Abstract
Cutaneous fungi are known to affect parts of the outermost skin layers of mammals, including the epidermis, stratum spinosum and stratum corneum, as well as mucocutaneous membranes, genitalia or external ears. Relatively little is known about fungal infections of Mysticete cetaceans and studies are needed to determine the fungal diversity associated with these marine mammals. This case report was aimed at identifying the fungi associated with the skin of a diseased neonatal southern right whale (Eubalaena australis) found stranded in the Western Cape Province of South Africa. Initial physical examination on site revealed hyperplasia of the follicular and epidermal epithelium. Preliminary examination of skin biopsies using scanning electron microscopy indicated that the skin was colonized by fungal hyphae. Isolation methods yielded a number of fungal isolates, which were identified using standard morphology and rDNA sequence data. These analyses confirmed colonization of the cutaneous layers by species belonging to the genera Fusarium, Chaetomium and Penicillium. Moreover, all isolates were capable of degrading keratin, indicating that skin may act as a natural substrate for these fungi. This study is the first report of the association of these fungi with southern right whale skin.
Introduction
In the field of clinical pathology, four main types of fungal infections are recognized: superficial colonization (without tissue response), cutaneous infection (skin, eyes, sinuses, oropharynx, external ears and vagina), subcutaneous infection and deep infection (localized or disseminated) (De Hoog et al. Citation2000; Wagner and Sohnle Citation1997). In case of superficial colonization, the fungus grows on compounds associated with mammalian skin, including lipids and keratinous materials such as hair, without provoking an immune response. These fungi are known as commensals. Other fungi can simply land on the mammal skin without growing or utilizing any host products as nutrients – these are known as contaminants. Dermatophytes (fungi classified in Epidermophyton, Microsporum or Trichophyton) are generally regarded as commensals due to the fact that they are dependent on keratin as their source of carbon and nitrogen. Cutaneous mycoses, which include both dermatophytoses of the skin and non-dermatophyte cutaneous infections, pertain to muco-cutaneous membranes, living outermost skin, genitalia and external ears (De Hoog et al. Citation2000; Matsumoto Citation2005).
Several mechanisms protect mammalian skin against colonization by pathogenic micro-organisms and their subsequent invasion into the epidermis or dermis. These include the physical barrier provided by hair, the epidermis, desquamation, the humidity of the skin, pH, niche filling by resident microflora, exposure to ultraviolet light and the presence of inhibitory substances on the skin surface (Cove et al. Citation1992; Wagner and Sohnle Citation1997). The latter are secreted by sebaceous and sweat glands and may include mediators of specific humoral immunity (Gebhart and Kersten Citation1992). In cetaceans, however, the external glabrous body is characterized by the absence of hair, sebaceous and sweat glands (Parry Citation1949; Sokolov Citation1982; Yablokov et al. 1974). A study on three delphinid species (Stenella attenuata, Delphinus delphis and Phocoena phocoena) by Meyers and Seegers (Citation2004) demonstrated that the enzyme, lysozyme, and the peptide group, ß-defensins, are products of the thick integument acting as non-specific defense against bacteria, fungi, algae and ecto-parasites.
Fungi form part of the normal skin microflora of marine mammals (Buck Citation1984; Haldiman et al. Citation1985; Henk and Mullan Citation1996; Migaki and Jones Citation1983). However, research conducted on mammals showed that fungi may also be opportunistic and secondary invaders of skin tissue (Migaki and Jones Citation1983). Moreover, animals that are immuno- (Moeller Citation1997) or integumentarily compromised (Miller et al. Citation2002), malnourished, on long-term antibiotic or immunosuppressive agent treatment, or suffering from co-morbid diseases are generally more susceptible to fungal infection (Fowler and Cubas Citation2001; Reidarson et al. Citation2001). Interestingly, fungal diseases have also been documented to cause death in captive and beach-cast marine mammals (Buck et al. Citation1987; Higgins Citation2000; Mayer Citation1988; Migaki and Jones Citation1983; Sweeney and Ridgway Citation1975; Sweeney et al. Citation1976; Stroud and Roffe Citation1979; Tangredi and Medway Citation1980). However, infections from pathogenic fungi are less common or documented in free-ranging cetaceans, since diseased animals generally die at sea, which hampers further investigations (Dhermain et al. Citation2002). In southern right whales specifically, scant literature exists on the incidence of mycotic dermatitis. One case of zygomycosis in a stranded calf has been reported (Best and McCully Citation1979) that presented with multiple suppurative granulomatous lesions in the left epaxial muscle as a result of colonization by a representative of the genus, Mucor. A yeast infection caused by Candida zeylanoides (Castellani) Langeron & Guerra (1942) has recently been documented in a beached southern right whale neonate (Mouton et al. Citation2009).
On 16 September 1999, the corpse of a beached 4.84-m long male southern right whale (SRW) neonate was reported from Voëlklip beach, Hermanus, South Africa (34° 25'S 19° 17.4'E). It was believed to have stranded overnight and was examined the next day. The calf suffered from a skin condition, possibly mycotic dermatitis, although its body condition was excellent (code 2: fresh carcass of Pugliares et al. Citation2007), with no signs of autolytic bloating or exfoliation of the outer layers of skin. The eyes were noted as small and sunken. On gross inspection, the skin appeared unusually formed in places, seemingly lacking epidermal covering, and harbouring a heavy infestation of orange-colored cyamids, Cyamus erraticus. Other features noted were that the heart was flaccid and the pericardium contained several litres of straw-coloured fluid, while the bladder was grossly distended. Histological analysis of renal and cardiac samples from another calf with similar post-mortem features indicated renal congestion and interstitial myocardial oedema. The contents of the stomach, intestine and rectum were noted as an opaque reddish-brown fluid, greenish-yellow mucus and light greenish faeces, respectively. The umbilicus was unhealed but granulating; the tracheoles of the lungs contained frothy air and an excised section of lung floated in water. These latter features plus the lack of meconium and the heavy external parasite infestation indicated that the animal must have been alive for some time (at least a few days) before stranding. These observations were very similar to the previously studied SRW neonate, where C. zeylanoides was isolated from all the sampling sites (Mouton et al. Citation2009). The aim of our present study was to investigate the fungal diversity associated with the skin of the stranded neonate and compare these results with previous findings.
Materials and methods
This work was carried out under annual permits issued to PBB under the terms of the Sea Fishery Act of South Africa, 1988 (Act no. 12 of 1998), dated 10th March 1998, and the Marine Living Resources Act of South Africa, 1998 (Act no. 18 of 1998), dated 29th January 1999 and 27th January 2000, respectively.
Sampling
Skin samples (approximately 5 cm2 and 3 cm deep) were taken from five equally spaced lesional areas along the mid-dorsal, lateral and mid-ventral surface, numbered 1 to 5 posteriorly from the neck region. Prior to cutting, each sampling site was first sterilized with 80% ethanol to exclude post-mortem contaminants. The samples were placed in sterile foil strips and frozen at −20 °C on return from the field.
Scanning electron microscopy
For scanning electron microscopy, samples were stored in 25% (v/v) glutaraldehyde solution for a minimum of 3 days and a maximum of 6 days, after which they were placed in buffer containing 2.5% (v/v) glutaraldehyde, 0.1 M NaH2PO4 and 0.2 M Na2HPO4. The samples were subsequently stored at −5 °C, until further analyses. Frozen samples were dehydrated in a graded ethanol (EtOH) (Merck AG ethanol; Merck & Co., Inc., Whitehouse Station, NJ, USA) series (30, 50, 70, 80, 90 and 100%), each solution being changed at 2-h intervals for 2.5 h per concentration. The samples were subjected to two additional washes in absolute ethanol for 2.5 h each. The samples were subsequently critical-point dried from 100% EtOH in CO2, mounted and coated with gold–palladium in a sputter coater and viewed using a JEOL JSM-5200 scanning microscope (JEOL-USA, Inc., Peabody, MA, USA) operating at 15 kV.
Isolation and culturing of fungi
For isolation, frozen skin samples were plated out on a range of microbial growth media, including Biolab malt extract agar (MEA; Biolab Diagnostics (Pty) Ltd., Wadeville, RSA), half strength Difco potato dextrose agar (PDA; Becton Dickenson, Sparks, MA, USA), Sabouraud glucose agar (SGA; Ronald Citation1993) and bird seed agar (BSA; Kurtzman and Fell Citation2000). These growth media are routinely used to cultivate a wide range of fungal taxa. After 2–7 days of incubation at 25°C, fungal colonies started to develop on some of the skin samples, which were subsequently selected for purification via repetitive culturing on Biolab malt extract agar at 25°C. The fungal isolates were examined morphologically using light microscopy. The Penicillium isolate was also inoculated onto Czapek yeast autolysate agar (CYA) (Pitt Citation1973) to investigate colony growth and characteristics. To test for the ability to utilize keratin as a source of carbon and nitrogen, the fungal isolates were inoculated onto keratin azure medium according to the method of Scott and Untereiner (Citation2004) and incubated at 25°C. Additionally, isolates were also inoculated onto malt extract agar (MEA) adjacent to a sterile whale skin section and incubated at 25°C to support the keratin azure experiment.
Fungal genomic DNA extraction
To screen for non-culturable fungi, genomic DNA was extracted directly from frozen skin samples according to the method described by Hoffman and Winston (Citation1987), while genomic DNA from fungal isolates was extracted using the ZR Fungal/Bacterial DNA Kit™ (Cat. No.: D6005; Zymo Research, Orange, CA, USA). Fragments of the nuclear rDNA gene complex were amplified using the polymerase chain reaction (PCR). These included the D1/D2 (600–650 bp) region of the large subunit (LSU, 26S) rDNA, as well as the internal transcribed spacer region (ITS1, ∼113–121 bp), 5.8S rDNA gene (∼161 bp) and ITS2 region (∼222–230 bp) (Lloyd-MacGilp et al. Citation1996). A similar procedure was followed for control reactions, where an equal volume of milliQ water was added to the reaction mixture instead of the template DNA. Universal fungal primer pairs were used in the PCR including F63 (5'-GCA TAT CAA TAA GCG GAG GAA AAG-3') and LR3 (5'-GGT CCG TGT TTC AAG ACG G-3') (Fell et al. 2000) for the 26S fragment, as well as ITS1 (5'-TCCGTAGGTGAACCTGCGG-3'; White et al. Citation1990) and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') (White et al. Citation1990) for the ITS1/5.8S/ITS2 fragment. To estimate the quantity of PCR product, the amplification products were separated electrophoretically on 1% (w/v) agarose gels, containing ethidium bromide (1 μg/ml) at 90 mV for 1 h. DNA fragments were immediately purified by means of a High Pure PCR product purification kit (Roche Molecular Biochemicals (Pty) Ltd. SA) following the manufacturer's protocol (Roche instruction manual, vol. 2, Sept. 1999; Roche Molecular Biochemicals).
Sequencing and data analysis
Sequencing of the rDNA fragments was performed employing a Hitachi 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA, USA) using F63/LR3 and ITS1/ITS4 as sequencing primers. The fungal isolates were initially identified using the sequences to perform a BLAST search on the NCBI database for each of the isolates. The results of these searches were used to identify species with high and lower similarity on Genbank, for further use in compiling the datasets.
Multiple consensus sequences (500–600 bp) were aligned using ClustalX version 2.0.10 with default settings and final manual editing was performed with Sequence Alignment Editor Version 2.0a11. The Chaetomium sequence dataset included partial 28S sequences (Untereiner et al. Citation2001) of our four isolates and closely related Chaetomium spp., as well as other more distant species (from GenBank), and outgroups included Daldinia concentrica (Bolton) Cesati & De Notaris (1863) and Xylaria curta (Fries 1851). A separate sequence dataset was compiled for the ITS1, 5.8S, ITS2 region of the Penicillium spp., including species with high similarity, as well as more distant spp., and the outgroup included Chaetomium globosum (Kunze ex Fries 1829) and Phialophora europae (de Hoog, Mayser & Haase Citation2000). The datasets were analyzed using Parsimony Analysis in PAUP 4.0b10 (Swofford Citation2002). Using heuristic searches, the ”Multrees” option was activated while performing tree bisection reconstruction (TBR) branch swapping to find the most parsimonious tree. Gaps were treated as missing data. Bootstrap support for nodes was evaluated from 1000 heuristic searches. Groups with a bootstrap frequency of 50% and higher were included in the consensus trees.
Results
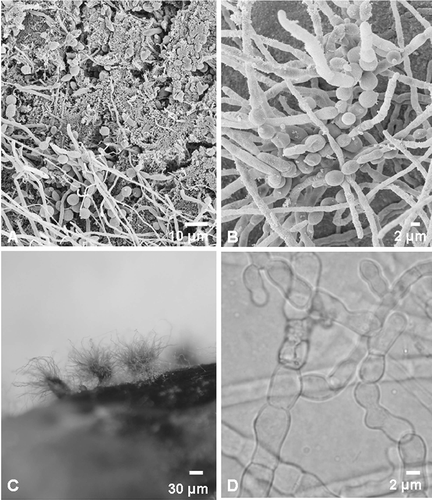
Examination of the skin using scanning electron microscopy revealed a mat of fungal mycelia, yeast-like cells and bacterial colonies covering the skin. A scanning electron micrograph showing the hyphal growth is presented in and B. However, no characteristic features could be recognized enabling species identification.
Figure 1. Micrographs of fungi associated with southern right whale neonate skin. (A, B) Scanning electron micrographs showing fungal growth on the whale skin. (B) Scanning electron micrograph of an early formation of a fruiting body. (C) Fruiting bodies (ascomata) of C. globosum on a section of whale skin. (D) Light micrograph of unusually formed cells of C. globosum starting to form the textura intrica/epidermoidea of the peridium (fruit body wall).
The range of skin sections on growth media yielded four fungal isolates from four different sampling sites on the animals' body. These isolates were identified using morphological characteristics as well as sequencing results of the LSU and the ITS1/5.8S/ITS2 regions of the fungal rDNA; results are presented in These include Chaetomium murorum Corda (W47, W49), C. globosum (W48) and Penicillium coprophilum (Berkeley & M.A. Curtis), Seifert & Samson (1985) (W50).
Table 1. Fungal isolates taken from different skin sections of a southern right whale neonate, cultured on different growth media, identified using morphology and sequencing data from the 26S and ITS regions of the ribosomal gene cluster
To test for the ability to utilize keratin as a source of carbon and nitrogen, the fungal isolates were inoculated onto keratin azure medium (Scott and Untereiner Citation2004), where we found that all the isolates were able to release the blue dye into the underlying clear medium indicating their ability to utilize keratin. Moreover, isolates were able to colonize the sterile whale skin section on the MEA and even formed sexual fruiting bodies on these ().
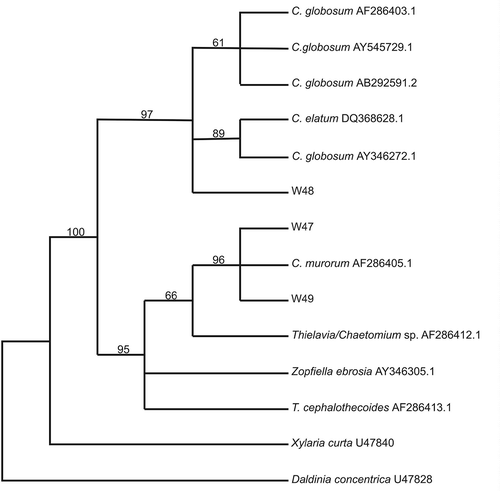
After a few days of incubation at 25°C on MEA, prior to the appearance of fruiting bodies, light microscopy of the Chaetomium isolates revealed the presence of unusual densely packed and irregular jig-saw-shaped cells (textura intrica/epidermoidea) (). These cells later form the peridium (fruit body wall) in these ascomycetes and were later recognized as the structure observed in the scanning electron micrographs (). One to two weeks later, light microscopy of the C. murorum (W47 and W49) isolates revealed violet-blue to black spherical to ovoidal ascomata. The ascomatal setae were long, flexuous often recurved at the apex, smooth, verrucose or spinulose. Asci were clavate containing eight ascospores. The ascospores were ellipsoidal to broadly fusiform, brown with a distinct apical pore and darker longitudinal bands. The C. globosum isolate (W48) was identified by its spherical to ovoidal ascomata with brown peridium. Numerous brownish, unbranched, septate and coiled ascomal hairs could be observed. The asci were clavate, containing eight ascospores. Brown ascospores were limoniform with an apical germ pore. The identities of the C. murorum, as well as C. globosum isolates, could be confirmed using partial sequences of the LSU ribosomal DNA (500–600 bp, 28S) (Spatafora et al., Citation1998). The phylogenetic tree () shows that the two C. murorum isolates group closely with a C. murorum isolate (MUCL 40179; CBS 163.52) (Genbank no.:AF286405.1) from an unknown substrate (96% Parsimony bootstrap). Other closely related species include a Chaetomium/Thielavia sp. from porcupine dung (MUCL 40242) (Genbank no.:AF286412.1), Zopfiella ebrosia (Genbank no.:AY346305.1) and T. cephalothecoides from mouse dung (MUCL 40270) (Genbank no.:AF286413.1) (95% Parsimony bootstrap). The C. globosum isolate grouped with other C. globosum isolates (97% Parsimony bootstrap), including species from canine skin (Genbank no.:AB292591.2), cow dung (Genbank no.:AY346272.1) and stored cotton (Genbank no.:AF286403.1).
Figure 2. Maximum parsimony phylogenetic tree based on rDNA partial large subunit sequences (±500 bp) of whale skin fungal isolates and selected sequences from Genbank. Numbers above branches indicate bootstrap values (>50%) from 1000 replicates.
The Penicillium isolate (W50) was characterized microscopically by an abundance of terverticillate mononematous conidiophores. Conidia were observed to be smooth-walled, ovoid to ellipsoidal, produced in chains from flask-shaped phialidic conidiogenous cells, narrowing towards the collulum. No sexual phase could be observed. Colonies were characterized by a dark brown reverse color on MEA, as well as a brown diffusible color on MEA. Colony diameter after 1 week on CYA was 21–24 mm at 30°C and 29–33 mm at 25°C. Using sequencing data from the internal transcribed spacer 1 region, the 5.8S rDNA gene and the internal transcribed spacer region 2, this species was identified as Penicillium coprophilum. This was confirmed with a phylogenetic analysis where this Penicillium isolate grouped closer to the P. coprophilum spp. than any of the other close related species, such as P. concentricum (Samson, Stolk & Hadlok 1976), P. chrysogenum (Thom, 1910) and P. commune (Thom, 1910).
Genomic DNA extracted directly from skin from the neonate's body and the internal transcribed spacer region of the rDNA was amplified with PCR using fungal specific primers. Sequence analyses of the ITS1/5.8S/ITS2 regions of the rDNA revealed the presence of Fusarium oxysporum (Schlechtendal 1824) (Genbank accession no.:GQ241936).
Discussion
Mycological and systematic analyses of fungal isolates from skin samples revealed the presence of fungi belonging to the genus Chaetomium, which are well known to occur in marine environments (Grishkan et al. Citation2003). Although infections caused by Chaetomium spp. have not yet been reported in marine mammals, Chaetomium spp. are well known to be causative agents of infections in humans: C. globosum, for example, has been related to cases of onychomycosis, cutaneous lesions and was isolated from the pleural fluid of a patient with leukemia (De Hoog et al. Citation2000). In addition, an infection of the subcutaneous tissue and dermis of the chest and abdominal area in a Chinese patient was attributed to C. murorum (De Hoog et al. Citation2000). Other infections caused by Chaetomium spp. include brain abscesses and peritonitis (De Hoog, et al. Citation2000; Guppy et al. Citation1998; Rock Citation1998; Sutton et al. Citation1998). P. coprophilum was also isolated from the cutaneous tissues of the calf although this species is not a documented pathogen. However, the keratin azure experiment clearly proved the ability of this species to utilize keratin, which would explain its presence on the whale skin. Fungi belonging to the genus Penicillium are commonly considered as contaminants but may cause infections, particularly in immuno-compromised hosts (Wong et al. Citation2001). In addition to their infectious potential, nearly all Penicillium spp. are active producers of mycotoxins (Pitt et al. Citation2000). This genus is widespread and represents the most abundant group of mesophilic fungi from temperate soil, decaying vegetation, and many species are airborne, explaining their ubiquity (De Hoog et al. Citation2000).
Results from molecular analyses of genomic fungal DNA directly from skin samples indicated the presence of F. oxysporum. The occurrence of Fusarium infections or fusariosis have been recorded in captive marine mammals (Higgins Citation2000), while an increased frequency of opportunistic infections in humans and in animals, such as reptiles, turtles, pinnipeds and dolphins, were reported over the years (Cabañes et al. Citation1997; Frasca et al. Citation1996). These include evidence of cutaneous nodules, presumed to be caused by F. oxysporum, in a stranded Atlantic white-sided dolphin (Lagenorhynchus acutus) and pygmy sperm whale (Kogia breviceps) along the northeast coast of the USA (Frasca et al. Citation1996). Moreover, similar nodules were found in a captive beluga (Bowenkamp et al. Citation2001).
Many marine mammal studies have proposed a linkage of immuno-suppression with contaminants, environmental stress, including anthropogenic impacts, and disease among marine mammals (Aguilar and Borrell Citation1994; Hutchinson and Simmonds Citation1994; Ross et al. Citation1996a, Citationb). When an animal is faced with a stressor, a variety of neurochemical and hormonal changes occur with notable physiological consequences that have been well-described (Burchfield Citation1985; Chrousos and Gold Citation1992; Guyton and Hall Citation1996). The latter may also increase the probability of infection from pathogenic agents (Solomon et al. Citation1985). This case study is the first report of the presence of the Chaetomium spp. and P. coprophilum with the skin of a southern right whale.
Schaeffer et al. (Citation1998) suggested that literature on pathology in marine mammals may contribute as a tool in assessing the stress and health of an ecosystem. Recognizing alterations in health, assessing and monitoring the effects of stress on marine mammal populations might provide an understanding of underlying causes of mortality among marine mammals (Fair and Becker Citation2000). Our previous study documented a yeast infection in a similar beached southern right whale neonate (Mouton et al. Citation2009), whereas this study provides evidence of the presence of four more fungal species associated with the skin of this second beached neonate. We believe that documenting our findings will contribute to the knowledge of fungi associated with southern right whale skin; thereby, expounding the role of these opportunists in this unique ecological niche.
Acknowledgements
Carolyn Angell, Marc Berkstresser, Leonie Hofmeyr-Juritz and Derek Kemp are acknowledged for their assistance with data collection; Linda Bisset for preparation, use and management of the scanning electron microscope at Iziko South African Museum; Dane Gernecke and Miranda Waldron at the Electron Microscopy Unit, University of Cape Town; Professor Conrad Matthee from the Stellenbosch University for help and training in phylogenetic inference; Dr Nicolene Botha for assistance with artwork. Funding was provided by the National Research Foundation, South Africa, and the Island Foundation, USA.
References
- Aguilar , A and Borrell , A. 1994 . Abnormally high polychlorinated biphenyl levels in striped dolphins (Stenella coeruleoalba) affected by the 1990–1992 Mediterranean epizootic . Sci Total Enviro. , 154 : 237 – 247 .
- Best , PB and McCully , RM. 1979 . Zygomycosis (phycomycosis) in a right whale (Eubalaena australis) . J Comp Pathol. , 89 : 341 – 348 .
- Bowenkamp , K , Frasca , S Jr. , Draghi , A II , Tsongalis , GJ , Koerting , C , Hinckley , L , De Guise , S , Montali , RJ , Goertz , CJ , St Aubin , DJ and Dunn , L. 2001 . Mycobacterium dermatitis and chronic pleuritis in a captive white whale (Delphinapterus leucas) with aortic rupture . J Vet Diag Invest. , 13 : 524 – 530 .
- Buck , JD. 1984 . Microbiological observations on two stranded live whales . J Wildlife Dis. , 20 : 148 – 150 .
- Buck , JD , Shepard , LL and Spotte , S. 1987 . Clostridium perfringens as the cause of death of a captive Atlantic bottlenosed dolphin (Tursiops truncatus) . J Wildlife Dis. , 23 : 488 – 491 .
- Burchfield , SR. 1985 . Stress: Psychological and physiological interactions , New York : Hemisphere Publishing Corporation .
- Cabanes , FJ , Alonso , JM , Castellà , G , Alegre , F , Domingo , M and Pont , S. 1997 . Cutaneous hyalophomycosis caused by Fusarium solani in a sea turtle (Caretta caretta L.) . J Clin Microbiol. , 35 : 3343 – 3345 .
- Chrousos , GP and Gold , PW. 1992 . The concept of stress and stress system disorders . J Am Med Assoc. , 267 : 1244 – 1252 .
- Cove , JH , Eady , EA , Tipper , JL and Cunliffe , WJ. 1992 . “ The role of the cutaneous microflora in host defense and its response to the environment ” . In The environmental threat to the skin , Edited by: Marks , R and Gerd , P . 385 – 389 . London : Martin Dunitz .
- De Hoog , GS , Guarro , J , Gene , J and Figueras , MJ. 2000 . “ Atlas of Clinical Fungi ” . In , 2nd , Vol. 1 , Utrecht, , The Netherlands : Centraalbureau voor Schimmelcultures .
- Dhermain , F , Soulier , L and Bompar , JM. Natural mortality factors affecting cetaceans in the Mediterranean Sea . Cetaceans of the Mediterranean and Black Seas: state of knowledge and conservation strategies. A report to the ACCOBAMS Secretariat . February 2002 , Monaco. Edited by: Notarbartolo di Sciara , G . Section 15
- Fair , PA and Becker , PR. 2000 . Review of stress in marine mammals . J Aquat Ecosyst Stress Recovery , 7 : 335 – 354 .
- Fowler , ME and Cubas , ZS. 2001 . Biology, Medicine, and Surgery of South American Wild Animals , Ames, IA : Blackwell Publishing .
- Frasca , S Jr , Dunn , JL , Cooke , JC and Buck , JD. 1996 . Mycotic dermatitis in an Atlantic white-sided dolphin, a pygmy sperm whale, and two harbor seals . J Am Vet Med Assoc. , 208 : 727 – 729 .
- Gebhart , W and Kersten , A. 1992 . “ The host's immunological contribution to the ecological battle on the skin surface ” . In The environmental threat to the skin , Edited by: Marks , R and Gerd , P . 391 – 393 . London : Martin Dunitz Ltd .
- Grishkan , I , Nevo , E and Wasser , SP. 2003 . Soil micromycete diversity in the hypersaline Dead Sea coastal area, Israel . Mycol Prog. , 2 : 19 – 28 .
- Guppy , KH , Thomas , C , Thomas , K and Anderson , D. 1998 . Cerebral fungal infections in the immunocompromised host: A literature review and a new pathogen - Chaetomium atrobrunneum: Case report . Neurosurgery , 43 : 1463 – 1469 .
- Guyton , AC and Hall , JE. 1996 . “ Textbook of Medical Physiology ” . In , 9th , 1 – 1148 . Philadelphia : W.B. Saunders .
- Haldiman , JT , Henk , WG , Henry , RW , Albert , TF , Abdelbhaki , YZ and Duffield , DW. 1985 . Epidermal and papillary dermal characteristics of the bowhead (Balaena mysticetus) . Anat Rec. , 211 : 391 – 402 .
- Henk , WG and Mullan , DL. 1996 . Common epidermal lesions of the bowhead whale, Balaena mysticetus . Scan Microsc Int. , 103 : 905 – 916 .
- Higgins , R. 2000 . Bacteria and fungi of marine mammals . Can Vet J. , 41 : 105 – 116 .
- Hoffman , CS and Winston , F. 1987 . A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli . Gene , 57 : 267 – 272 .
- Hutchinson , JD and Simmonds , MP. 1994 . Organochlorine contamination in pinnipeds . Rev Environ Contam Toxicol. , 136 : 123 – 167 .
- Kurtzman , CP and Fell , JW. 2000 . The yeasts. A taxonomic study , Amsterdam : Elsevier .
- Lloyd-Macgilp , SA , Chambers , SM , Dodd , JC , Fitter , AH , Walker , C and Young , JPW. 1996 . Diversity of the ribosomal internal transcribed spacers within and among isolates of Glomus mosseae and related mycorrhizal fungi . New Phytol. , 133 : 103 – 111 .
- Matsumoto , T. 2005 . “ Fungal disease in dermatology ” . In Principles and practice of clinical mycology , Edited by: Kibbles , C , MacKenzie , DWR and Odds , FC . 103 – 129 . New York : Wiley .
- Mayer , S. 1988 . “ A review of the scientific justifications for the maintaining Cetaceans in captivity ” . In A report for the Whale and Dolphin Conservation Society , Bath, , UK : WSCS .
- Meyer , W and Seegers , U. 2004 . A preliminary approach to epidermal antimicrobial defense in the Delphinidae . Mar Biol. , 144 : 841 – 844 .
- Migaki , G and Jones , SR. 1983 . “ Mycotic diseases in marine mammals ” . In Pathobiology of marine mammal diseases , Edited by: Howard , EB . Vol. II , 1 – 27 . Boca Raton, FL : CRC Press .
- Miller , WG , Padhye , AA , Van Bonn , W , Jensen , E , Brandt , ME and Ridgway , SH. 2002 . Cryptococcosis in a Bottlenose Dolphin (Tursiops truncates) caused by Cryptococcus neoformans var. gattii . J Clin Microbiol. , 40 : 721 – 724 .
- Moeller , RB. Jr. 1997 . “ Pathology of marine mammals with special reference to infectious diseases ” . In Diseases of Marine Mammals , Edited by: Vos , J.S. London : Taylor and Francis .
- Mouton , M , Reeb , D , Botha , A and Best , PB. 2009 . Yeast infection in a beached southern right whale (Eubalaena australis) neonate . J Wildlife Dis. , 45 : 692 – 699 .
- Parry , DA. 1949 . The structure of whale blubber, and a discussion of its thermal properties . Q J Microsc Sci. , 90 : 13 – 25 .
- Pitt , JI . 1973 . An appraisal of identification methods for Penicillium species: Novel taxonomic criteria based on temperature and water relations . Mycology , 65 : 1135 – 1157 .
- Pitt , JI , Basilico , JC , Abarca , ML and Lopez , C. 2000 . Mycotoxins and toxigenic fungi . Med Mycol. , 38 : 41 – 46 .
- Pugliares , KR , Bogomolni , A , Touhey , KM , Herzig , SM , Harry , CT and Moore , MJ. Marine mammal necropsy: an introductory guide for stranding responders and field biologists . Woods Hole Oceanographic Institution Technical Report, WHOI-2007-06 . 2007 .
- Reidarson , TH , McBain , JF , Dalton , LM and Rinaldi , MG. 2001 . “ Mycotic diseases ” . In CRC Handbook of Marine Mammal Medicine: Health, disease and rehabilitation , 2nd , Edited by: Dierauf , LA and Gulland , FMD . Boca Raton, FL : CRC Press .
- Rock , JP. 1998 . Cerebral fungal infections in the immunocompromised host: A literature review and a new pathogen – Chaetomium atrobrunneum: Case report – Comment . Neurosurgery , 43 : 1469
- Ronald , M. 1993 . Handbook of microbiological media , Boca Raton, FL : CRC Press .
- Ross , PS , De Swart , RL , Timmerman , HH , Reijinders , PJH , Vos , JG , Van Loveren , H and Osterhaus , ADME. 1996a . Suppression of natural killer cell activity in harbour seals (Phoca vitulina) fed Baltic Sea herring . Aquat Toxicol. , 34 : 71 – 84 .
- Ross , PS , De Swart , RL , Addison , RF , Van Loveren , H , Vos , JG and Osterhaus , ADME. 1996b . Contaminant-induced immunotoxicity in harbour seals: Wildlife at risk? . Toxicology , 112 : 157 – 169 .
- Schaeffer , DJ , Herricks , EE and Kerster , HW. 1988 . Ecosystem health: 1. Measuring ecosystem health . Environ Manag. , 12 : 445 – 455 .
- Scott , JA and Untereiner , WA. 2004 . Determination of keratin degradation by fungi using keratin azure . Med Mycol. , 42 : 239 – 246 .
- Sokolov , VE. 1982 . Mammal Skin , Berkeley, CA : University of California Press .
- Solomon , GF , Amkraut , AA and Rubin , RT. 1985 . “ Stress, hormones, neuroregulation, and immunity ” . In Stress: Psychological and physiological Interactions , Edited by: Burchfield , SR . 207 – 221 . Washington, DC : Hemisphere Publishing .
- Spatafora , JW , Volkmann-Kohlmeyer , B and Kohlmeyer , J. 1998 . Independent terrestrial origins of the Halosphaeriales (marine Ascomycota) . Am J Bot. , 85 : 1569 – 1580 .
- Stroud , RK and Roffe , TJ. 1979 . Causes of death in marine mammals stranded along the Oregon coast . J Wildlife Dis. , 15 : 91 – 97 .
- Sutton , DA , Fothergill , AW and Rinaldi , MG. 1998 . Guide to clinically significant fungi , Baltimore, MD : Williams & Wilkins .
- Sweeney , JC and Ridgway , SH. 1975 . Common diseases of small cetaceans . Journal of the American Vet Med Assoc. , 167 : 533 – 540 .
- Sweeney , JC , Migaki , G , Vainik , PM and Conklin , RH. 1976 . Systemic mycoses in marine mammals . J Am Vet Med Assoc. , 169 : 946 – 948 .
- Swofford , DL. 2002 . PAUP*: Phylogenetic analysis using Parsimony (and other methods) 4.0 Beta. CD-ROM , Sunderland, MA : Sinauer .
- Tangredi , BP and Medway , W. 1980 . Post-mortem isolation of Vibrio alginolyticus from an Atlantic white-sided dolphin (Lagenorhynchus acutus) . J Wildlife Dis. , 16 : 329 – 331 .
- Untereiner , WA , Dèbois , V and Naveau , FA. 2001 . Molecular systematics of the ascomycete genus Farrowia (Chaetomiaceae) . Can J Bot. , 79 : 321 – 333 .
- Wagner , DK and Sohnle , PG. 1997 . “ Cutaneous defense mechanisms against fungi ” . In Fungal disease. Biology, immunology and diagnosis , Edited by: Jacobs , PH and Nall , L . 161 – 189 . New York : Marcel Dekker .
- White , TJ , Bruns , T , Lee , S and Taylor , DJ. 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR protocols , Edited by: Innes , MA , Gelfand , DH , Sninsky , JJ and White , TJ . 315 – 322 . San Diego, CA : Academic Press .
- Wong , SSY , Wong , KH , Hui , WT , Lee , SS , Lo , JYC , Cao , L and Yuen , KY. 2001 . Differences in clinical and laboratory diagnostic characteristics of penicilliosis marneffei in human immunodeficiency virus (HIV) – and non-HIV-infected patients . J Clin Microbiol. , 39 : 4535 – 4540 .
- Yablokov , AV , Bel'kovich , VM and Vi , Borisov I . 1972 . “ Kity I Del'finy Izd-vo. Moscow: Nauka (translated in 1974 as ‘Whales and Dolphins’ ” . In Part I and II , Arlington, VA : Joint Publications Research Service .