Lett. , 35 : 1569 – 1572 .
- Kawagishi , H , Kojima , F and Okamoto , K. 1995 . JP 7070168 .
- Kawagishi , H , Simada , A , Shizuki , K , Mori , H , Sakamoto , H and Furukawa , S. 1996a . Erinacine D. A Stimulator of NGF-synthesis from the mycelia of Hericium erinaceum . Heterocycl Commun. , 2 : 51 – 54 .
- Kawagishi , H , Simada , A , Shizuki , K , Mori , H , Sakamoto , H and Furukawa , S. 1996b . Erinacines E, F, and G, stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum . Tetrahedron Lett. , 41 : 7399 – 7402 .
- Kawagishi , H , Masui , A , Tokuyama , S and Nakamura , T. 2006 . Erinacines J and K from the mycelia of Hericium erinaceum . Tetrahadron , 62 : 8463 – 8466 .
- Kenmoku , H , Sassa , T and Kato , N. 2000 . Isolation of erinacine P, a new parental metabolite of cyathane-xylosides, from Hericium erinaceum and its biomimetic conversion into erinacines A and B . Tetrahedron Lett. , 41 : 4389 – 4393 .
- Kenmoku , H , Kato , N , Shimada , M , Omoto , M , Mori , A , Mituhashi , W and Sassa , T. 2001 . Isolation of (–)-cyatha-3,12-diene, a common biosynthetic intermediate of cyathane diterpenoids, from an erinacine-producing basidiomycete, Hericium erinaceum . Tetrahedron Lett. , 42 : 7439 – 7422 .
- Kenmoku , H , Shimai , T , Toyomasu , T , Kato , N and Sassa , T. 2002 . Erinacine Q, a new erinacine from Hericium erinaceum, and its biosynthetic route to erinacine C in the basidiomycete . Biosci Biotechnol Biochem. , 66 : 571 – 575 .
- Kenmoku , H , Tanaka , K , Okada , K , Kato , N and Sassa , T. 2004 . Erinacol (cyatha-3,12-dien-14β-ol) and 11-O-acetylcyathin A3, new cyathane metabolites from an erinacine Q-producing Hericium erinaceum . Biosci Biotechnol Biochem. , 68 : 1786 – 1789 .
- Kimura , Y , Nishibe , M , Nakajima , H , Hamasaki , T , Shimada , A , Tsuneda , A and Shigematsu , N. 1991 . Hericerin, a new pollen growth inhibitor from the mushroom Hericium erinaceum . Agric Biol Chem. , 55 : 2673 – 2674 .
- Kita , T , Takaya , Y and Oshima , Y. 1998 . Scabronines B, C, D, E and F, novel diterpenoids showing stimulating activity of nerve growth factor synthesis, from the mushroom Sarcodon scabrosus . Tetrahedron , 54 : 11877 – 11886 .
- Lee , EW , Shizuki , K , Hosokawa , S , Suzuki , M , Suganuma , H , Inakuma , T and Kawagishi , H. 2000 . Two novel diterpenoids, erinacines H and I from the mycelia of Hericium erinaceum . Biosci Biotechnol Biochem. , 64 : 2402 – 2405 .
- Ma , BJ , Zhou , Y , Li , LZ , Li , HM , Gao , ZM and Ruan , Y. 2008 . A new cyathane-xyloside from the mycelia of Hericium erinaceum . Z Naturforsch. , 63b : 1241 – 1242 .
- Marcotullio , MC , Pagiott , R , Maltese , F , Obara , Y , Hoshino , T , Nakahata , N and Curini , M. 2006 . Neurite outgrowth activity of cyathane diterpenes from Sarcodon cyrneus, cyrneines A and B . Planta Med. , 72 : 819 – 823 .
- Marcotullio , MC , Pagiotti , R , Maltese , F , Oball-Mond Mwankie , GN , Hoshino , T , Obara , Y and Nakahata , N. 2007 . Cyathane diterpenes from Sarcodon cyrneus and evaluation of their activities of neuritegenesis and nerve growth factor production . Bioorg Med Chem. , 15 : 2878 – 2882 .
- Mizuno , T. 1999 . Bioactive substances in Hericium erinaceus (Bull.: Fr.) Pers. and its medicinal utilization . Int J Med Mushrooms , 1 : 105 – 119 .
- Mori , K , Obara , Y , Hirota , M , Azumi , Y , Kinnugasa , S , Inatomi , S and Nakahata , N. 2008 . Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells . Biol Pharm Bull. , 9 : 1727 – 1732 .
- Obara , Y and Nakahata , N. 2002 . The signaling pathway of neurotropic factor . Drug News Perspect. , 15 : 290 – 298 .
- Obara , Y , Nakahata , N , Kita , T , Takaya , Y , Kobayashi , H and Oshima , Y. 1999 . Stimulation of neurotrophic factor secretion from 1321N1 human astrocytoma cells by novel diterpenoids, scabronines A and G . Eur J Pharmacol. , 370 : 79 – 84 .
- Obara , Y , Hoshino , T , Marcotullio , MC , Pagiotti , R and Nakahata , N. 2007 . A novel cyathane diterpene, cyrneine A, induces neurite outgrowth in a Rac1-dependent mechanism in PC12 cells . Life Sci. , 80 : 1669 – 1677 .
- Ohta , T , Kita , T , Kobayashi , N , Obara , Y , Nakahata , N , Takaya , Y and Oshima , Y. 1998 . Scabronine A, a novel diterpenoid having potent inductive activity of the nerve growth factor synthesis, isolated from the mushroom Sarcodon scabrosus . Tetrahedron Lett. , 39 : 6229 – 6232 .
- Saito , T , Aoki , F , Hirai , H , Inagaki , T , Matsunaga , Y , Sakakibara , T , Sakemi , S , Suzuki , Y , Watanabe , S , Suga , O , Sujaku , T , Smogowicz , AA , Truesdell , SJ , Wong , JW , Nagahisa , A , Kojima , Y and Kojima , N . 1998 . Erinacine E as a kappa opioid receptor agonist and its new analogs from a basidiomycete, Hericium ramosum . J Antibiot. , 51 : 983 – 990 .
- Shimada , A , Kawagishi , H and Furukawa , A. 1996 . JP 8073486 .
- Shimbo , M , Kawagishi , H and Yokogoshi , H. 2005 . Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats . Nutr Res. , 25 : 617 – 623 .
- Takano , M , Umino , A and Nakad , M. 2004 . Synthetic studies on cyathins: enantioselective total synthesis of (+)-allocyathin B2 . Org Lett. , 26 : 4897 – 4900 .
- Takei , N , Tsukui , H and Hatanaka , H. 1989 . Intracellular storage and evoked release of acetylcholine from postnatal rat basal forebrain cholinergic neurons in culture with nerve growth factor . J Neurochem. , 53 : 1405 – 1410 .
- Trost , BM , Dong , L and Schroeder , GM. 2005 . Total synthesis of (+)-allocyathin B2 . J Am Chem Soc. , 127 : 2844 – 2845 .
- Ueda , K , Tsujimori , M , Kodani , S , Chiba , A , Kubo , M , Masuno , K , Sekiya , A , Nagai , K and Kawagishi , H. 2008 . An endoplasmic reticulum (ER) stress-suppressive compounds and its analogues from the mushroom Hericium erinaceum . Bioorg Med Chem. , 16 : 9467 – 9470 .
- Wright , DL and Whitehead , CR. 2000 . Recent progress on the synthesis of cyathane type diterpenes . Org Prep Proc Int. , 32 : 307 – 331 .
- Yaoita , Y , Danbara , K and Kikuchi , M. 2005 . Two new aromatic compounds from Hericium erinaceum (Bull.:Fr.) Pers . Chem Pharm Bull. , 53 : 1202 – 1203 .