Abstract
A locally isolated strain of Trichoderma harzianum was studied for production of xylanase (EC 3.2.1.8) using lignocellulosic substrates for solid-state fermentation. Among the different substrates used, wheat straw produced the highest yields (146 IU/ml). The influence of temperature, pH, moistening agents, moisture level, carbon sources, nitrogen sources, pretreatments and metal ions were evaluated with respect to xylanase production. Highest xylanase production was obtained using wheat straw after 12 days of incubation. Different substrate combination ratios and the effect of particle size were also checked against xylanase production. Maximum xylanase production was observed in a wheat straw/rice straw ratio of 1:1, with a particle size of 0.45–0.5 mm. Media supplementation with xylose as a carbon source in a ratio 1:5 (carbon source/substrate) gave maximum activity (157 IU/ml). Nitrogen supplementations from yeast extract produced xylanase activity of 141 IU/ml. Optimum xylanase production was observed at 30°C and pH 5.0. Xylanase production was enhanced in the presence of Ca2+ and Zn2+ ions. Ammonium sulfate fractionation (20–80% saturation) of partially purified xylanase yielded 76.5% of the enzyme with a 3.53-fold purification. The molecular weight of xylanase was found to be ca. 29,000 Da by SDS–PAGE.
Introduction
Cellulose, hemicelluloses and lignin are the main components of wood and plant cell walls and a major reservoirs of energy and nutrients (Taiz and Zeiger Citation1991). Xylan is the major constituent of hemicellulose and the second most abundant biopolymer after cellulose. It is a major renewable source with high potential for degradation into useful end-products (Kohli et al. Citation2001). Chemically, the structure of xylan in the plant cell walls may differ greatly depending on the origin. Xylan consists of β-1-4-linked d-xylopyranosyl residues on a backbone (Bakri et al. Citation2001), which contains side chains, including arabinosyl, glucuronosyl, 4-O-methyl glucuronosyl, and acetyl residues (Subramaniyan and Prema Citation2002). The breakdown of xylan requires synergistic action of many enzymes. The enzymes involved in degradation of the main polysaccharide chain are endo-1,4-β-xylanase (EC 3.2.1.8), β-xylosidase (EC 3.2.1.37) and α-arabinofuranosidase (EC 3.2.1.55), acetylxylan esterase (EC 3.1.1.72) and α-glukuronidase (EC 3.2.1.139), which remove side substituents in heteroxylans (Rogalski et al. Citation2001; Shallom and Shallom Citation2003).
Haltrich et al. (Citation1996), in an overview of fungal xylanases, showed that the enzyme can be produced by a number of microorganisms, including bacteria, yeasts and filamentous fungi such as Aspergillus, Aureobasidium, Bacillus, Chaetomium, Cryptococcus, Fusarium, Humicola, Penicillium, Phanerochaete, Rhizomucor, Talaromyces, Trichoderma, amongst others. Microbial cellulase-free xylanases have potential application in the selective removal of hemicellulose from Kraft pulp to give brightness to the paper (Beg et al. Citation2001). This eco-friendly technology effectively addresses concerns over the environmental hazards of conventionally used chlorine in the paper and pulp industries, leading to release of organo-chlorines, toxic to all forms of life (Beg et al. Citation2001; Kohli et al. Citation2001). Xylanases show great potential for industrial applications, mainly for the bioconversion of lignocelluloses to sugar, ethanol and other useful substances, clarification of juices and wines, improving the nutritional quality of silage and green feed and the de-inking processes of waste paper (Viikari et al. Citation2001). It is not unrealistic to foresee that coal and crude oil are likely to be substituted by biomass in another 50 years (Goheen Citation1981, Kulkarni et al. Citation1999).
Most research on xylanase production has used submerged fermentation, but solid-state fermentation offers distinct advantage in terms of less space requirements, lower costs and the abundance of agricultural waste as a substrate for the production of enzymes. Moreover, solid-state fermentation involves simplicity of the fermentation media, with fewer requirements for complex machinery, equipment or control systems. It also offers a greater compactness of the fermentation vessel owing to a lower water volume, greater product yield, reduced energy demand, lower capital, and low recurring costs under industrial operation. Easy scale-up processes, less downstream processing and superior yield, absence of foam build-up, and easier control of contaminants due to the low moisture levels in the system are additional advantages of solid-state fermentation (Pandey Citation1992).
India, being an agricultural country, has abundant agricultural waste produced from different crop residues. Thus, the use of agricultural waste in xylanese production will decrease the impact of agricultural waste on the environment. Agricultural waste, such as wheat straw, rice straw, husks, corn straw and paddy straw, contribute to millions of tonnes of waste yearly. Based on the enormous amount of wastes, there is an urgent need to manage bulk wastes effectively and economically. At the same time, it is also necessary to generate value-added products from these wastes. Solid-state fermentation has been used for the production of fine chemicals of commercial value from microbial sources, such as enzymes, antibiotics, flavoring compounds and microbial biomass for use as animal feed (Pang and Ibrahim Citation2005).
The industrial xylanase-producing strains are mainly species of Aspergillus and Trichoderma (Dobrev et al. Citation2007), and other enzymes synthesized by these two genera are well characterized and most often applied in industrial practice (Romanowska et al. Citation2006). In view of the aforementioned applications, the present investigation was undertaken: (i) to purify and characterize xylanase predominantly produced by a natural isolate of Tricoderma harzianum and (ii) to investigate the effect of carbon/nitrogen sources, metal ions, moistening agents, stability, different substrate concentration and different substrates on the production of xylanase.
Material and methods
Fungal strain and maintenance
Isolation and screening of fungi for xylanase
Fungal cultures were isolated from 30 different samples collected from Gir forest, Junagadh (Western India). Isolation and establishment of pure cultures of these strains was carried out by routine methods. Initially, these samples were plated on potato dextrose agar plates (PDA) and purified cultures were maintained at 4ºC in a refrigerator on PDA containing 1% xylan. Based on the zone of clearance shown by the cultures on PDA plates supplemented with 1% xylan and their individual xylanase activity, the KSR-2 strain was selected for further studies. The strain was identified by morphotaxonomic features/characters and was found to be Tricoderma harzianum (Class Sordariomycetes; Hypocreales; Hypocreaceae). Identification of the strain was confirmed by Agharkar Research Institute, Mycology and Plant Pathology Division, Pune (India).
Solid substrates and culture conditions
Solid substrates and combinations
Five different types of agricultural waste, i.e. wheat straw, corncobs, rice straw and soyabean waste, has been collected from local farms, while sugar cane bagasse was collected from Sugar Industries near Vadodara. Solid-state fermentation was initially carried out using all the substrates individually and xylanase production was tested using selected combinations of different solid substrates at a ratio of 1:1. Wheat straw was combined individually with rice straw and xylanase production was tested. Combinations of sugarcane bagasse and soyabean waste, corncobs and wheat straw have also been checked for production of xylanase.
Xylanase production and extraction
The production media contained 5 g of solid substrate moistened with distilled water. The moisture level of lignocellulosic substrate was adjusted to 1:5 (substrate/medium, w/v) with distilled water. The flasks were inoculated with 1 ml of 106 spores/ml suspension prepared from a week-old PDA slant of the Trichoderma harzianum grown at 30°C. Inoculated production media were incubated under static conditions at 30°C and xylanase production was checked every 24 h for 18 days. Xylanase was extracted in 50 ml of 0.1 M sodium acetate buffer on a rotary shaker at 250 rpm for 30 min. The content was filtered through Whatmann No. 1 filter paper and the filtrate used as the xylanase source.
Study of physio-chemical factor on xylanase activity
Spore suspension was prepared in 0.1% Tween 80 from a 7-day-old culture of Trichoderma harzianum. Inocula with 105, 106 and 107 spores/ml in solution were added in the media to check production of xylanase. Carbon sources were glucose, maltose, lactose, sucrose, fructose and xylose; nitrogen sources were peptone, malt extract, beef extract, yeast extract; the inorganic nitrogen source was sodium nitrate, ammonium sulfate and urea, added as individual components in ratios of 1:5 (source/media) in the production media. In addition to distilled water, tap water, MS medium, basal salt solution and modified MS medium were added as moistening agents to check their influence on xylanase production. To study the influence of initial moisture, the ratio of substrate to moistening medium (distilled water) was adjusted from 1:1 to 1:6. To study the influence of pretreatment of substrate on xylanase production, wheat straw were soaked in 0.1 M HCl, 0.1 M NaOH, 0.1 M HF and then thoroughly washed until neutral, and dried. Steam treatment was given at 100°C for 1 h.
Parameters for xylanase assay
Xylanase production
Xylanase activity was determined by incubating a mixture of 0.1 ml aliquots of each enzyme source and 1% Birchwood xylan dissolved in 0.1 M sodium acetate buffer as a substrate. The release of reducing sugars in 30 min at 30°C, pH 5.0 (0.1 M acetate buffer) was measured as xylose equivalents using the dinitrosalicylic acid method (Miller Citation1959). One unit of enzyme activity (IU) is defined as the amount of enzyme liberating 1 μmol of xylose per min. All sets were performed in triplicates and the standard errors reported.
Optimization of enzyme activity
Birchwood xylan used in assay buffer system for the enzyme–substrate reaction was supplemented at six different concentrations as: 0.5, 1, 1.5, 2, 2.5 and 3%. To check optimum temperature for xylanase activity, enzymes were incubated with xylan in 0.1 M acetate buffer, pH 5 as substrate at 16, 30, 37, 40, 50, 60, 70 and 80°C. Once optimum temperature was noted, for checking thermostability, enzymes were maintained at 70°C and assayed for xylanase activity every 5 min. Enzyme activity was checked individually at different pH values of 2, 3, 4, 4.5, 5, 6 and 7.
Effect of metal ions on xylanase activity
To check the effect of metal ions (Mn2+, Zn2+, Cu2+, Ca2+, K2+, Mg2+) on enzyme activity, a 1-mM concentration of these metal ions was added to the reaction mixture and observed at 540 nm. After incubation, enzyme activities were determined.
Partial purification and molecular weight determination
All purification steps were done at 4ºC. Partial purification of xylanase was carried out by ammonium sulfate precipitation (20–80% saturation). Pellets of enzymes were dissolved in acetate buffer pH 5.0 and subjected to dialysis. SDS–polyacrylamide gel with 12% gel was performed following the procedure of Lammeli (Citation1970) using a Medox electrophoresis unit (Medox India Pvt. Ltd., Ahmedabad, India). Native PAGE was performed as described in (Sambrook and Russell Citation2001) with 1% xylan as the substrate. Once electrophoresis was completed gels were further incubated for 30 min (at room temperature) in a 0.1% Congo red solution; the gels were destained in 1 M NaCl until clearance bands of xylanase activity were obtained.
Result and discussion
Xylanase production using solid substrates
The agro-industrial residues – wheat straw, rice straw, corn cobs, sugar cane bagasse and soyabean waste – were used for solid-state fermentation at 30°C using distilled water as the moistening agent in a ratio of 1:5 (substrate/liquid). Maximum xylanase yield (146 IU/ml) was obtained from wheat straw (5 g) after 12 days (). In corncobs, rice straw, sugarcane baggase and soybean meal, there was a relative decrease in enzyme activity ().
Table 1. Effect of various substrates on enzyme production
Gupta et al. (Citation2009) also found that an increase in concentration of substrate lead to a decrease in enzyme activity due to the fact that a high substrate concentration led to increased viscosity, which influenced medium components and oxygen transfer. When wheat straw was used, there was a significant difference in xylanase titers and this fact may be attributed to its hemicellulose nature and favorable degradability (Sonia et al. Citation2005). Wheat straw has been known for being ideally suitable for xylanase production in Thermoascus aurantiacus, Penicillium canescens and P. citrinum cultures (Kalogeris et al. Citation1998; Bakri et al. 2008; Nair et al. Citation2008).
Effect of incubation time on xylanase production and activity
Xylanase activity was determined after 24 h of incubation until 18 days to determine the optimum incubation period for the maximum production of xylanase. Enzyme production, however, started after 48 h of inoculation and showed maximum production (146 IU/ml) on the 12th day of incubation (at 30°C). In some fungi, high xylanase production has been shown to be closely linked to cellulase production due to the time-course or incubation period (Haltrich et al. Citation1996; Christakopoullos et al. Citation1999; Kang et al. Citation2004). A further increase in the incubation period resulted in a decrease in enzyme production. The decrease in enzyme production may be due to the susceptible portion of xylan molecules that are rapidly digested and only the crystalline portion remains (Roose Citation1963; Jing et al. Citation1998). The optimum incubation time for maximum activity (146 IU/ml) with enzyme–substrate reaction mixture was 30 min ().
Table 2. Activity of enzyme–substrate reaction at various incubation times
Influence of temperature and pH
Xylanase was produced with distilled water as the moistening agent in the temperature range 20–37°C, showing maximum activity (146 IU/ml) after the 12th day of incubation at 30°C. A further increase in temperature above 40°C not only inhibited fungal growth but also the production of xylanase. Production of xylanase is closely related to the growth of fungus as the optimum temperature for xylanase production is similar to optimum temperature for the growth of fungus. Similarly, the highest xylanase titers in fungal systems have been reported to occur generally at temperatures that are optimal for growth of cultures in solid-state fermentation (Biswas et al. Citation1990; Christakopoullos et al. Citation1999). Dialysed xylanase has been kept at different temperatures and maximum activity was obtained at 30°C ().
Table 3. Effect of temperature on enzyme activity
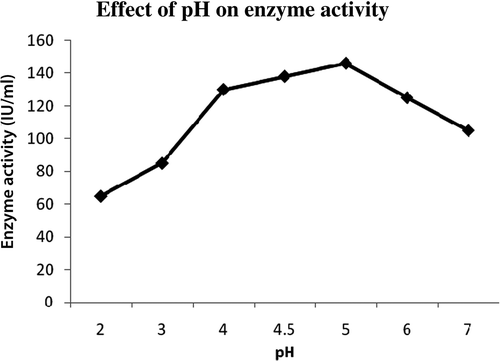
Most fungi are able to grow at a wide pH range of 5.0–8.0 (Paredes et al. Citation1998). The initial pH of the medium was adjusted to a variable pH range by adding 0.1 N HCl and production of enzyme was tested at different pH range from 3.0 to 7.0. The production of xylanase was found to be best at pH 5.0 with xylanase activity of 146 IU/ml. There was no marked difference in xylanase titers at pH 3.0 or 4.0, but activity starts declining from pH 6.0. Similarly, the highest xylanase titers in fungal systems have been reported to occur generally at pH 5.0 for growth of cultures in solid-state fermentation (Shah and Dutta Citation2005). Dialysed xylanase shows maximum activity at pH 5.0 ().
Influence of moistening agents and moisture level
Shah et al. (Citation2005) reported that xylanase, cellulase and protein production were sensitive to the composition of the moistening agent. In the present study, all media with different moistening agents supported enzyme production but maximum xylanase production (146 IU/ml) was recorded when distilled water was used as the moistening agent (). When tap water, MS medium, basal salt solution or modified MS medium were used as moistening agents relatively less xylanase was produced. There are reports that mineral salt solution is a better inducer of high production of xylanase (Reese et al.Citation1969; Haq et al. Citation1993; Shah and Dutta Citation2005). In the present study, the use of distilled water as a moistening agent gave better results compared to mineral solution. Butt et al. (Citation2002) also found that distilled water is a better moistening agent for production of xylanase.
Table 4. Effect of various moistening agents on xylanase activity
Moisture content in solid-state fermentation is a crucial factor, which determines the success of enzyme production in solid-state fermentation (Ramesh and Lonsane Citation1990). A higher than optimum moisture level causes decreased porosity, alteration in particle structure, gummy texture, lower oxygen transfer and enhancement of aerial mycelia (Raimbault and Alazard Citation1980). Moreover, it leads to conglomeration of the substrate or sticking of particles to the wall of the reactor, resulting in possible bacterial contamination (Lonsane et al. Citation1985). Xylanase production was optimized by adjusting the initial moisture content of solid-state fermentation from 1:1 to 1:6 (wheat straw/distilled water), corresponding 50–86% moisture level. Increase in moisture level increases xylanase production by up to 1:5 (80%), while higher concentrations do not increase xylanase production due to increase in protein levels. Maximum activity (146 IU/ml) was obtained with moisture levels adjusted to 1:5 ().
Table 5. Effect of moisture levels on xylanase production
Influence of carbon and nitrogen sources
Enzyme production by microorganism mainly depends on growth conditions and nutrient availability. Therefore, improving the nutritional value of wheat straw by supplementation of carbon should also improve the growth of fungus and, subsequently, enzyme production. Flasks containing media were supplemented with carbon sources (glucose, xylose, maltose, lactose, fructose, and sucrose) at a ratio of 1:5 (carbon source/substrate). Maximum activity (156 IU/ml) was obtained using xylose (), which is slightly higher than using media without any carbon, while flasks supplemented with maltose showed minimum production of xylanase (135 IU/ml). Xylose has been described as an effective inducer and carbon source for xylanase production in several microorganisms, including Fusarium solani (Gupta et al. Citation2009) Aspergillus pullulans (Preim et al. Citation1991), Fusarium oxysporium (Chirstakopoullos et al. Citation1999) and Trichoderma lanuginosus (Purkarthofer et al. Citation1993).
Table 6. Effect of carbon sources on enzyme production
Xylanase with minimal cellulases can be produced using a low nitrogen-to-carbon ratio (Gerber et al., Citation1997). In the present study, the effect of both organic (yeast extract, malt extract, beef extract peptone) and inorganic nitrogen sources (urea, ammonium sulfate, sodium nitrate, ammonium nitrate) on xylanase production was studied. Xylanase activity (141 IU/ml) was evident when yeast extract was added. These results agree with those reported in the literature where fungi were found to produce higher xylanase activities on organic nitrogen sources (Purkarthofer et al. Citation1993; Lemos et al. Citation2001; Yang et al. Citation2006; Bakri et al. 2008).
Pretreatment of substrates
Several structural and compositional factors affect fermentability of lignocellulosic materials, which include cellulose protection by lignin, hemicellulose sheathing, degree of hemicellulose acetylation and cellulose crystallinity. These barriers differ in relative significance, depending on the material (Teh-An Citation1996). The alteration of the substrate using pretreatment techniques leads to a change in the physical nature of lignin, increases available surface area and pore sizes, causes partial depolymerization of hemicellulose, decrystallization of cellulose and deacetylation of hemicellulose, all of which can enhance availability of the substrate (Shah and Dutta Citation2005).
In the present work, wheat straw was treated with mild acid, mild alkali and steam. None of these pretreatments increased xylanase production. A decrease in mycelial growth on the media ultimately leads to decreased enzyme production. This decrease could also be caused by formation of toxic inhibitors (acids and phenolics derived from carbohydrates and lignins) during chemical pretreatment (Teh-An 1996).
Influence of metal ions
Some metal ions occurring in pulp were included in the present investigation due to the potential use of the xylanase in the pulp and paper industries. Ca2+ and Zn2+ slightly enhanced xylanase production (). There is no significant effect from other metal ions on enzyme activity. Similar experimental results are reported for Trichoderma harzianum (Seyis and Nilufer. Citation2005), Aspergillus terreus (Ghanem et al. Citation2000) and Trichoderma lanuginosus (Cesar and Mirsa Citation1996). These studies have shown that Ca2+ enhances xylanase activity, while Mn2+ and Zn2+ ions also had a positive effect on xylanase activity (Cesar and Mirsa Citation1996; Castro et al. Citation1997).
Table 7. Effect of metal ions on enzyme activity
Influence of different combinations and particle size of substrate
Wheat straw of different particle sizes were tested to determine their effects on xylanase production. Particle size affected xylanase production (data not shown). The highest titer of (146 IU/ml) xylanase was produced from wheat straw of particle size 0.45–0.5 mm, whereas lower activities were produced on the wheat straw of other sizes. To some extent, our study is in agreement with Yang et al. (Citation2006), where maximum production was obtained at mesh size of 0.35–0.4 mm. These findings also confirmed that the size of the carbon source is an important factor in xylanase production (Kalogeris et al. Citation1998).
Combinations of wheat straw to rice straw have been used to check production profiles of xylanase. Ratios of 1:1, 1:2 and 1:3, and visa versa, were used and tested. Maximum enzyme production (146 μmol/ml/min) resulted from a combination of 1:1 (wheat straw/rice straw).
Partial purification of xylanase, and characterization using SDS–PAGE and native gels
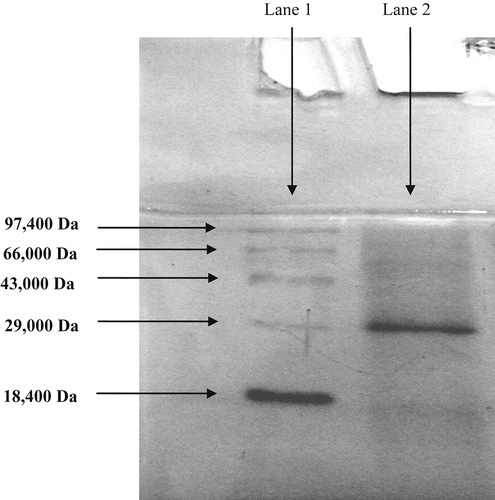
The ammonium sulfate fractionation (20–80% saturation) of a crude xylanase yielded 76.5% of the enzyme with a 3.53-fold purification (data not shown). Gawade and Kamat (Citation1999) reported that xylanases from two Aspergillus species were concentrated from 30 to 80% ammonium sulfate saturation with 62 and 67% yield. The partially purified fraction was subjected to SDS–PAGE and native PAGE analysis. It was found that xylanase has a homogenous nature in band pattern (mw 29,000 Da) (). Kulkarni et al. (Citation1999) reported that microbial xylanase are single subunit proteins within a range of 8–145 kDa. Gupta et al. (Citation2009) reported on xylanase from Fusarium solani F7 89 kDa, while Sardar (Citation2000) reported 24 kDa xylanase from Aspergillus niger.
Thermostability of xylanase
Utilization of enzymes in industrial processes often encounters the problem of thermal inactivation of the enzyme (Shah and Dutta Citation2005). In the present study, xylanase exhibited nearly 90% activity at 65°C over 30 min at pH 5.0, while at 70°C it showed 75% activity at pH 5.0 over 30 min. Thermostability has previously been reported for xylanase from Thermomyces lanuginosus and Paecilomyces themophila (Li et al. Citation2005; Yang et al. Citation2006).
Storage stability
Xylanases used in industrial applications are stored at different temperatures, i.e. at room temperature, cooled or frozen (Shah and Dutta Citation2005). Enzyme retained 95% activity at 4–5 ºC after storage for 1 month. Enzyme retained 75% activity when stored at room temperature for 3 days. A 2–3-ml aliquot of xylanase was frozen for 3 weeks and residues of semisolid lyophilized enzyme retained nearly 70% activity.
In conclusion, the isolate Trichoderma harzianum was an active producer of xylanases with negligible levels of cellulase and is easily cultivated on inexpensive substrates, such as wheat straw, under solid-state fermentation.
Acknowledgement
Authors thank DBT, Department of Biotechnology, New Delhi for financial support and Dr Singh (Agharkar Research Institute, Pune) for identification of the culture.
References
- Bakir , U , Yavascaoglu , S , Guvenc , F and Ersayin , A. 2001 . An endo-β-1,4- xylanase from Rhizopus oryzae: production, partial purification and biochemical characterization . Enzym Microb Technol. , 29 : 328 – 334 .
- Bakri , Y , Jacques , P and Thonart , P. 2003 . Xylanase production by Penicillium canescens 10-10c in solid-state fermentation . Appl Biochem Biotechnol. , 105 : 737 – 748 .
- Beg , QK , Kapoor , M , Mahajan , L and Hoondal , GS. 2001 . Microbial xylanases and their industrial applications a review . Appl Microbiol Biotechnol. , 56 : 326 – 338 .
- Biswas , SR , Jana , SC , Mishra , AK and Nanda , G. 1990 . Production, purification and characterization of xylanase from a hyperxylanolytic mutant of Aspergillus ochraceus . Biotechnol Bioeng. , 35 : 244 – 251 .
- Butt , WA , Haq , IU and Iqbal , J. 2002 . Biosynthesis of xylanase by UV-treated mutant strain of Aspergillus niger GCBMX-45 . Biotechnology , 1 : 10 – 14 .
- Castro , LPM , Aguilar , BA and Osorio , GA. 1997 . Thermostable xylanases produced at 37°C and 45°C by a thermotolerant Aspergillus strain . FEMS Microbiol Lett. , 146 : 97 – 102 .
- Cesar , T and Mirsa , V. 1996 . Purification and properties of the xylanase produced by Thermomyces lanuginosus . Enzym Microb Technol. , 19 : 289 – 296 .
- Christakopoullos , P , Mamma , D , Nerinckxw , W , Kekos , D , Macris , B and Claeyssens , M. 1999 . Production and partial characterization of xylanase from Fusarium oxysporium . Bioresour Technol. , 58 : 115 – 119 .
- Dobrev , GT , Pishtiyski , IG , Stanchev , VS and Mircheva , R. 2007 . Optimization of nutrient medium containing agricultural wastes for xylanase production by Aspergillus niger B03 using optimal composite experimental design . Bioresour Technol. , 98 : 2671 – 2678 .
- Gawande , PV and Kamat , MY. 1999 . Production of Aspergillus xylanase by lignocellulosic waste fermentation and its application . J Appl Microbiol. , 87 : 511 – 519 .
- Gerber , PJ , Heitmann , JA and Joyee , TW. 1997 . Purification and characterization of xylanases from Trichoderma . Bioresour Technol. , 61 : 127 – 140 .
- Ghanem , NB , Yusef , HH and Mahrouse , HK. 2000 . Production of Aspergillus terreus xylanase in solid-state cultures: application of the Plackett Burman experimental design to evaluate nutritional requirements . Bioresour Technol. , 73 : 113 – 121 .
- Goheen , DW. 1981 . Chemicals from wood and other biomass. Part 1: Future supply of organic chemicals . J Chem Educ. , 58 : 465 – 468 .
- Gupta , VK , Gaur , R , Gautam , N , Kumar , P , Yadav , IJ and Darmwal , NS. 2009 . Optimization of xylanase production from Fusarium solani F7 . Am J Food Technol. , 4 ( 1 ) : 20 – 29 .
- Haltrich , D , Nidetzky , B , Kulbe , KD , Steiner , W and Zupancic , S. 1996 . Production of fungal xylanases . Bioresour Technol. , 58 : 137 – 161 .
- Haq , I , Iqbal , SH , Butt , WA and Qadeer , MA. 1993 . Production of xylanase and CMC cellulose by mould culture . Pak J Biotechnol. , 4 : 403 – 409 .
- Jing , C , Wu Zhang , J and Ruipen , R. 1998 . Production, properties and applications of xylanase from Aspergillus niger . Az Ann NV Acad Sci. , 864 : 214 – 218 .
- Kang , SW , Park , YS , lee , JS , Hong , SI and Kim , SW. 2004 . Production of cellulose and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass . Bioresour Technol. , 91 : 153 – 156 .
- Kalogeris , E , Christakopoullos , P , Kekos , D and Macris , BJ. 1998 . Study on the solid-state production of thermostable endoxylanases from Thermoascus aurantiacus. Characterization of two isozymes . J Biotechnol. , 60 : 155 – 163 .
- Kohli , U , Nigam , P , Singh , H and Chaudhary , K. 2001 . Thermostable, alkalophilic and cellulase free xylanase production by Thermoactinomyces thalophilus subgroup C . Enzym Microb Technol. , 28 : 606 – 610 .
- Kulkarni , N , Shendya , A and Rao , M. 1999 . Molecular and biotechnological aspects of xylanases . FEMS Microbiol Rev. , 23 : 411 – 456 .
- Laemmli , UK. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Lemos , JLS , Fontes , MCA and Pereira , NJ. 2001 . Xylanase production by Aspergillus awamori in solid-state fermentation and influence of different nitrogen sources . Appl Biochem Biotechnol. , 91 : 681 – 689 .
- Li , XT , Jiang , ZQ , Li , LT , Yang , SQ , Feng , WY , Fan , JY and Kusakabe , I. 2005 . Characterization of a cellulase-free, neutral xylanase from Thermomyces lanuginosus CBS 288.54 and its biobleaching effect on wheat straw pulp . Bioresour Technol. , 96 : 1370 – 1379 .
- Lonsane , BK , Ghildyal , NP , Budiatman , S and Ramakrishna , SV. 1985 . Engineering aspects of solid state fermentation . Enzym Microb Technol. , 7 : 258 – 265 .
- Miller , GL. 1959 . Use of dinitrosalicylic acid reagent for determination of reducing sugars . Anal Chem. , 31 : 426 – 428 .
- Nair , SG , Sindhu , R and Shashidhar , S. 2008 . Fungal xylanase production under solid state and submerged conditions . Afr J Microbiol Res. , 2 : 82 – 86 .
- Pandey , A. 1992 . Recent process developments in solid-state fermentation . Process Biochem. , 27 : 12 – 17 .
- Pang , PK and Ibrahim , CO. 2005 . Xylanase production by a local fungal isolate, Aspergillus niger USM AI 1 via solid state fermentation using palm kernel cake (PKC) as substrate . Songklanakarin J Sci Technol. , 27 ( 2 ) : 326 – 336 .
- Paredes , LO , Maldonado , SH and Solis , A. 1998 . “ Solid substrate fermentation: a biotechnological approach to bioconversion of wastes ” . In Bioconversion of Waste Materials to Industrial Products , 2nd , Edited by: Martin , A.M. 103 – 153 . Dordrecht : Kluwer .
- Purkarthofer , H , Sinner , M and Steiner , W. 1993 . Cellulase-free xylanase from Thermomyces lanuginosus: Optimization of production in submerged and solid- state culture . Enzym Microb Technol. , 15 : 677 – 682 .
- Priem , B , Dobberstein , J and Emeis , CC. 1991 . Production of β-1,4-xylanase in continuous culture by Aureobasidium pullulans CBS 58475 . Biotechnol Lett. , 13 : 149 – 154 .
- Raimbault , M and Alazard , D. 1980 . Culture method to study fungal growth in solid fermentation . Eur J Appl Microbiol Biotechnol. , 9 : 199 – 209 .
- Ramesh , MV and Lonsane , BK. 1990 . Critical importance of moisture content of the medium in alpha-amylase production by Bacillus licheniformis M 27 in a solid state fermentation system . Appl Microbiol Biotechnol. , 33 : 501 – 505 .
- Romanowska , I , Polak , J and Bielecki , S. 2006 . Isolation and properties of Aspergillus niger IBT-90 xylanase for bakery . Appl Microbiol Biotechnol. , 69 : 665 – 671 .
- Reese , E , Lolad , T and Rarrish , JE. 1969 . Modified substrates and modified products as inducers of carbohydrates . J Biotechnol. , 47 : 1145 – 1151 .
- Rogalski , J , Leszek , M and Tokarzewska-Zadora , J. 2001 . Purification and characterization of two endo-1,4-β-xylanases and β-xylosidases from Phelbia radiata . Acta Microbiol Pol. , 50 : 117 – 128 .
- Roose , FJ. 1963 . Advances in enzymatic hydrolysis of cellulose and related material , 50 – 53 . London : Pergamon Press .
- Sardar , M , Roy , I and Gupta , MN. 2000 . Simultaneous purification and immobilization of Aspergillus niger xylanase on the reversibly soluble polymer Euragit TM L-100 . Enzym Microb Technol. , 27 : 672 – 679 .
- Sambrook , J and Russell , DW. 2001 . Molecular cloning. A laboratory manual , 3rd , Cold Spring Cold Spring Harbor, NY : Laboratory Press .
- Seyis , I and Nilufer , A. 2005 . Investigation of Factors Affecting Xylanase Activity from Trichoderma harzianum 1073 D3 . Braz Arch Biol Technol. , 48 ( 2 ) : 187 – 193 .
- Shah , A and Datta , M. 2005 . Xylanase production under solid-state fermentation and its characterization by an isolated strain of Aspergillus foetidus in India . World J Microbiol Biotechnol. , 21 : 233 – 243 .
- Shallom , D and Shallom , Y. 2003 . Microbial cellulases . Curr Opin Microbiol. , 6 : 219 – 228 .
- Sonia , KG , Chadha , BS and Saini , HS. 2005 . Sorghum straw for xylanase hyper-production by Thermomyces lanuginosus (D2W3) under solid state fermentation . Bioresour Technol. , 96 : 1561 – 1569 .
- Subramaniyan , S and Prema , P. 2002 . Biotechnology of microbial xylanases, enzymology, molecular biology and application . Crit Rev Biotechnol. , 22 : 33 – 64 .
- Taiz , L and Zeiger , E. 1991 . “ Plant and cell architecture ” . In Plant Physiology , Edited by: Taiz , L. and Zeiger , E. 9 – 25 . Redwood City, CA : Pearson Benjamin Cummings .
- Teh-An Hsu . 1996 . “ Pretreatment of Biomass ” . In Handbook on Bioethanol: Production and Utilization , Edited by: Wyman , C.E. 179 – 212 . New York : Taylor and Francis .
- Viikari , L , Kantelinen , A , Sundqvist , J and Linko , M. 2001 . Xylanases in bleaching: from an idea to the industry . FEMS Microbiol Rev. , 13 : 335 – 350 .
- Yang , SQ , Yang , QJ , Jiang , SQ , Li , LT , Tian , HM and Wang , YZ. 2006 . High-level of xylanase production by the thermophilic Paecilomyces themophila J18 on wheat straw in solid-state fermentation . Bioresour Technol. , 97 : 1794 – 1800 .