Abstract
Ten species of Pluteus are described and illustrated from specimens recently collected at three remnants of Atlantic forest in São Paulo State, Southeast Brazil. Two new taxa are proposed: Pluteus aureovenatus and P. dominicanus var. hyalinus, as well as a new status, P. sublaevigatus to P. chrysophlebius subsp. sublaevigatus. The occurrence of P. fuligineovenosus, P. jamaicensis, and P. riberaltensis var. conquistensis represent the first records from Brazil. Pluteus albostipitatus, P. fluminensis, P. harrisii and P. xylophilus are also described and illustrated. In addition, a molecular study was performed based on parsimony analyses of nLSU and ITS + 5.8S sequences of Brazilian and other Pluteus species. The generated phylogenetic trees revealed a well-supported clade with all Pluteus species. In the nLSU analyses, it was possible to identify a clade with all species of section Pluteus and another including members of both sections Hispidoderma and Celluloderma. In the ITS + 5.8S analyses, it was possible to identify three major clades, each of them corresponding to the majority representatives of sections Celluloderma, Hispidoderma and Pluteus.
Introduction
Pluteus Fr. is a large and widely distributed genus of presumably saprophytic and non-ectomycorrhizal species (Singer Citation1986; Banerjee and Sundberg Citation1995). The genus as defined by Singer (Citation1986) is characterised by free lamellae, pinkish spore print, absence of both annulus and volva, inamyloid basidiospores, cystidia metuloid or not and inverse hymenophoral trama. However, recently Minnis et al. (Citation2006) and Corriol and Moreau (Citation2007), supported by molecular and morphological data, transferred to Pluteus two annulate species previously classified in the genus Chamaeota (W.G. Sm.) Earle. Thus, the concept of Pluteus must be amended to also accommodate species with partial veil.
The infrageneric classification of Pluteus currently in use subdivides the genus into three sections (Pluteus, Hispidoderma Fayod and Celluloderma Fayod) based on morphological features such as the structure of the pileipellis and characteristics of the pleurocystidia (Singer Citation1958, Citation1986). A hymeniform layer of spheropedunculate cells characterise the pileipellis of the section Celluloderma, while the other two sections exhibit pileipellis with elongate, pilose or hyphous elements. Members of the section Pluteus differ from those of section Hispidoderma by the presence of metuloids and thin-walled pleurocystidia, respectively (Singer Citation1958, Citation1986). Singer (Citation1956, Citation1958, Citation1986) also considered two subsections in section Celluloderma: Mixtini Singer and Eucellulodermini Singer, differed by a pileipellis composed of uniform or heteromorphic elements, respectively.
Vellinga and Schreurs (Citation1985) also proposed an infrageneric classification for Pluteus that maintains the section Pluteus according to Singer's classification and introduces the new section Villosi Schreurs & Vellinga to include the species with pileipellis differentiated as a cutis combined with thin-walled pleurocystidia. The section Celluloderma also was maintained but divided into three subsections according to the shape of the pileipellis cells. The two subsections, Mixtini and Eucellulodermini, proposed by Singer (Citation1956, Citation1958, Citation1986), were maintained and a third subsection was proposed, Hispidodermini (Fayod) Vellinga & Schreurs, which is characterized by a trichodermic pileipellis or by a hymeniderm with cylindrical to fusiform elements, grouping mostly members of section Hispidoderma sensu Singer (Singer Citation1958, Citation1986).
The knowledge of Pluteus in Brazil started with Hennings (Citation1900) with the description of P. scruposus Henn. from Mato Grosso State. Later, Hennings (Citation1904a, Citationb) described P. cervinus var. griseoviridis Henn. from São Paulo State and P. termitum Henn. from Amazonas State. Most of these early species described from Brazil were not preserved and may not be Pluteus, as mentioned by Saccardo and Trotter (Citation1912), who recognised that P. termitum was a possible synonym of Collybia eurhiza (Berk.) Höhn., and by Singer (Citation1958), who established P. scruposus as a synonym of Oudemansiella canarii (Jungh.) Höhn. and P. termitum as a probable Lepiota sp.
Rick (Citation1907, Citation1919, Citation1930, Citation1938, Citation1961) also described and recorded some problematic species. In total, 21 Pluteus taxa from Brazil (Rio Grande do Sul State), including six species (P. cristatulus Rick, P. fibrillosus Rick, P. leptonia Rick, P. sensitivus Rick, P. straminellus Rick, and P. velatus Rick) and two varieties [P. exiguus var. venosus Rick and P. nanus var. podospileus (Sacc. & Cub.) Rick] with Brazilian types. However, Singer (Citation1953, Citation1958) considered several of Rick's collections as misidentifications, nomina dubia, synonyms, or species of other genera.
Another 10 Pluteus taxa described as new from Brazilian materials were described by Bresadola (Citation1920) and Singer (Citation1953, Citation1956, Citation1958, Citation1973, Citation1989): P. amazonicus Singer, P. cervinus var. brasiliensis Bres., P. fluminensis Singer, P. hylaeicola Singer, P. melanopotamicus Singer, P. paraensis Singer, P. riograndensis Singer, P. subfibrillosus Singer, P. umbrinoalbidus Singer, and P. varzeicola Singer. Recently, Menolli and Capelari (Citation2010) described two new species (P. densifibrillosus Menolli & Capelari and P. puttemansii Menolli & Capelari) from materials collected in the Atlantic forest in São Paulo State.
Grandi et al. (Citation1984), Singer (Citation1984, Citation1989), Raithelhuber (Citation1991), Stijve and Meijer (Citation1993), Stijve (Citation1995), Pegler (Citation1997), Meijer (Citation2001, Citation2006, 2008), and Drechsler-Santos et al. (Citation2007) also contributed to increasing the knowledge about Pluteus in Brazil. However, these works are just lists and do not include complete descriptions of the species.
Wartchow et al. (Citation2004, Citation2006) recorded and provided a complete description of P. albostipitatus (Dennis) Singer, P. aquosus Singer, P. beniensis Singer, P. globiger Singer, P. nigrolineatus Murrill, and P. thomsonii (Berk. & Broome) Dennis for Rio Grande do Sul State. However, Rodríguez et al. (Citation2008) considered that the European species P. thomsonii recorded by Wartchow et al. (Citation2004) is probably P. neotropicalis Rodr.-Alcánt., which was recently described (Rodríguez et al. Citation2008) based on Mexican material previously misidentified as P. thomsonii (Rodríguez and Guzmán-Dávalos Citation1999).
Moreover, Meijer (Citation2008) and Menolli and Capelari (Citation2010) recently provided complete descriptions of one species [P. xylophilus (Speg.) Singer] from Paraná State and four [P. densifibrillosus, P. longistriatus (Peck) Peck, P. puttemansii and P. umbrinoalbidus] from São Paulo State, respectively. Meijer (Citation2008) also mentioned that 34 species of Pluteus occur in Paraná State, but he did not mention the names of them. In addition, in his major papers including Pluteus (Stijve and Meijer Citation1993, Meijer Citation2001, Citation2006, Citation2008), it was only possible to localize about 24 Pluteus taxa. According to Meijer (2009, personal communication), the remaining 10 taxa mentioned are different from any other known Pluteus species and so far they have not been formally described.
All records of Pluteus from Brazil are very confusing and incomplete due to poor descriptions, synonyms, misidentifications, the lack of preserved material, and lists lacking descriptions. So far, 70 epithets have been recorded, distributed over nine States (Amazonas, Bahia, Mato Grosso, Pará, Paraná, Rio de Janeiro, Rio Grande do Sul, Rondônia, and São Paulo), with 23 of them represented by Brazilian types. However, only 23 are known for certain or are at least cited with a complete description. The remainders represent misidentifications, synonyms, species of other genera, unpreserved specimens, or are presented in lists.
Molecular studies involving Pluteus are restricted and they do not include South American species. Moncalvo et al. (Citation2002) and Matheny et al. (Citation2006) conducted a broad study of Agaricales, wherein the Pluteus clade was superficially discussed. Later, Minnis et al. (Citation2006) added a Chamaeota sequence to the nLSU analysis done by Moncalvo et al. (Citation2002) and proposed a new combination in Pluteus. Malysheva et al. (Citation2009) and Rodríguez et al. (Citation2009) performed analyses of the ITS +5.8S region with the placement of new species or new records from Russia and Mexico, respectively.
Here, we report, describe and illustrate 10 species of Pluteus recently collected in the remnants of the Atlantic forest in Southeast Brazil. We proposed two new taxa, a new status and three new records for Brazil. Moreover, for the first time in Pluteus, molecular analyses with sequences of South American species based on nLSU and ITS + 5.8S data are presented.
Material and methods
Sampling
The studied materials were collected at the Parque Estadual da Cantareira, São Paulo city; Parque Estadual das Fontes do Ipiranga, São Paulo city; and Reserva Biológica de Paranapiacaba, Santo André city, all of them are remnants of the Atlantic forest in São Paulo State, Southeast Brazil. When necessary, materials from other localities were examined.
Morphological study
The macroscopic descriptions and illustrations of basidiomata were based on fresh material. Colour terms are according to Küppers (Citation1979). For microscopic analyses, the dried materials were rehydrated in 70% ethanol followed by 5% KOH or Melzer's reagent. All microscopic illustrations were made with the aid of a drawing tube. The notation “[a/b/c]” at the beginning of a set of basidiospores data is to be read as “a basidiospores were measured from b basidiomata taken from c collections”. The basidiospores were measured in lateral view, always 20 basidiospores from each basidioma, and the terms denoting their shapes were according to Bas (Citation1969). Q represents the range of the length/width quotient for all the measured spores, Qm represents the average of all computed Q values for all the measured basidiospores and Lm (Wm) represents the average of all lengths (widths) of the measured basidiospores. The terms used to characterize the pleurocystidia as Cervinus- and Magnus-types are according to Singer (Citation1958) and Banerjee and Sundberg (Citation1995). For the pileipellis cells, the length of the pedicel was included in the measurement of the sphaeropedunculate cells, and its length is also reported separately. A separate measurement is given when an apical projection was present. The specimens and the voucher of the sequences are deposited at the herbarium SP. Generic and infrageneric concepts follow Singer (Citation1986); for comparison, the classification proposed by Vellinga and Schreurs (Citation1985) was also considered.
Molecular study
DNA extraction
Procedures for DNA extraction were done according to an adapted protocol by Zolan and Pukkila (Citation1986) using lyophilised basidiomata previously ground to a fine powder in liquid nitrogen. The sample was resuspended in 50 μl of TE, incubated at 37 °C for 30 min after the addition of RNAse (0.01 mg/μl), and stored at −20 °C.
PCR amplification and DNA sequencing
The ITS + 5.8S region was amplified using the primer set ITS1-F and ITS4 (Vilgalys and Hester Citation1990; White et al. Citation1990; Gardes and Bruns Citation1993), and the nLSU rDNA was amplified using the primer set LR0R and LR5 (Moncalvo et al. Citation2000). The PCR reaction, containing 2.0 U of Platinum® Taq DNA Polymerase (Invitrogen), 0.2 mM of each dNTP, 1.5 mM of MgCl2, and 0.2 μM of each primer of the selected region in a total of 100 μl, was performed in a Eppendorf thermocycler, using the following program: 94 °C for 5 min, 40 cycles at 94 °C for 40 s, 55 °C for 30 s, and 72 °C for 60 s, and then 72 °C for 5 min. Amplification products were purified using the PureLink PCR Purification Kit (Invitrogen). DNA sequencing reactions were performed with the DYEnamic ET Dye Terminator Kit in a MegaBACE 1000 DNA sequencer (GE Healthcare) according to the manufacturer's instructions. The samples were sequenced in both directions. The sequences were deposited in GenBank ().
Table 1. Collection data and GenBank accession number of the Pluteus and outgroups taxa analysed
Data analysis
To elucidate the relationship of Pluteus species found in São Paulo State, Brazil, we conducted phylogenetic analyses using nLSU and ITS + 5.8S sequences determined in this study with sequences available in GenBank ().
The sequences were automatically aligned using the Clustal W version (Thompson et al. Citation1994) in the BioEdit version 7.0.5.3 (Hall Citation1999). Parsimony analysis was performed with PAUP version 4.0b10 (Swofford Citation2003). Parsimony trees were obtained by heuristic searches with simple sequence addition in 1000 replicates, employing tree-bisection-reconnection (TBR) branch-swapping algorithm. Characters from the extreme 5′ and 3′ ends of the sequences were deleted from all taxa to obtain individual datasets that had identical start and end positions, gaps were treated as missing, all characters were unordered and equally weighted, and multistate taxa was interpreted as uncertainty. Starting trees were obtained via stepwise addition, with one tree held at each step during stepwise addition and the steepest descent option not in effect. Also, the initial MaxTrees were set to auto-increase, branches of zero length were collapsed (creating polytomies), and MulTrees options were in effect.
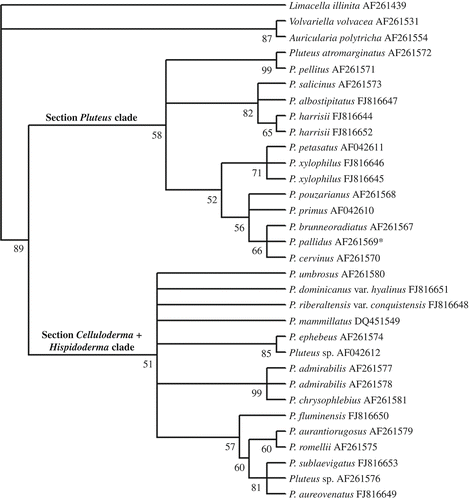
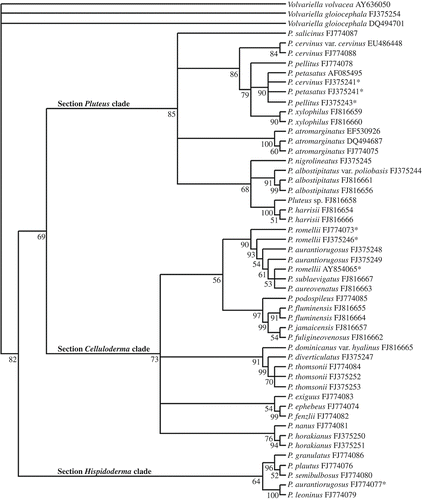
Branch and branch node supports were determined using 1000 BS replicates. Estimated levels of homoplasy and phylogenetic signal (retention and consistency indexes) were determined. The generated trees were rooted to Limacella illinita (Fr.) Maire, Auricularia polytricha (Mont.) Sacc. and Volvariella volvacea (Bull.) Singer as the outgroup taxa for nLSU analysis following Minnis et al. (Citation2006), and V. gloiocephala (DC.) Boekhout & Enderle and V. volvacea for the ITS +5.8S analysis ().
Results and discussion
Sequence data from the nLSU region
In the nLSU analysis, 29 sequences of 26 taxa plus three outgroups were aligned. The alignment dataset consisted of 1304 characters, including gaps. Prior to analysis, 515 characters from 5′ and 3′ ends of the sequences were excluded. Of the 789 characters included in the analysis, 590 characters were constant, 109 variable characters were parsimony-uninformative and 90 were parsimony-informative.
The heuristic searches retained 24 equally most parsimonious trees resulting in a bootstrap 50% majority-rule consensus tree with the following scores: tree length = 380 steps, CI = 0.592, RI = 0.703, RC = 0.416, and HI = 0.408.
The MP tree generated from the nLSU sequence data () revealed a well-supported clade (89% BS) including all Pluteus species. Two clades can be distinguished with low BS support: one clade (58% BS) clustering species that belong to section Pluteus, and the other revealing a polytomy including members of sections Hispidoderma and Celluloderma (51% BS).
Sequence data from the ITS + 5.8S region
In the ITS + 5.8S analysis, 48 sequences of 31 taxa plus three outgroups were aligned. The alignment dataset consisted of 1397 characters, including gaps and portions of nSSU and/or nLSU. Prior to analysis, 898 characters from the 5′ and 3′ ends of the sequences were excluded. Out of the 499 characters included in the analysis, 152 characters were constant, 103 variable characters were parsimony-uninformative and 244 were parsimony informative.
The heuristic searches retained two equally most parsimonious trees resulting in a bootstrap 50% majority-rule consensus tree with the following scores: tree length = 1425 steps, CI = 0.451, RI = 0.711, RC = 0.321, and HI = 0.549.
The MP tree generated from the ITS +5.8S sequence data also revealed a well-supported clade (82% BS), including all Pluteus species, divided into three major clades (). Species from sections Pluteus and Celluloderma are clustered into two sister-clades with 85 and 73% BS support, respectively. In an external branch of these clades there is a third clade that groups the majority species from section Hispidoderma with 64% BS support.
Phylogenetic analyses from the rDNA sequence data
It was possible to confirm the monophyletic origin of Pluteus from both analyses (nLSU and ITS + 5.8S), as also shown by Moncalvo et al. (Citation2002) and Matheny et al. (Citation2006). Moreover, especially in the ITS + 5.8S () it was possible to recognize three major clades, each one corresponding to the sections proposed by Singer (Citation1958, Citation1986).
All species from section Pluteus clustered in the same clade ( and ), forming a well-supported group in accordance with the infrageneric classification of Singer (Citation1958, Citation1986).
The clustering of species from section Celluloderma in one clade was observed in both analyses ( and ). The nLSU analysis for section Celluloderma () showed a polytomy within a clade with low BS support (51%) including members of section Hispidoderma, such as P. umbrosus (Pers.) P. Kumm. and P. riberaltensis var. conquistensis Singer, and also including members of the section Villosi, viz. P. ephebeus (Fr.) Gillet, and species with partial veil traditionally classified in the genus Chamaeota, viz. P. mammillatus (Longyear) Minnis, Sundb. & Methven. The relationship between members of section Celluloderma and section Hispidoderma was also demonstrated by Moncalvo et al. (Citation2002) in a well-supported clade (92% BS).
In the ITS + 5.8S analysis (), the majority members of the section Celluloderma are clustered in a branch connected to section Pluteus clade, while the members of the section Hispidoderma are mostly in an external branch from the clades with members of the sections Pluteus and Celluloderma. Some species formerly classified in the section Hispidoderma, such as P. ephebeus, P. exiguus (Pat.) Sacc. and P. fenzlii (Schulzer) Corriol & P.-A. Moreau (an annulated species), formed a clade with all species of section Celluloderma.
For the members of the section Hispidoderma (including Villosi and Chamaeota members), it will be necessary to perform a detailed study to elucidate the evolutionary history of the group and to find characters that are shared with other members of section Celluloderma (or also of section Pluteus, if the position of P. albostipitatus and P. nigrolineatus are considered, as discussed below). Until now, it has not been possible to recognise the monophyly of section Hispidoderma as proposed by Singer (Citation1958, Citation1986), and perhaps this represents an artificial group delimited by morphological characters. However, one should not disregard the possibility that the section Hispidoderma is a subsection of Celluloderma, as proposed by Vellinga and Schreurs (Citation1985).
In the ITS +5.8S analysis (), it is possible to observe the presence of two species currently classified in section Hispidoderma (P. albostipitatus and P. nigrolineatus) but positioned in the section Pluteus clade. Rodríguez et al. (Citation2009) had already demonstrated the affinities between section Pluteus and section Hispidoderma (89% BS) due to the presence of these species in the same clade with other representative species of section Pluteus. Morphological characters, such as the presence of a slightly thick-walled pleurocystidia and sometimes with poorly developed prongs and/or a repent epicutis, could be used to make assumptions about the position of P. albostipitatus and P. nigrolineatus in the section Pluteus () and to consider that these species probably represent a transition between sections Hispidoderma and Pluteus (see discussion under P. albostipitatus). However, it is not possible to confirm this assumption with ITS + 5.8S analysis because the internal structure of the section Pluteus is unresolved. A molecular study is necessary which would show a well-resolved topology for section Pluteus, placing these species as sister group of all other species in section Pluteus, to confirm this phylogenetic relation (Justo 2009, personal communication).
In both analyses, it is possible to recognize some species or species-complexes that need revision to confirm the scope and/or the monophyly of the section. The nLSU analysis showed the presence of P. pallidus Homola, a species of section Celluloderma, in the section Pluteus clade (). This fact was also observed by Moncalvo et al. (Citation2002) but the authors did not comment the position of P. pallidus and considered the section Pluteus as a well-supported group. However, a re-examination of the voucher material, demonstrated that it actually represents a species of section Pluteus because it has the typical horned and metuloidal pleurocystidia. This was confirmed by Bonnard (Citation2001), who cited the voucher material (JB 90/27) in the original description of P. albineus Bonnard.
In the clade including members of the Cervinus group (section Pluteus clade), based on the ITS + 5.8S analysis (), there is also some species for which identification must be revised. Two sequences of P. cervinus (Schaeff.) P. Kumm., representative (EU486448 and FJ774088) of different countries (USA and Russia, respectively), clustered in a well-supported clade (84% BS), while the Mexican material of P. cervinus (FJ375241) also clustered in a well-supported clade (90% BS), but with sequences of P. pellitus (Pers.) P. Kumm. and P. petasatus (Fr.) Gillet, both from Mexico (FJ375243 and FJ375242, respectively), and with another sequence of P. petasatus from the Czech Republic (AF085495, origin of voucher strain CBS441.85 obtained at http://www.cbs.knaw.nl/databases/). Rodríguez et al. (Citation2009) already reported molecular affinities (96% BS) between their sequences of P. cervinus, P. pellitus and P. petasatus, and also mentioned macroscopic similarities between them. Previous morphological relations between P. pellitus and P. petasatus were indicated by Vellinga and Schreurs (Citation1985) and Rodríguez and Guzmán-Dávalos (Citation2000). The sequence of P. pellitus from Russia (FJ774078), which probably represents this species, formed a separate branch from the Mexican sequence of P. pellitus. The sequences of P. petasatus clustered in a well-supported clade (90% BS), despite their origins being from different regions of the world (Mexico and Czech Republic).
Considering these data, it is probable that the Mexican materials forming the P. cervinus/pellitus/petasatus complex could represent only one species, most likely P. petasatus. Thus, the collections of all P. cervinus, P. pellitus and P. petasatus present in the analysis must be revised because sequences from different collections around the world and with the same identification are not grouped together or species with different names clustered in the same clade. Moreover, many species of the Cervinus group most likely represent a complex of species with morphological similarities, which means that different worldwide species are erroneously named with the same epithet, e.g. P. cervinus.
For the Celluloderma and Hispidoderma clades () some collections of P. romellii (Britzelm.) Sacc. and P. aurantiorugosus (Trog) Sacc. also need revision to confirm their GenBank identification. The sequences of P. romellii (FJ774073, FJ375246 and AY854065) from different countries (Russia, Mexico and USA, respectively) were grouped in the same clade with P. aurantiorugosus, P. aureovenatus Menolli & Capelari and P. sublaevigatus (Singer) Menolli & Capelari (both of the latter herein described) but in different branches. The three sequences of P. romelli need revision, especially the material from the USA (California), which segregated from the other P. romelli sequences and clustered, despite the low support (53% BS), with the morphologically different P. aureovenatus and P. sublaevigatus (see discussion under P. aureovenatus and P. sublaevigatus).
The Russian collection of P. aurantiorugosus (FJ774077) also needs revision because it grouped with 100% BS with P. leoninus (Schaeff.) P. Kumm. in the section Hispidoderma clade (). Both species, despite some ecological and macromorphological similarities pointed out by Malysheva et al. (Citation2009), have different pileipellis structures and are consequently traditionally classified in different sections, Hispidoderma and Celluloderma, respectively. Malysheva et al. (Citation2009) obtained the same support (100% BS) for these species groupings. However, considering this high support and that the other sequences of P. aurantiorugosus (FJ375249 and FJ375246) from different countries clustered in another clade with all species of the section Celluloderma, it is likely that the Russian collection named as P. aurantiorugosus in the GenBank actually represents P. leoninus.
The presence of P. semibulbosus (Lasch) Quél. in the section Hispidoderma clade and the clustering with P. plautus (Weinm.) Gillet (52% BS) should also be regarded with caution, because Singer (Citation1986) considered the first as a member of section Hispidoderma, while Orton (Citation1986) classified it in the section Celluloderma. These data are in accordance with Malysheva et al. (Citation2009), whom already reported molecular affinities (96% BS) between them, and with Vellinga and Schreurs (Citation1985), whom considered P. semibulbosus sensu Singer as synonymous of P. plautus, a member of section Hispidoderma. In addition, Vellinga and Schreurs (Citation1985) considered P. semibulbosus sensu Orton as a P. inquilinus Romagn., which in fact is a member of section Celluloderma.
Taxonomy
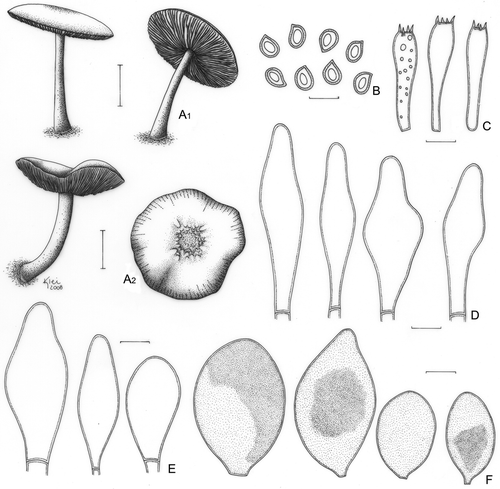
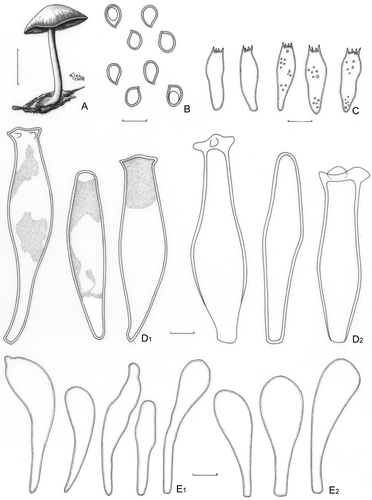
Pluteus albostipitatus (Dennis) Singer, Lloydia 21: 240 (1959) ().
Figure 3. Pluteus albostipitatus. A. Basidioma (Menolli Jr. et al. NMJ128). B. Basidiospores; B1. F. Karstedt & M. Capelari FK782; B2. Menolli Jr. et al. NMJ128. C. Basidia (Menolli Jr. et al. NMJ128). D. Pleurocystidia; D1. F. Karstedt & M. Capelari FK782; D2. Menolli Jr. et al. NMJ128. E. Cheilocystidia; E1. F. Karstedt & M. Capelari FK782; E2. Menolli Jr. et al. NMJ128. Bars (A) = 1 cm; (B–E) = 10 μm.
≡ Pluteus spilopus var. albostipitatus Dennis, Bull. Soc. Mycol. Fr. 69: 195, Citation1953.
Pileus 34–37 mm diam, plane, slightly to deeply umbonate, light brown to greyish brown (N60A50M40 to N80A40M50), darker with coffee shades at the centre (N90A50M60), covered by appressedly and radially arranged fibrils, more concentrated at the centre and dissolving outside of the central disc, finely pruinose at the centre, margin sulcate-striate. Lamellae free, white then pinkish, crowded, with lamellulae. Stipe 35–54 × 2(apex)–4(base) mm, slightly tapering towards the apex, central, occasionally flexuous, white to pale cream, with greyish to slightly silver base, slightly longitudinally striate, with scanty basal mycelium. Odour, taste, and flesh colour not recorded. Basidiospores [40/2/2] (6.2–)7.5–10.0(−11.2) × 6.2–7.5 (−8.7) μm [Q = 1.00–1.29(−1.40); Qm = 1.14; Lm = 8.4 μm; Wm = 7.4 μm], globose to broadly ellipsoid, occasionally subglobose or ellipsoid, widely variable in form, especially among different collections, inamyloid, hyaline, smooth, thick-walled, sometimes guttulate. Basidia (20–) 25–31(−35) × 8.7–12.5 μm, versiform to clavate, thin-walled, four-spored, sometimes with small scattered guttules. Pleurocystidia (39–)50–83(−92) × (11.2–)15.0–24 μm, slightly ventricose with a rounded to subcapitate apex or slightly strangulated at the apex, thin- to relatively thick-walled, sometimes confused with a metuloid with a evenly thickened wall up to 1 μm wide, colourless, hyaline and moderately numerous. In some cases a second and rare type of pleurocystidia is observed, with two short lateral prongs, almost similar in size to the most common type, but rarely shorter in length with approximately 27 μm high. Cheilocystidia (25–)31–52(−55) × (6.2–)8.7–13.7(−15.0) μm, clavate, rarely slightly ventricose, sometimes with a well developed pedicel at the base, colourless, hyaline, thin-walled, abundant. Lamellar edge sterile. Lamellar trama inverse, up to 62 μm wide, composed of thin or slightly thick-walled hyphae, 2.5–10.0(−12.5) μm diam, hyaline. Pileus context undifferentiated, thin, approximately 38 μm thick, composed of thin- or slightly thick-walled hyphae, 3.7–10(−18.7) μm diam, hyaline. Pileipellis a repent epicutis up to 62 μm thick, composed of thin or slightly thick-walled hyphae, 2.5–6.2 μm diam, elongated, septate, with brown vacuolar content. Clamp connections absent in all parts examined.
Habitat and habit: Solitary, on wood.
Material examined: BRAZIL, São Paulo State, São Paulo city, Parque Estadual da Cantareira, Núcleo Engordador, 24 Apr. 2007, Menolli Jr. et al. NMJ128 (SP); 23 Oct. 2006, F. Karstedt & M. Capelari FK782 (SP).
Comments: Pluteus albostipitatus was described from Trinidad (Dennis Citation1953 as P. spilopus var. albostipitatus Dennis), and since then, it was also reported for several countries: Argentina (Singer Citation1956, Citation1958, Horak Citation1964), Bolivia (Singer Citation1958), Martinique (Pegler Citation1983), Galapagos Islands (Reid et al. 1981) and the French Guyana (Courtecuisse Citation1991). For Brazil, this species was already recorded from Paraná (Stijve and Meijer Citation1993 as P. cf. albostipitatus; Meijer Citation2001, Citation2006), Rio Grande do Sul (Wartchow et al. Citation2006) and São Paulo States (Pegler Citation1997).
The two materials herein studied differ themselves in basidiospore, pleuro- and cheilocystidia shapes (). The collection Menolli Jr. et al. NMJ128 has predominantly globose basidiospores, pleurocystidia with evenly thickened walls up to 1.0 μm and rarely with two subacute lateral prongs, and cheilocystidia usually clavate and without a long pedicel at the base. On the other hand, the collection F. Karstedt & M. Capelari FK782 has usually slightly ellipsoid basidiospores, pleurocystidia that sometimes have two rounded lateral prongs and with thin to slightly thickened walls but that are not distinctly thick, and cheilocystidia frequently with a well developed pedicel at the base. These morphological differences observed in our two collections probably represent intraspecific variations, and it will be necessary to study more materials to determine the frequency of these characters and to or not attribute a varietal status to them.
Pluteus albostipitatus is probably a taxon with wide micromorphological variations, especially regarding the basidiospore, pleuro- and cheilocystidia shapes. Singer (Citation1958) observed a variation in the basidiospore shape from subglobose to broad ellipsoid, but never globose, and recorded the presence of short obtuse or subacute small prongs in the pleurocystidia, in accordance with our material, except for the absence of globose basidiospores. Pegler (Citation1983) also observed a variation in basidiospore shape from subglobose to ellipsoid and recorded the presence of a narrow pedicelate base in the cheilocystidia, but observed pleurocystidia without prongs. Horak (Citation1964) and Courtecuisse (Citation1991) described pleurocystidia with rounded or truncate apex, without mention of the presence or absence of prongs and cheilocystidia with a long basal pedicel. However, Horak (Citation1964) recorded basidiospores as round-oval to almost globose as shown in the illustrations presented, like those observed in our collections. Courtecuisse (Citation1991) recorded small basidiospores (5.5–6.5 × 4.5–5.2 μm), but had performed their measurements in an immature material.
Moreover, the materials herein analysed have minor differences when compared to the Brazilian collections studied by Wartchow et al. (Citation2006). They recorded P. albostipitatus with a larger pileus (50–55 mm) and basidiospores varying from subglobose to broad ellipsoid, but never globose or ellipsoid, as observed in our collections. The pleuro- and cheilocystidia are also moderately longer [56.7–97.6(−113) × 12–24 μm and 35.2–71.2 × 8–18.4 μm, respectively], and for the pleurocystidia, there is no mention of the two short lateral prongs and either the wall is slightly thickened and not relatively thick (nearly to metuloid), as observed in our materials. Wartchow et al. (Citation2006) did not describe, but illustrated a different shape of pleurocystidia, with round and small several finger-like protuberances at the apex.
Singer (Citation1973) described P. albostipitatus var. poliobasis from Mexico (Veracruz State), which differs from the type variety only by the pearl-gray colour (griseo) of the stipe base. Regarding this variety characterization, Singer (Citation1956, Citation1958) described the stipe of P. albostipitatus as “white, towards the base mostly pale sordid greyish” or “white, finally becoming somewhat silvery-white to glassy-cinereous”, respectively. The stipe colour of our material resembles the descriptions of Singer (Citation1956, Citation1958), and the stipe base of P. albostipitatus var. poliobasis probably has a darker grayish shade, as mentioned by Rodríguez and Guzmán-Dávalos (Citation2000, Citation2001) from material recollected in Jalisco State, Mexico.
Pluteus albostipitatus is close to P. melanopotamicus Singer described from Amazonas State, Brazil (Singer Citation1989). The latter species is macroscopically the same as P. albostipitatus, and according to the protologue, the main differences between them are the dimorphic basidiospores of P. melanopotamicus, which for us is irrelevant, because type 2 are only 0.5 μm longer than type 1 and there can be a gradual variation in size as observed in P. albostipitatus. The pleurocystidia of P. melanopotamicus were described (Singer Citation1989) as non-metuloidal, with wall thicknesses varying from 0.3 to 0.6 μm, and with round and small several finger-like protuberances at the apex. In our collections of P. albostipitatus, a relatively thickened wall, up to 1.0 μm, was observed which could be compared to those of P. melanopotamicus. On the other hand, the pleurocystidia with apical ornamentation of P. melanopotamicus were not found, but they were observed in the P. albostipitatus collections studied by Wartchow et al. (Citation2006). Probably, either P. melanopotapicus is a later synonym of P. albostipitatus or the material identified by Wartchow et al. (Citation2006) as P. albostipitatus is actually P. melanopotamicus. These materials should be revised to solve this question.
In the ITS + 5.8S analysis (), P. albostipitatus clustered (68% BS) in a clade with P. nigrolineatus, P. albostipitatus var. poliobasis, P. harrisii and Pluteus sp. (section Pluteus). The molecular relation of P. albostipitatus with P. nigrolineatus and P. harrisii may indicate the transitional position of P. albostipitatus between section Hispidoderma and section Pluteus, because P. nigrolineatus belongs to section Hispidoderma, and P. harrisii, although classified in section Pluteus, has pleurocystidia with poorly developed prongs. Pegler (Citation1983) also observed this intermediary position for P. albostipitatus, due to the unusual pleurocystidia shape as reported by Singer (Citation1958), which could indicate an intermediate relationship with the metuloidal pleurocystidia of the section Pluteus.
In the nLSU analysis (), P. albostipitatus clustered (82% BS) with P. salicinus. Despite the results of the molecular analyses, P. albostipitatus is considered distinct from all these species because, among other characteristics, P. harrissi has a glabrous pileus without appressedly fibrils and metuloidal pleurocystidia (Murrill Citation1911; Singer Citation1956, Citation1958; Pegler Citation1983; Banerjee and Sundberg Citation1995), P. nigrolineatus is a clamped species (Singer Citation1956, Citation1961; Wartchow et al Citation2006), and P. salicinus is also clamped and has characteristically metuloid pleurocystidia (Singer Citation1956; Orton Citation1986; Banerjee and Sundberg Citation1995).
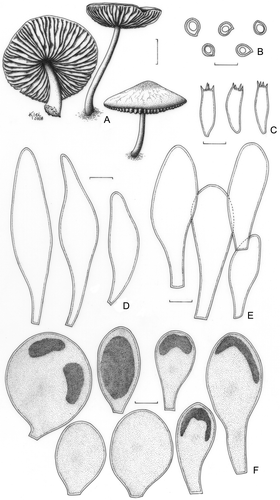
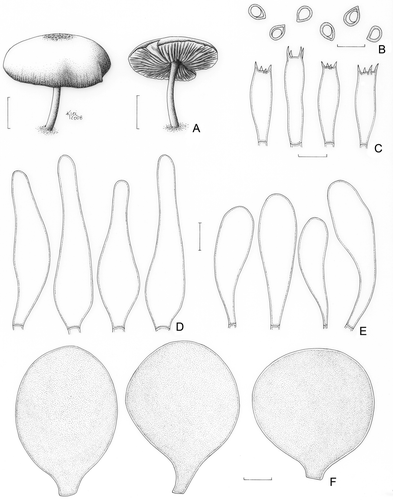
Pluteus aureovenatus Menolli & Capelari, sp. nov. ()
Figure 4. Pluteus aureovenatus (holotype). A. Basidioma. B. Basidiospores. C. Basidia. D. Pleurocystidia. E. Cheilocystidia. F. Pileipellis cells. Bars (A) = 1 cm; (B–F) = 10 μm.
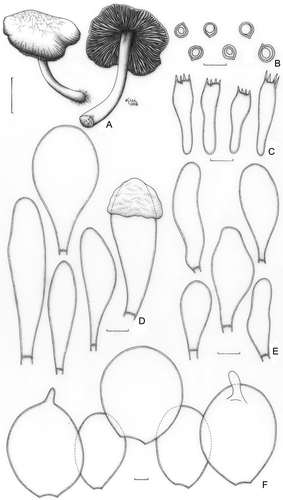
MycoBank: MB 513078
Etym.: The name refers to the colour and ornamentation of the pileus surface.
Pileus 22 mm diam, conicus vel convexus, aureoflavus, hygrophanus, venosus ad rugulosum, venae rubro-luteae, margo leviter sulcata, striata et leviter erosa. Lamellae subliberae, luteae. Stipes 30 × 1(apex)–3(basis) mm, curvus, leviter compressus, subbulformis in basi, centralis, luteus. Basidiosporae 6.2(−7.5) × (5.0–)6.2(−7.5) μm, globosae, hyalinae, inamiloydeae, laeves, crassis parietibus. Pleurocystidia (50–)56–68(−64) × (12.5–)15.0–22(−31) μm, clavata, hyalina, cum parietibus tenuibus. Cheilocystidia (25–)31–49(−61) × (8.7–)10.0–17.0(−21) μm, clavate vel versiformi-clavata, interdum leviter ventricosa, hyalina, cum parietibus tenuibus. Pileipellis cellularis a strato unico composita ex cellulis subglobosis, 54–70 × (36–)46–58 μm, hyalineis, incoloratis, absque pedicellis, interdum papilla apicali munitis. Fibulae absentes.
Holotypus : BRAZIL, São Paulo State, São Paulo city, Parque Estadual das Fontes do Ipiranga, 24.I.2008, F. Karstedt & L.A.S. Ramos FK1045 (SP).
Pileus 22 mm diam, conic to convex, deep yellowish-orange, hygrophanous, veined-rugulose all over except at the margin, vein reddish-orange, margin slightly sulcate, translucently striate and slightly eroded. Lamellae sub-free, yellow, crowded, with lamellulae. Stipe 30 × 1(apex)–3(base) mm, curved, slightly compressed, with a subbulbous base, central, yellow, smooth, slightly longitudinally striate. Odour, taste, and flesh colour not recorded. Basidiospores [20/1/1] 6.2(−7.5) × (5.0–)6.2(−7.5) μm [Q = 1.00(−1.24); Qm = 1.03; Lm = 6.4 μm; Wm = 6.2 μm] globose, rarely broadly ellipsoid, inamyloid, hyaline, smooth, thick-walled, guttulate. Basidia 27–36 × 7.5–8.7 μm, clavate, slim, thin-walled, four-spored. Pleurocystidia (50–)56–64(−68) × (12.5–)15–22(−31) μm, clavate, sometimes slightly fusoid, frequently with apical mucilage in KOH, colourless, hyaline, thin-walled, moderately abundant. Cheilocystidia (25–)31–49(−61) × (8.7–)10–17(−21) μm, clavate to versiform-clavate, sometimes slightly ventricose, colourless, hyaline, thin-walled, moderately abundant. Lamellar edge heteromorphous. Lamellar trama inverse, up to 44 μm wide, composed of thin-walled hyphae, some commonly inflated, 2.5–10.0(–18.7) μm diam, hyaline, septate, sometimes with oleiferous hyphae up to 3.7 μm diam. Pileus context undifferentiated, up to 230 μm thick, composed of thin-walled hyphae, 3.7–8.7 μm diam, hyaline, septate. Pileipellis cellular approximately 56 μm, composed of one layer of subglobose cells, 54–70 × (36–) 46–58 μm, non-pedicelate, thin-walled, colourless, hyaline, occasionally with an apical extension digitate to papillate up to 20 μm long. Clamp connections absent in all parts examined.
Habitat and habit: Solitary, on wood.
Material examined: BRAZIL, São Paulo State, São Paulo city, Parque Estadual das Fontes do Ipiranga, 24 Jan. 2008, F. Karstedt & L.A.S. Ramos FK1045 (SP—holotype).
Comments: Pluteus aureovenatus is characterised by a deep yellowish-orange and veined pileus, conic to convex and margin translucently striate, sub-free and yellow lamellae, globose basidiospores, large non-pedicelate pileipellis cells, and frequently clavate pleuro- and cheilocystidia.
This species is closely related to P. aurantiacus Murrill, P. aurantiorugosus, and P. laetifrons (Berk. & Curt.) Sacc. However, according to the type description (Murrill, Citation1917) and Smith and Stuntz (Citation1958), P. aurantiacus is different because the pileus is convex umbonate, the basidiospores are subglobose to broadly ellipsoid, the pleuro- and cheilocystidia are similar and slightly shorter [28–38 (−50) × 9–14 × 5–10 μm] when compared to P. aureovenatus, and the cells of the pileipellis are clavate-pedicellate and much smaller (15–33 μm). Singer (Citation1956) considered P. aurantiacus as a synonym for P. aurantiorugosus. However, Smith and Stuntz (Citation1958) recognised them as distinct species, since P. aurantiorugosus has an orange stipe and slightly longer and more elongated basidiospores. Regardless, P. aurantiorugosus differs from P. aureovenatus because, according to Homola (Citation1972), P. aurantiorugosus has a whitish to yellowish stipe at first and then orange to red tints at the base, a orange to yellowish-orange pileus in the dried specimens instead of beige-straw for P. aureovenatus, basidiospores that are not predominantly globose (6–7 × 4.5–5 μm), and cells of the pileipellis that are vesiculose, clavate, or occasionally subfusoid-ventricose and much shorter (22–39 × 22–31 μm) than P. aureovenatus.
Pluteus laetifrons is another species with orange-red pileus related to P. aureovenatus. However, Dennis (Citation1953) and Singer (Citation1958) described P. laetifrons with slightly smaller basidiospores (spheric with 5–6 μm and 4.5–5.5 × 4.3–5.3 μm, respectively) and according to Dennis (Citation1953) much smaller cells of the pileipellis (20–35 μm). Singer (Citation1958) described a new variety of P. laetifrons from Bolivia, viz. P. laetifrons var. bolivianus Singer, that differs from the type variety due to its larger and almost geometrically globose basidiospores (5.3–6.8 × 5.3–6.8 μm) and its darker, deeply coloured spots on the pileus that consist of pigmented pileipellis cells. Nevertheless, P. aureovenatus has pileipellis cells that are always colourless and basidiospores that are occasionally broadly ellipsoid and slightly longer than those described for P. laetifrons var. bolivianus. Moreover, Singer (Citation1958) did not mention the size of pileipellis cells for P. laetifrons var. bolivianus, which are approximately 20–35 μm, as described in the type variety and they are, therefore, much shorter than those observed for P. aureovenatus.
The relationship of P. aureovenatus with P. aurantiacus, P. aurantiorugosus and P. laetifrons is probably comparable to the relation between P. chrysophlebius and P. sublaevigatus (see discussion under P. sublaevigatus), where these species are separated by molecular data and have some micro-morphological differences such as pileipellis cell size and basidiospores that differ slightly in size and shape.
In the molecular analyses ( and ), P. aureovanatus clustered in the clade with all species of section Celulloderma and together with P. romellii (from USA – California) and P. sublaevigatus. However, P. romellii is described to have a different pileus colour (date-brown, umber or snuff-brown), much longer pleuro- and cheilocystidia [52–100 × (16–)20–40 μm and 30–80 × 12–40(−53) μm, respectively] and pedicelate pileipellis cells with brownish vacuolar contents (Orton Citation1986). Pluteus sublaevigatus differs from P. aureovenatus because it has a yellow pileus that is slightly rugulose just at the centre and without orange tints or reddish-orange veins all over, a sterile lamellar edge and shorter [(35–)44–51(−55) × (22–) 29–34 μm] pedicelate pileipellis cells (see description under P. sublaevigatus). Pluteus aureovenatus belongs to section Celluloderma.
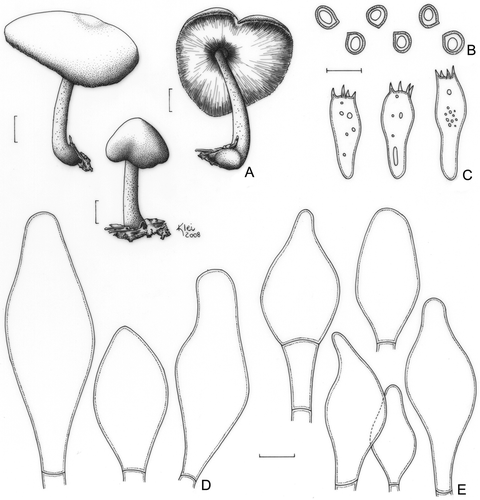
Pluteus dominicanus var. hyalinus Menolli & Capelari, var. nov. ()
Figure 5. Pluteus dominicanus var. hyalinus (holotype). A. Basidioma. B. Basidiospores. C. Basidia. D. Pleurocystidia. E. Cheilocystidia. F. Pileipellis cells. Bars (A) = 1 cm; (B–F) = 10 μm.
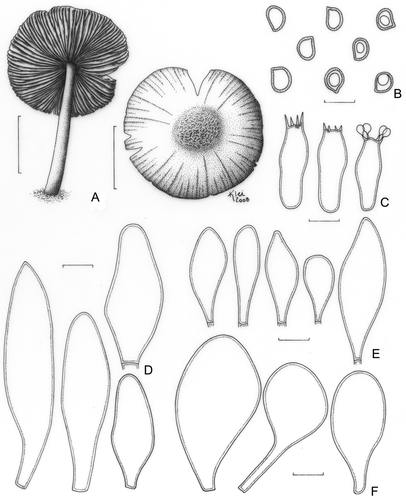
MycoBank: MB 513080
Etym.: The varietal name refers to the hyaline and colourless pileipellis cells.
Similis Pluteus dominicanus var. dominicanus Singer sed cellulis hyalinis et achromaticis pileipellis differt.
Holotypus : BRAZIL, São Paulo State, São Paulo city, Parque Estadual da Cantareira, Núcleo Engordador, 2.I.2008, F. Karstedt et al. FK1058 (SP).
Pileus 31 mm diam, plane-convex, slightly umbonate, beige to light brown, slightly rugose at the centre, margin translucently striate, slightly sulcate or sometimes eroded. Lamellae free, pinkish, subdistant, with lamellulae. Stipe 54 × 2(apex)–5(base) mm, tapering towards the apex, central, whitish to beige, smooth. Odour, taste, and flesh colour not recorded. Basidiospores [20/1/1] 6.2–7.5(−8.7) × 5.0–6.2(−7.5) μm [Q = 1.16–1.24(−1.40); Qm = 1.22; Lm = 7.3 μm; Wm = 6.0 μm], broadly ellipsoid, rarely ellipsoid, inamyloid, hyaline, smooth, thick-walled, sometimes guttulate. Basidia 20–29(−34) × 7.5–8.7 μm, clavate to versiform, thin-walled, four-spored. Pleurocystidia (35–)42–61(−69) × (10.0–)11.2–16.2(−17.5) μm, slightly ventricose, lancelate or sometimes fusiform-clavate, colourless, hyaline, sparse and few seen, thin-walled. Cheilocystidia, like the pleurocystidia, but smaller and very rare, (20–)29–43(−45) × (8.7–)10.0–14.0 μm. Lamellar edge heteromorphous. Lamellar trama inverse, up to 50 μm wide, composed of thin- or slightly thick-walled hyphae, 2.5–7.5(−15.0) μm diam, hyaline. Pileus context undifferentiated, approximately 280 μm thick, composed of thin- or slightly thick-walled hyphae, 3.7–7.5(−18.7) μm diam, hyaline. Pileipellis cellular up to 44 μm thick, composed of one layer of clavate to spheropedunculate cells, (26–) 32–47(−53) × (13.7–)16.2–21(−25) μm, with a short to long pedicel (6.2–25 μm long), thin-walled, colourless, hyaline. Clamp connections absent in all parts examined.
Habitat and habit: Solitary, on wood.
Material examined: BRAZIL, São Paulo State, São Paulo city, Parque Estadual da Cantareira, Núcleo Engordador, 02 Jan. 2008, F. Karstedt et al. FK1058 (SP—holotype).
Comments: Pluteus dominicanus var. hyalinus is proposed as a variety of P. dominicanus especially because of the presence of hyaline and colourless pileipellis cells. P. dominicanus is known only from its type locality (Venezuela) in its original description (Singer Citation1961) and was subsequently recorded by Dennis (Citation1970) based on the same material. Singer (Citation1961) established P. dominicanus as “cheilocystidia not seen” and having pileipellis cells “with deep brownish-melleous dissolved intracellular pigment”, instead of the Brazilian material in which the cheilocystidia are rarely seen and the pileipellis cells are hyaline and colourless. The re-examination of the type of P. dominicanus (K!) demonstrated that the major difference of the new variety is, in fact, regarding the colour of the pileipellis cells. The type of P. dominicanus () has broadly ellipsoid or rarely subglobose basidiospores [20/1/1] 7.5–8.7(−10.0) × 6.2–7.5(−8.7) μm [Q = (1.15–) 1.16(−1.21); Qm = 1.17; Lm = 8.7 μm; Wm = 7.4 μm]; slightly lancelate to ventricose pleurocystidia, 44–55 × 15.0–19.0 μm; cheilocystidia present, 36–55 × 12.5–20(−24) μm, like the pleurocystidia, but that are very rare and only possible to see in tangential view; and pileipellis composed of vesiculose to espheropedunculate cells, 37–48(−57) × 22–34(−39) μm, with a short to medium pedicel (2.5–10.0 μm long) and condensed or dissolved brownish pigment.
Figure 6. Pluteus dominicanus (holotype). A. Basidiospores. B. Basidia. C. Pleurocystidia. D. Cheilocystidia. E. Pileipellis cells. Bars = 10 μm.
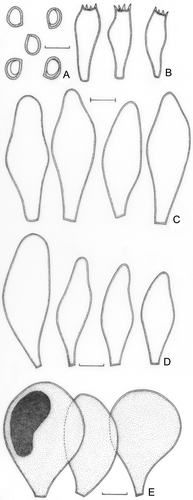
Pluteus dominicanus var. hyalinus is also related to the North American species P. pallidus and P. umbrinodiscus, but it differs from these especially in the pileus colour and the frequency of pleuro- and cheilocystidia.
Pluteus dominicanus belongs to section Celluloderma and clustered with all other species from section Celluloderma in both molecular analyses ( and )
Pluteus fluminensis Singer, Lloydia 21: 292 (1959). ()
Pileus 27–31 mm diam, plane-convex, eventually slightly depressed at the centre or concave, not umbonate, deep sepia (N80A90M60) to porphyry brown, darker brown and rugose to venose at the centre, slightly paler towards the margin, margin sulcate or finely cracking and showing the white flesh. Lamellae free to sub-free, white then pinkish, close, with lamellulae. Stipe 30–38 × 2–3(apex)–5(base) mm, tapering towards the apex, with or without a small bulb, central, hollow, pale cream slightly greyish or translucent, finely pruinose and with finely brownish fibrils at base, slightly longitudinally striate, with scanty basal mycelium. Odour, taste, and flesh colour not recorded. Basidiospores [80/4/4] 6.2–7.5 × 5.0–6.2 μm (Q = 1.21–1.24; Qm = 1.23; Lm = 6.8 μm; Wm = 5.5 μm), broadly ellipsoid, inamyloid, hyaline, smooth, thick-walled, guttulate. Basidia 21–37 × 7.5–8.7(−10.0) μm, clavate to versiform, sometimes more slender, thin-walled, four-spored. Pleurocystidia (41–)47–65(−69) × 12.5–21(−25) μm, ventricose or slightly ventricose to lageniform, rarely vesiculose-saccate, colourless, hyaline, thin-walled, sparse, moderately abundant to scattered. Cheilocystidia (25–)32–50(−56) × (10.0–)11.2–18.7 (−22)μm, like the pleurocystidia or rarely subclavate to clavate, difficult to see, moderately numerous. Lamellar edge heteromorphous. Lamellar trama inverse, up to 62 μm wide, composed of thin- or slightly thick-walled hyphae, 1.2–8.7 (−15.0) μm diam, hyaline, septate. Pileus context undifferentiated, up to 125 μm thick, composed of thin or slightly thick-walled hyphae, 3.7–5.0 μm diam, hyaline, septated, sometimes with oleiferous up to 3.7 μm diam. Pileipellis cellular approximately 45 μm thick, composed of one layer of subglobose, vesiculose or subclavate cells, (20–) 25–46(−52) × (17.5–) 20–32(−37) μm, frequently non-pedicelate, often with a short to medium pedicel (1.2–7.5 μm long), thin-walled, with condensed or dissolved brown pigment. Clamp connections absent in all parts examined.
Habitat and habit: Solitary or in pairs, on wood.
Material examined: BRAZIL, São Paulo State, Cananéia city, Ilha do Cardoso, between Morro Três Irmãos and Sítio Grande, 23 Oct. 1984, M. Capelari MC164 (SP); near Morro Três Irmãos, 18 Dec. 1984, M. Capelari & R. Maziero MC212 (SP); São Paulo city, Parque Estadual da Cantareira, Núcleo Engordador, 24 Apr. 2007, Menolli Jr. et al. NMJ127 (SP); 24 Jan. 2008, F. Karstedt & L.A.S. Ramos FK1046 (SP).
Additional specimen examined: BRAZIL, Rio de Janeiro State, Angra dos Reis, 31 Sep. 1953, R. Singer B432 (F—holotype).
Comments: Pluteus fluminensis was described from Brazil (Rio de Janeiro State) and was also reported for Bolivia and USA (Florida) in its original description (Singer Citation1958). Later, Pegler (Citation1997) reported it for São Paulo State and Stijve and Meijer (Citation1993) and Meijer (Citation2006) for Paraná State as P. cf. fluminensis. The basidiospores of P. fluminensis were described in the protologue (Singer, Citation1958) as “subglobose or rarely a few piriform, (4.5–)5–6.3(−7) × (4–)4.3–5.3(−5.8) μm”; however, upon re-examination of the type material (F!), it was demonstrated that the basidiospores are broadly ellipsoid, [20/1/1] 6.2–7.5 × 5.0–6.2 μm (Q = 1.21–1.24; Qm = 1.23; Lm = 6.8 μm; Wm = 5.5 μm). The pleuro- and cheilocystidia of the type are probably ventricose, but they had collapsed and it was not possible to confirm their size and shape. The pileipellis cells of the type are subglobose to vesiculose and slightly longer [(37–)41–57(−61) × (29–) 31–38(−42) μm] than those of the other collections herein studied.
Pluteus jamaicensis is closely related to P. fluminensis, but, according to Singer (Citation1958), the first has epicuticular elements with dissolved internal pigment instead of the pigmented condensations observed in P. fluminensis. The occurrence of pileipellis cells with condensed brown pigment was confirmed in the re-examination of the type and in additional collections of P. fluminensis herein examined. Singer (Citation1958) also considered a future possibility of a subspecies status for P. fluminensis in P. jamaicensis, but herein they are considered as two distinct species.
Pluteus fluminensis belongs to section Celluloderma and clustered with all other species from section Celluloderma in both molecular analyses ( and ).
Pluteus fuligineovenosus Horak, Nova Hedwigia 8: 190 (Citation1964). ()
Pileus 14–38 mm diam, plane to slightly conic, apparently subumbonate, dark brown, slightly paler towards the margin and darker to blackish at the centre, veined all over especially around the centre then radially arranged towards the margin, vein darker, margin apparently neither sulcate nor striate. Lamellae free to slightly sinuate, white then pinkish, subdistant, with few lamellulae. Stipe 8–31 × 1–4 mm, slightly tapering towards the apex, central to slightly excentric, slightly flexuous, apparently hollow, translucent white, slightly longitudinally striate, with scanty basal mycelium. Odour, taste, and flesh colour not recorded. Basidiospores [20/1/1] 6.2–7.5 × (5.0–)6.2–7.5 μm [Q = 1.00(−1.24); Qm = 1.06; Lm = 6.5 μm; Wm = 6.2 μm], globose, occasionally broadly ellipsoid, inamyloid, hyaline, smooth, thick-walled, guttulate. Basidia 18.7–21(−25) × 6.2–7.5 μm, clavate to versiform, thin-walled, 4-spored. Pleurocystidia (51–)57–77 × 11.2–18.7 μm, fusoid-ventricose to lancelate, colourless, hyaline, thin-walled, sparse and moderately abundant. Cheilocystidia (37–)45–63 × (12.5–)15.0–20 μm, clavate to elongate-clavate, colourless, hyaline, thin-walled, not numerous. Lamellar edge apparently sterile. Lamellar trama inverse, up to 31 μm wide, composed of thin- or slightly thick-walled hyphae, 3.7–11.2 μm diam, hyaline, few septa seen, with oleiferous hyphae up to 5.0 μm diam. Pileus context undifferentiated, approximately 47 μm thick, composed of thin or slightly thick-walled hyphae, 5.0–7.5 μm diam, hyaline, few septa seen. Pileipellis cellular up to 100 μm thick, composed of one layer of vesiculose to spheropedunculate cells, (32–) 39–50(−62) × (17.5–)20–32 (−39) μm, with a short to long pedicel (2.5–23 μm long), thin-walled, with condensed or dissolved brown to chestnut-brown pigment. Clamp conncetions absent in all parts examined.
Habitat and habit: Gregarious to disperse, on wood.
Material examined: BRAZIL, São Paulo State, São Paulo city, Parque Estadual da Cantareira, Núcleo Engordador, 12 Dec. 2006, F. Karstedt & M. Capelari MC826 (SP).
Comments: Pluteus fuligineovenosus is characterised by a brown and veined pileus, with darker to blackish centre and veins, globose basidiospores, and spheropedunculate cells of the pileipellis with condensed or dissolved brown to chestnut-brown pigment.
This species is known from Chile where it was originally described (Horak Citation1964), and later from Argentina (Singer Citation1969). The Brazilian collection has basidiospores that are slightly bigger than those mentioned in the original description (5.6–6.6 × 5.2–5.6 μm), but more similar in size [5.8–7(−7.8) × 5−6.2 μm] to those from the Argentinean collections (Singer Citation1969), pleurocystidia that are more frequently fusoid ventricose, and stipe that is apparently hollow and with scanty mycelium at the base, instead of solid stipe and a lack of basal micelium as described from the Chilean material (Horak Citation1964). This work reports for the first time P. fuligineovenosus from Brazil, and it is the second collection since its description.
In the ITS + 5.8S analyses (), P. fuligineovenosus clustered in the clade with other species from section Celluloderma, forming a well-supported clade (99% BS) with P. fluminensis and P. jamaicensis. However, P. fuligineovenosus can be distinguished from P. fluminensis and P. jamaicensis by the basidiospore shape (preponderantly globose in P. fuligineovenosus instead of mostly broadly ellipsoid in P. fluminensis and P. jamaicensis) and the pileus ornamentation, because P. fuligineovenosus is characterised by a pileus with a darker to blackish centre and veins all over, especially around the centre and then radially arranged towards the margin.
Pluteus harrisii Murrill, Mycologia 3: 277 (1911). ()
Pileus 20–33(−55) mm diam, conic to convex, slightly umbonate, hazel (N60A80M70) to brown or with coffee shades, slightly darker at the centre, with or without finely white punctuation especially at the centre, margin finely sulcate to sulcate-striate. Lamellae free, white then pinkish, attachment partially unequal, crowded, with lamellulae. Stipe 30–48(−62) × 2(apex)–5(base) mm, subequal, central, occasionally flexuous, apparently hollow, white to whitish grey, slightly pruinose at the apex, longitudinally striate, with basal mycelium. Odour, taste, and flesh colour not recorded. Basidiospores [80/4/4] 7.5–8.7(−10.0) × (5.0–)6.2–7.5 (−8.7) μm [Q = (1.15–)1.16–1.21(−1.50); Qm = 1.23; Lm = 8.2 μm; Wm = 6.7 μm], broadly ellipsoid, occasionally ellipsoid or rarely subglobose, inamyloid, hyaline, slightly pinkish in KOH, smooth, thick-walled, sometimes guttulate. Basidia 24–30 × 7.5–10.0 μm, versiform to clavate, thin-walled, four-spored, sometimes with small scattered guttules. Pleurocystidia (40–)52–82(−91) × 15.0–24 μm, fusoid-ventricose, colourless, hyaline, sparse, moderately numerous to abundant, moderately thick-walled, wall usually uniform up to 1.2 μm wide or sometimes thinner towards the base and thickening up to 2.5 μm wide at the apex, apices usually with two to four versiform lateral prongs (up to 5.0 μm long), prongs rarely with a secondary bifurcation, occasionally without prongs and with a rounded or deformed apex, sometimes a colourless internal condensed content is present. Cheilocystidia (31–)36–59 × 8.7–15(−17.5) μm, clavate to versiform-clavate, usually with a moderately long pedicel, colourless, hyaline, moderately abundant, thin-walled. Lamellar edge sterile. Lamellar trama inverse, up to 62 μm wide, composed of thin or slightly thick-walled hyphae, 2.5–13.7 μm diam, sometimes branched or with terminal elements slightly inflated up to 17.5 μm diam, hyaline, septate. Pileus context undifferentiated, up to 200 μm thick, composed of thin- or slightly thick-walled hyphae, 3.7–8.7 μm diam, sometimes inflated up to 25 μm diam, hyaline, septate. Pileipellis a repent epicutis, up to 100 μm thick, composed of thin- to slightly thick-walled hyphae, 5.0–12.5 μm diam, elongated, septate, with light to dark brown content, sometimes with terminal elements slightly inflated. Clamp connections absent in all parts examined.
Habitat and habit: Solitary, on leaf of palm tree or decaying wood.
Material examined: BRAZIL, São Paulo State, São Miguel Arcanjo city, Parque Estadual Carlos Botelho, 25 Apr. 1986, M. Capelari & V.L.R. Bononi MC10 (SP); Santo André city, Reserva Biológica de Paranapiacaba, 11 Apr. 1990, M. Capelari et al. MC3282 (SP); 22 Mar. 2007, Menolli Jr. et al. NMJ122 (SP); São Paulo city, Parque Estadual da Cantareira, Núcleo Engordador, 31 Jan. 2008, F. Karstedt et al. FK1066 (SP).
Comments: Pluteus harrisii is characterised by a dark brown pileus, a white stipe with greyish tones, and metuloids with poorly developed apical prongs. This species was originally described from Jamaica by Murrill (Citation1911), who established P. harrisii without cystidia. However, Singer (Citation1956, Citation1958), Pegler (Citation1983) and Banerjee and Sundberg (Citation1995) found thick-walled pleurocystidia with short apical prongs in the holotype.
Besides the type locality, P. harrisii was also found in Cuba (Murrill Citation1911), Trinidad (Baker and Dale Citation1951 as “P. cervinus var. bambusinus”), USA – Florida (Singer Citation1956, Citation1958), Guadeloupe (Pegler Citation1983), Mexico (Vargas Citation1993; Rodríguez and Guzmán-Dávalos Citation2001), and Brazil (Pegler Citation1997; Meijer Citation2006 as P. aff. harrisii).
The Brazilian materials herein studied has basidiospores moderately larger than those found by Singer (Citation1956, Citation1958) from the Central and North American materials (6.0–9.8 × 5.0–6.5 μm) and pleurocystidia sometimes with a colourless amorphous substance, as recorded by Banerjee and Sundberg (Citation1995) in the type revision. Moreover, it has a stipe that is apparently hollow instead of a solid stipe as recorded by Singer (Citation1956, Citation1958) and Pegler (Citation1983).
Two of the studied collections, Menolli Jr. et al. NMJ122 and F. Karstedt et al. FK1066, have the same nLSU sequence and differ only in two base pairs in the ITS + 5.8S sequence. However, Menolli Jr. et al. NMJ122 differs from F. Karstedt et al. FK1066 by the presence of a colourless internal condensed content in the pleurocystidia and fewer cheilocystidia that are usually versiform. It will be necessary to study more collections to determine whether these characters are constant in successive fruitings to verify if they constitute intraspecific variations or if is sufficient to consider them as independent taxa.
The molecular analyses ( and ) show the sequence of P. harrisii clustered with all other species of the section Pluteus and forming a low-supported clade (68% BS), albeit in a separate branch, with P. albostipitatus. However, P. albostipitatus is currently classified in the section Hispidoderma (see discussion under P. albostipitatus) and differs from P. harrisii as it has a densely fibrillose pileus and pleurocystidia that are not distinctly metuloid and frequently without prongs. Pluteus harrisii belongs to section Pluteus.
Pluteus jamaicensis Murrill, Mycologia 3: 278 (1911)()
Pileus 17–34 mm diam, campanulate-convex, sometimes slightly umbonate, dark beige to dark brown (N70A80M70), slightly paler towards the margin, finely rivulose all over, rugose to rivulose at the centre, margin slightly sulcate and sometimes finely cracking and showing the white flesh. Lamellae free to sinuate-remote, white then pinkish, with numerous lamellulae. Stipe 20–30 × 1–2 mm, equal, central, hollow, cream to translucent white, slightly greyish at base. Odour, taste, and flesh colour not recorded. Basidiospores [20/1/1] 6.2(−7.5) × 5.0(−6.2) μm [Q = (1.21–)1.24; Qm = 1.24; Lm = 6.4 μm; Wm = 5.2 μm], broadly ellipsoid, inamyloid, hyaline, smooth, thick-walled, sometimes guttulate. Basidia 25–29(−31) × 6.2–8.7 μm, clavate to versiform, thin-walled, four-spored. Pleurocystidia (46–)50–56 × 10.0–13.7(−17.5) μm, fusoid-ventricose to lancelate, colourless, hyaline, thin-walled, sparse and not abundant. Cheilocystidia (25–)31–45(−50) × (7.5–)10.0–12.5 μm, clavate to elongate-clavate, colourless, hyaline, thin-walled, numerous. Lamellar edge heteromorphous with few basidia. Lamellar trama inverse, up to 62 μm wide, composed of thin- or slightly thick-walled hyphae, 3.7–13.7 μm diam, hyaline. Pileus context undifferentiated, approximately 62 μm thick, composed of thin- or slightly thick-walled hyphae, hyaline, 3.7–10.0 μm diam. Pileipellis cellular up to 125 μm, composed of one or more layers of spheropedunculate cells, (37–)45–54 × (31–)37–42 μm, with a short pedicel (5.0–10.0 μm long), thin-walled, with dissolved brown cytoplasmic content. Clamp connections absent in all parts examined.
Habitat and habit: Gregarious, on wood.
Material examined: BRAZIL, São Paulo State, São Paulo city, Parque Estadual da Cantareira, Núcleo Engordador, 24 Apr. 2007, Menolli Jr. et al. NMJ130 (SP).
Comments: Pluteus jamaicensis was described from Jamaica (Murrill Citation1911), and since then, it was reported from Trinidad (Dennis Citation1953 as P. aethalus var. jamaicensis; Pegler Citation1983), Argentina (Singer and Digilio Citation1951 as “P. phlebophorus var. ?”; Singer Citation1956, Citation1958), Venezuela ? (Dennis Citation1970), and Martinique and Guadalupe (Pegler Citation1983). Moreover, according to Singer (Citation1958), P. phlebophorus (Ditmar) P. Kumm. sensu Rick (Citation1938) reported from São Leopoldo, Rio Grande do Sul State, Brazil, is perhaps P. jamaicensis. This species was originally described without cystidia (Murrill Citation1911), but posterior revisions of the type confirmed the presence of marginal and facial cystidia (Singer Citation1958; Smith and Stuntz Citation1958; Pegler Citation1983; Banerjee and Sundberg Citation1993). The Brazilian collection has a slightly sulcate pileus margin, as reported by Singer (Citation1956, Citation1958) from Argentinean materials; preponderantly clavate cheilocystidia, as described by Pegler (Citation1983); and broadly ellipsoid basidiospores that are slightly longer than those described by Smith and Stuntz (Citation1958) in the revision of the type specimen (subglobose, 5–6 × 5–5.5 μm).
In the ITS + 5.8S analysis (), P. jamaicensis clustered with P. fluminensis and P. fuligineovenosus. However, they are morphologically distinct (see discussion under P. fluminensis and P. fuligineovenosus). This work reports P. jamaicensis for the first time from Brazil. As supported by the molecular () and the morphological data, Pluteus jamaicensis belongs to section Celluloderma.
Pluteus riberaltensis var. conquistensis Singer, Lloydia 21: 255 (1959). ()
Pileus 27–53 mm diam, campanulate when young, then convex, umbonate, dark brown, slightly darker in the centre, slightly radially coloured, finely pruinose all over and finely cracking especially around the centre, margin not striate and showing the whitish flesh between the fibrils. Lamellae free, pinkish, moderately crowded, with lamellulae. Stipe 53–65 × 4–9 mm, slightly tapering towards the apex, base subbulbous, central to slightly eccentric, white-cream with brown fibrils over the surface especially when young, then brownish and slightly longitudinally striate, with scanty mycelium at base. Odour, taste, and flesh colour not recorded. Basidiospores [20/1/1] 6.2–7.5 × 6.2 μm (Q = 1.00–1.21; Qm = 1.08; Lm = 6.7 μm; Wm = 6.2 μm), globose or occasionally broadly ellipsoid, inamyloid, hyaline, smooth, thick-walled, guttulate. Basidia 21–27(−31) × (7.5–)8.7–10.0 μm, clavate, thin-walled, four-spored, with small scattered guttules. Pleurocystidia (44–)48–69(−78) × (15.0–)17.5–28(−35) μm, slightly ventricose to lageniform, sparse and not abundant. Cheilocystidia (27–)30–47(−57) × (12.5–)17.7–27 μm, like pleurocystidia, but very rooting on the hymenium and hard to see. Lamellar edge heteromorphous with abundant basidia. Lamellar trama inverse, up to 37 μm wide, composed of thin- or thick-walled hyphae, 3.7–10.0 μm diam, hyaline, few septa seen, with oleiferous hyphae up to 3.7 μm diam. Pileus context undifferentiated, approximately 125 μm thick, composed of thin- or thick-walled hyphae, 3.7–7.5 μm diam, hyaline, septate. Pileipellis a cutis, approximately 100 μm thick, composed of thin-walled hyphae, 10–20 μm diam, elongated, septate, with dissolved brown vacuolar content, sometimes with the terminal elements slightly inflated and often ascendant. Clamp connections absent in all parts examined.
Habitat and habit: In pairs on wood.
Material examined: BRAZIL, São Paulo State, São Paulo city, Parque Estadual das Fontes do Ipiranga, 24 Jan. 2008, F. Karstedt & L.A.S. Ramos FK1043 (SP).
Comments: This variety was described from Bolivia (Singer Citation1958) and differs from the type variety almost exclusively by the umber-grey fibrils on the stipe. The Brazilian specimen is characterised by brown fibrils over the stipe surface, especially when young, as described for this variety. Also, there are some cavities on the pileus surface showing the flesh, probably caused by insects. However, our material has moderately longer pleurocystidia than those described for P. riberaltensis var. conquistensis (38–41 × 14–22.5 μm), and these are more similar to those of the type variety (41–86 x 12.5–39 μm), as described in the protologue (Singer Citation1958).
Singer (Citation1961) described another variety, viz. P. riberaltensis var. missionensis Singer, based on an Argentinean specimen that has a white stipe similar to the type variety but that differs from the latter by the conspicuous incrustation of the cystidia, and the missing differentiation in size of cheilo- and pleurocystidia. Pluteus riberaltensis and its varieties were described based on single collections and no other records have been reported. This work is the first to mention P. riberaltensis var. conquistensis from Brazil and reports the first collection since its description.
Pluteus riberaltensis var. conquistensis, as well as other species formerly classified in the section Hipidoderma (P. ephebeus, P. exiguus, P. umbrosus), clustered in the clade including members of the section Celluloderma. As discussed above, these species probably share morphological similarities, and the scope of the section Hispidoderma must be revised. For now, Pluteus riberaltensis var. conquistensis has been classified in the section Hispidoderma.
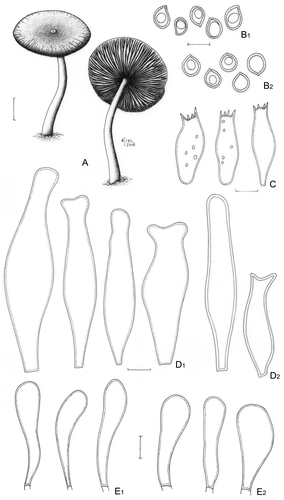
Pluteus sublaevigatus (Singer) Menolli & Capelari, stat. nov. ()
Figure 12. Pluteus sublaevigatus (F. Karstedt et al. FK1085). A. Basidioma. B. Basidiospores. C. Basidia. D. Pleurocystidia. E. Cheilocystidia. F. Pileipellis cells. Bars (A) = 1 cm; (B–F) = 10 μm.
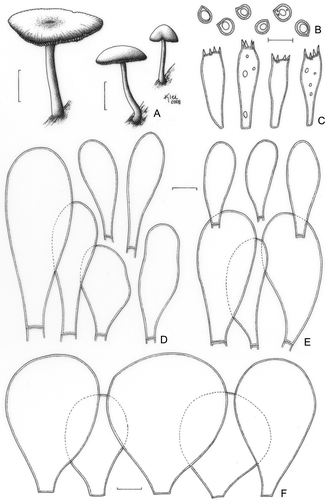
MycoBank: MB 513081
Basionym: Pluteus chrysophlebius subsp. sublaevigatus Singer, Lloydia 21: 278 (1959).
Pileus 11–28 mm diam, campanulate when young, then convex to plane, not umbonate, sometimes slightly depressed at the centre, yellow (N00A30M40 to N00A80M30), hygrophanous, slightly rugulose at the centre, margin translucently striate, sometimes eroded. Lamellae free to subfree, close, yellowish, moderately crowded, with lamellulae. Stipe 28–33 × 1–4 mm, curved, cylindric with a subbulbous base, central, whitish yellow, more yellowish at the base, surface smooth, sometimes with white punctuations and white pruinose mycelium at the base. Odour, taste, and flesh colour not recorded. Basidiospores [40/2/1] (5.0–)6.2–7.5 × 5.0–6.2(−7.5) μm (Q = 1.00–1.21(−1.24); Qm = 1.08; Lm = 6.5 μm; Wm = 6.1 μm), preponderantly globose, sometimes broadly ellipsoid, inamyloid, hyaline, smooth, thick-walled, guttulate, sometimes with small scattered guttules. Basidia (20–)22–27 (−30) × 7.5–8.7(−10.0) μm, clavate, thin-walled, four-spored. Pleurocystidia (34–)36–50(−65) × 12.5–17.5(−25) μm, clavate to vesiculose, sometimes slightly ventricose, very translucent and difficult to see in KOH, sparse and very rare, thin-walled. Cheilocystidia (22–)31–49(−54) × (7.5–)10.0–21(−26) μm, like the pleurocystidia, clavate to vesiculose, very translucent, abundant, thin-walled. Lamellar edge sterile. Lamellar trama inverse, up to 62 μm wide, composed of thin- or slightly thick-walled hyphae, 3.7–10 μm diam, hyaline, septate. Pileus context undifferentiated, up to 187 μm thick, composed of thin- or slightly thick-walled hyphae, 2.5–6.2(−16.2) μm diam, hyaline, septate. Pileipellis cellular up to 50 μm thick, composed of one layer of clavate, vesiculose or subglobose cells, (35–) 44–51(−55) × (22–)29–34 μm, with a short pedicel (2.5–7.5 μm long), thin-walled, colourless, hyaline. Clamp connections absent in all parts examined.
Habitat and habit: Gregarious on decaying wood.
Material examined: BRAZIL, São Paulo State, São Paulo city, Parque Estadual da Cantareira, Núcleo Engordador, 19 Feb. 2008, F. Karstedt et al. FK1085 (SP).
Additional specimen examined: BOLIVIA, Dpto La Paz, Prov. Nor-Yungas, Charobamba, 13 Feb. 1956, R. Singer 1147 (LIL—holotype).
Comments: The frequently globose basidiospores and the large subglobose cells of the pileipellis, along with the results of the molecular analyses, are the basis to propose the new status for P. chrysophlebius ssp. sublaevigatus.
Singer (Citation1958) used the frequently globose basidiospores as the distinctive character to propose P. chrysophlebius ssp. sublaevigatus based on material collected in Bolivia. The Brazilian collection is slightly different because it has a pileus slightly rugose at the centre and pleurocystidia predominantly clavate to vesiculose and slightly longer than those described for P. chrysophlebius ssp. sublaevigatus, which has a pileus with “the disc seemingly smooth when wet, but in dry or dried condition becoming rugulose” and cystidia “about 35 × 15 μm if vesiculose, about 53 × 9.5 μm if ampullaceous” (Singer, Citation1958). These are minor differences that we consider intraspecific variations between the two collections. Moreover, the re-examination of the type of P. sublaevigatus (LIL!) demonstrated that it has basidiospores that are preponderantly globose and rarely broadly ellipsoid, [20/1/1] 6.2–7.5 × 6.2–7.5 μm [Q = 1.00(−1.21); Qm = 1.01; Lm =7.0 μm; Wm = 7.0 μm], as well as a pileipellis composed of vesiculose or subglobose cells, 35–54 × 21–37 μm, characterised as thin-walled, colourless, hyaline and by have a short pedicel (2.5–7.5 μm long). The pleuro- and cheilocystidia of the type are collapsed and it was not possible to confirm their size and shape.
Pluteus sublaevigatus is close to P. chrysophlebius and P. admirabilis in morphology, but the molecular analysis of the nLSU gene () showed that they clustered in separated clades, with P. chrysophlebius and P. admirabilis in one clade and P. sublaevigatus in another clade with the Brazilian yellow species P. aureovenatus.
The morphological re-examination of the three collections (DAOM!) of P. admirabilis and P. chrysophlebius used in the molecular analyses showed some differences in microcharacters among themselves and when compared to P. sublaevigatus (F. Karstedt et al. FK1085), especially in the pileipellis cells (). The pileipellis cells from these collections are usually clavate-vesiculose to subglobose, ranging in size and in the presence or absence of apical projections (). Moreover, the size and shape of basidiospores are a constant character; the pleurocystidia are constantly slightly ventricose with few differences in size; and the cheilocystidia are frequently clavate-vesiculose to slightly ventricose (). These microcharacters partly agree with the type study results presented by Banerjee and Sundberg (Citation1993) and Singer (Citation1956) for P. admirabilis and P. chrysophlebius, and they differ from those observed in the Brazilian collection of P. sublaevigatus ().
Table 2. Micromorphological comparison between collections of Pluteus admirabillis, P. chrysophlebius and P. sublaevigatus
Figures 13–15. 13. Pluteus admirabilis (DAOM193532). 14. P. admirabilis (DAOM197226). 15. P. chrysophlebius (DAOM190194). A. Basidiospores. B. Basidia. C. Pleurocystidia. D. Cheilocystidia. E. Pileipellis cells. Bars = 10 μm.
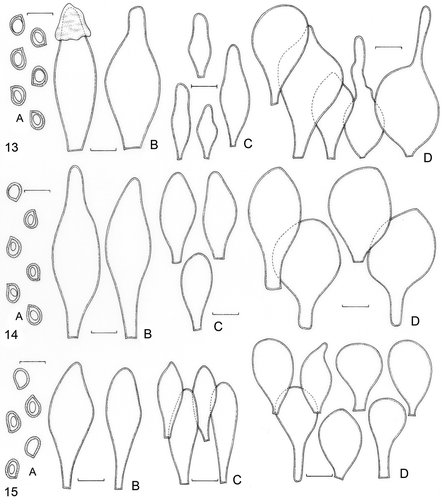
These differences are probably due to intraspecific variations because in our analysis, the nLSU sequences of P. chrysophlebius and P. admirabilis from GenBank clustered in a clade with 99% BS. This relationship between the sequences was already reported by Moncalvo et al. (Citation2002) and Minnis et al. (Citation2006). Then, if these collections really are representatives of P. chrysophlebius and P. admirabilis, according to the morphological and molecular data presented, they cannot be considered independent species. This fact is in accordance with Murrill (Citation1917) and Singer (Citation1956), who already considered them as synonyms.
The study of the voucher collections from the GenBank sequences was important for establishing the morphological differences between them and the Brazilian collections, as well as for supporting the results obtained with the molecular analysis. Pluteus sublaevigatus differs from the vouchers of P. chrysophlebius and P. admirabilis because it has close and not remote lamellae, basidiospores that are frequently globose, larger pileipellis cells, and similar pleuro- and cheilocystidia that are predominantly clavate-vesiculose ().
In the ITS + 5.8 analyses (), P. sublaevigatus clustered with low BS support (53%) together with P. aureovenatus and P. romellii. However, as to P. aureovenatus, P. romellii can be distinguished from P. sublaevigatus by the pileus colour and the size of the pleuro- and cheilocystidia. Pluteus aureovenatus can also be separated from P. sublaevigatus as discussed under the description of P. aureovenatus.
Stijve and Meijer (Citation1993) and Meijer (Citation2006) recorded in a checklist the occurrence of P. chrysophlebius subsp. bruchii Singer from Paraná State, Brazil, which probably represents P. sublaevigatus. The records of P. chrysophlebius for Martinique and Guadeloupe by Pegler (Citation1983) also probably represent P. sublaevigatus, due to the description and illustrations presented. Supported by the molecular ( and ) and the morphological data, Pluteus sublaevigatus belongs to section Celluloderma.
Pluteus xylophilus (Speg.) Singer, Lilloa 22: 405 (1951). ()
Figure 16. Pluteus xylophilus. A. Basidioma; A1. Menolli Jr. et al. NMJ138 ; A2. Menolli Jr. et al. NMJ143. B, C, D. Menolli Jr. et al. NMJ143. B. Basidiospores. C. Basidia. D. Pleurocystidia; D1. Normal Cervinus-type; D2. Modified Cervinus-type; D3. Magnus-type. E. Cheilocystidia (Menolli Jr. et al. NMJ138); E1. Short vesiculose; E2. Long-cylindrical-clavate. Bars (A) = 1 cm; (B–E) = 10 μm.
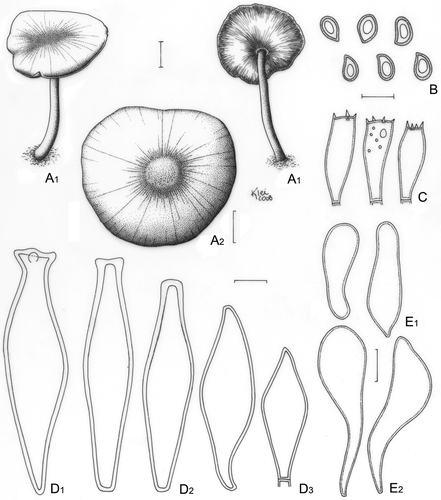
≡ Entoloma xylophilum Speg., Bol. Ac. Nac. Cord. 28: 305, 1926.
Pileus 30–70(−110) mm diam, plane-convex, occasionally slightly concave, sometimes slightly umbonate, light brown (N60A60M50 to N60A40M40), darker at the centre (N80A50M50), paler and discolouring radially towards the margin, margin sometimes splitting and apparently not sulcate or striate. Lamellae remote, slightly pinkish to pinkish, crowded, with lamellulae. Stipe 35–60(−120) × 3–10(apex)–9–20(base) mm, tapering towards the apex, rarely equal with 3 mm diam, central, sometimes flexuous, cream with brown fibrils over the surface and especially at base, longitudinally striate, with scanty mycelium at base. Odour, taste, and flesh colour not recorded. Basidiospores [160/8/7] (5.0–)6.2–7.5(−8.7) × 3.7–6.2 μm [Q = (1.21–)1.24–1.68(−1.74); Qm = 1.38; Lm = 6.7 μm; Wm = 4.9 μm], broadly ellipsoid to ellipsoid, occasionally elongate, inamyloid, hyaline, smooth, thick-walled, guttulate. Basidia (18.7–)21–26 × 6.2–8.7 μm, clavate or versiform, thin-walled, four-spored, with small scattered guttules. Pleurocystidia of three types: (I) the normal Cervinus-type, (46–)52–75(−87) × 12.5–23(−25) μm, fusoid-ventricose, colourless, hyaline, apices usually with two to six lateral prongs, moderately thick-walled especially in the apical region and tapering towards the base, sparse, and abundant to very abundant, frequently with adhered basidiospores on many pleurocystidial apices; (II) the modified Cervinus-type as found in P. harrisii, usually without prongs and subcapitate or slightly strangulated at the apex, or sometimes with two short lateral prongs, size similar to type I; (III) preponderantly of the Magnus-type, usually shorter especially in length, 38–62 × (8.7–)11.0–28 μm, lageniform to fusoid, with acute apex or rarely with a rounded apex, colourless, hyaline, thin- to moderately thick-walled, rare. Cheilocystidia dimorphic, not abundant, very rooting on hymenium, and hard to see: (I) short vesiculose, clavate to subclavate, 21–37 × 8.7–15 μm; (II) long-cylindrical-clavate, sometimes with a moderately long pedicel, 40–69 × 10.0–15.0 μm; sometimes transitions between the two extreme forms are found that are usually slightly ventricose or versiform and moderately deformed. Lamellar edge heteromorphous. Lamellar trama inverse, up to 62 μm wide, composed of thin or slightly thick-walled hyphae, sometimes moderately inflated, 1.2–13.7 μm diam, hyaline, septate. Pileus context undifferentiated, approximately 187 μm thick, occasionally very thick more than 1,000 μm thick, composed of slightly thick-walled hyphae, sometimes inflated, 3.7–21 μm diam, hyaline, septate. Pileipellis a repent epicutis up to 125 μm thick, composed of thin- to slightly thick-walled hyphae, 3.7–6.2 μm diam, elongated, septate, with dissolved brown vacuolar content. Clamp connections absent in all parts examined.
Habitat and habit: Solitary to scattered on wood.
Material examined: BRAZIL, São Paulo State, São Paulo city, Parque Estadual das Fontes do Ipiranga, 10 Nov. 1982, G. Guzmán 22986 (SP); 16 Jan. 1987, Pegler et al. 3712 (SP); 20 June 2006, F. Karstedt et al. FK683 (SP); 30 May 2007, Menolli Jr. et al. NMJ138 (SP); 18 Oct. 2007, Menolli Jr. & F. Karstedt NMJ150 (SP); Parque Estadual da Cantareira, Núcleo Engordador, 21 Aug. 2007, Menolli Jr. et al. NMJ143 (SP); 23 Oct. 2008, M. Capelari & L.A.S. Ramos MC4397 (SP).
Comments: Pluteus xylophilus is characterised by a moderately large pileus, three types of pleurocystidia, and two types of cheilocystidia. The specimens herein studied have surfaces usually with some cavities, probably caused by insects, showing the white flesh.
Singer (Citation1958) described two varieties, viz. P. xylophilus var. tucumanensis Singer and P. xylophilus var. major Singer, based on the size and pigmentation differences of the pileus and the predominance of each cheilocystidia type. However, he emphasises that it is necessary to check whether each of these characters remains constant in successive fruitings and to verify if these morphological variations constitute a forma, varieties, or independent microspecies. Singer's varieties are not considered in this work because considerable morphological variations were not observed.
Singer (Citation1956, Citation1958) accepted two varieties of P. cervinus, viz. P. cervinus var. brasiliensis and P. cervinus var. tucumanensis Singer, as synonyms of P. xylophilus, and also considered P. cervinus sensu Rick (Citation1938), which was recorded for Brazil, as a probable P. xylophilus.
The geographic distribution of P. xylophilus includes Argentina (Spegazzini Citation1925 as Entoloma xylophilum; Singer and Digilio Citation1951 as P. cervinus var. tucumanensis and P. cervinus var. brasiliensis; Singer Citation1958), Bolivia (Singer Citation1958), Brazil (Bresadola Citation1920 as P. cervinus var. brasiliensis; Grandi et al. Citation1984; Stijve and Meijer Citation1993 as P. xylophilus and P. xylophilus var. tucumanensis; Pegler Citation1997; Meijer Citation2006, Citation2008) and Peru (Singer Citation1958).
The molecular analyses ( and ) confirm the position of P. xylophilus in the section Pluteus and relate it with the other species of the Cervinus-group, forming a well-supported clade (86% BS, ).
Acknowledgements
The authors thank Dr Alfredo Justo (Clark University) for critical comments and presubmission review of the manuscript; Dr Tarciso S. Filgueiras (Reserva Biológica do IBGE) and Francisco Kuhar (FCEN Universidad de Buenos Aires) who kindly revised the Latin diagnoses; the curators of DAOM, F, K, LAU and LIL for the loan of collections; Dr Maria Helena Pelegrinelli Fungaro (Universidade Estadual de Londrina) for some DNA sequencing; Fernanda Karstedt (Instituto de Botânica) for collecting some specimens; Klei R. Sousa for preparing the illustrations; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the support and grant to the first author; and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP grant 04/04319-2) for financial support.
References
- Baker , REP and Dale , WT. 1951 . Fungi of Trinidad and Tobago . Mycol Pap , 33 : 1 – 123 .
- Banerjee , P and Sundberg , WJ. 1993 . Reexamination of Pluteus type specimens: types housed at the New York Botanical Garden . Mycotaxon , 49 : 413 – 435 .
- Banerjee , P and Sundberg , WJ. 1995 . The genus Pluteus section Pluteus (Pluteaceae, Agaricales) in the Midwestern United States . Mycotaxon , 53 : 189 – 246 .
- Bas , C. 1969 . Morphology and subdivision of Amanita and a monograph on its section Lepidella . Persoonia , 5 : 285 – 579 .
- Bonnard , J. 2001 . Pluteus albineus sp. nov. (Agaricales, Basidiomycetes) . Mycol Helv , 11 : 131 – 136 .
- Bresadola , G. 1920 . Selecta mycologica . Ann Mycol , 18 : 26 – 70 .
- Corriol , G and Moreau , P-A. 2007 . Agaricus (Annularia) fenzlii redécouvert das les Pyrénées. Notes sur le genre Chamaeota en Europe . Persoonia , 19 : 233 – 250 .
- Courtecuisse , R. 1991 . Eléments pour un inventaire mycologique des environs du saut Pararé (Arataye) et de L´Inselberg des Nouragues, (Guyane française) V. Pluteaceae. (Pluteales, Basidiomycota) . Cryptogam Bot , 2 : 136 – 152 .
- Dennis , RWG. 1953 . Les Agaricales de L'Ile de la Trinité: Rhodosporae-Ochrosporae . Bull Soc Mycol Fr , 69 : 145 – 198 .
- Dennis , RWG. 1970 . Fungus flora of Venezuela and adjacent countries . Kew Bull Addit Ser , 3 : 1 – 531 .
- Drechsler-Santos , ER , Pastorini , LH and Putzke , J. 2007 . Primeiro relato de fungos Agaricales em fragmento de mata nativa em Frederico Westphalen – RS . Rev Bras Bioc , 5 : 471 – 473 .
- Gardes , M and Bruns , TD. 1993 . ITS primers with enhanced specificity for Basidiomycetes – application to the identification of mycorrhizae and rusts . Mol Ecol , 2 : 113 – 118 .
- Grandi , RAP , Guzmán , G and Bononi , VL. 1984 . Adições às Agaricales (Basidiomycetes) do Parque Estadual das Fontes do Ipiranga, São Paulo, SP, Brasil . Rickia , 11 : 27 – 33 .
- Hall , TA. 1999 . BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT . Nucleic Acids Symp Ser , 41 : 95 – 98 .
- Hennings , P. 1900 . Fungi mattogrossenses a Dr. R. Pilger collecti 1899 . Hedwigia , 39 : 134 – 139 .
- Hennings , P. 1904a . Fungi amazonici I. a cl. Ernesto Ule collecti . Hedwigia , 43 : 154 – 186 .
- Hennings , P. 1904b . Fungi S. Paulenses III a cl. Puttemans collecti . Hedwigia , 43 : 197 – 209 .
- Homola , RL. 1972 . Section Celluloderma of the genus Pluteus in North America . Mycologia , 64 : 1211 – 1247 .
- Horak , E. 1964 . Fungi austroamericani II. Pluteus Fr . Nova Hedwigia , 8 : 163 – 199 .
- Küppers , H. 1979 . Atlas de los colores , Barcelona : Editorial Blume .
- Lim , YW and Jung , HS. 1998 . Phylogenetic relationship of Amanita species based on ITS1–5.8S rDNA–ITS2 region sequences . J Microbiol , 36 : 203 – 207 .
- Malysheva , EF , Malysheva , VF and Krasilnikova , AA. 2009 . Morphological and molecular approaches to study the genus Pluteus Fr . Mikol Fitopatol , 43 : 216 – 231 .
- Matheny , PB , Curtis , JM , Hofstetter , V , Aime , MC , Moncalvo , J-M , Ge , ZW , Yang , ZL , Sot , JC , Ammirati , J , Baroni , TJ , Bougher , NL , Hughes , KW , Lodge , DJ , Kerrigan , RW , Seidl , MT , Aanen , DK , DeNitis , M , Daniele , GM , Desjardin , DE , Kropp , BR , Norvell , LL , Parker , A , Vellinga , EC , Vilgalys , R and Hibbett , DS. 2006 . Major clades of Agaricales: a multilocus phylogenetic overview . Mycologia , 98 : 982 – 995 .
- Meijer AAR de . 2001 . Mycological work in the Brazilian State of Paraná . Nova Hedwigia , 72 : 105 – 159 .
- Meijer AAR de . 2006 . Preliminary list of the macromycetes from the Brazilian State of Paraná . Bol Mus Bot Munic , 68 : 1 – 55 .
- Meijer AAR de . 2008 . Notable macrofungi from Brazil's Paraná Pine forest , Colombo : Embrapa Florestas .
- Menolli , N Jr and Capelari , M. 2010 . Notes on Pluteus (Pluteaceae, Agaricales) from Brazil including two new species and a new record . Mycologia , 102 : 697 – 707 .
- Minnis , AM , Sundberg , WJ , Methven , AS , Sipes , SD and Nickrent , DL. 2006 . Annulate Pluteus species: a study of the genus Chamaeota in the United States . Mycotaxon , 96 : 31 – 39 .
- Moncalvo , J-M , Lutzoni , FM , Rehner , SA , Johnson , J and Vilgalys , R. 2000 . Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences . Syst Biol , 49 : 278 – 305 .
- Moncalvo , J-M , Vilgalys , R , Redhead , SA , Johnson , JE , James , TY , Aime , MC , Hofstetter , V , Verduin , SJW , Larsson , E , Baroni , TJ , Thorn , RG , Jacobsson , S , Clémençon , H and Miller , OK. Jr . 2002 . One hundred and seventeen clades of euagarics . Mol Phylogenet Evol , 23 : 357 – 400 .
- Murrill , WA. 1911 . The Agaricaceae of tropical North America . Mycologia , 3 : 271 – 282 .
- Murrill , WA. 1917 . Agaricales Agaricaceae (pars) Agariceae (pars) . North Am Flora , 10 : 77 – 144 .
- Murrill , WA. 1939 . Additions to Florida Fungi - I . Bull Torrey Bot Club , 66 : 29 – 37 .
- Orton , PD. 1986 . Bristish Fungus Flora Agarics and Boleti 4—Pluteaceae: Pluteus & Volvariella , Edinburgh : Royal Botanic Garden .
- Pegler , DN. 1983 . Agaric flora of the Lesser Antilles . Kew Bull Addit Ser , 9 : 1 – 668 .
- Pegler , DN. 1997 . The agarics of São Paulo , Kew : Royal Botanic Gardens .
- Raithelhuber , J. 1991 . “ Flora Mycologica Argentina ” . In Hongos III , Buenos Aires : Stuttgart .
- Rick , J. 1907 . Contributio ad monographiam Agaricearum et Polyporacearum Brasiliensium . Broteria , 6 : 65 – 92 .
- Rick , J. 1919 . Contributio II ad monographiam Agaricinorum brasiliensium . Broteria , 17 : 101 – 111 .
- Rick , J. 1930 . Contributio IV ad monographiam Agaricearum Brasiliensium . Broteria , 24 : 97 – 118 .
- Rick , J. 1938 . Agarici riograndenses . Lilloa , 3 : 399 – 455 .
- Rick , J. 1961 . Basidiomycetes eubasidii in Rio Grande do Sul – Brasilia. 5. Agaricaceae . Iheringia Ser Bot , 8 : 296 – 450 .
- Rodríguez , O , Galván-Corona , A , Villalobos-Arámbula , AR , Vargas , G and Guzmán-Dávalos , L. 2009 . Pluteus horakianus, a new species from Mexico, based on morphological and molecular data . Sydowia , 61 : 39 – 52 .
- Rodríguez , O and Guzmán-Dávalos , L. 1997 . New additions to the genus Pluteus (Pluteaceae, Agaricales) for Mexico . Micol Neotrop Apl , 10 : 83 – 91 .
- Rodríguez , O and Guzmán-Dávalos , L. 1999 . Nuevos registros del género Pluteus (Pluteaceae) en México . Doc Mycol , 29 : 67 – 78 .
- Rodríguez , O and Guzmán-Dávalos , L. 2000 . Algunas especies del género Pluteus Fr. (Pluteaceae, Agaricales) citadas en Nueva Galicia, México . Bol Inst Bot Univ Guadalajara , 7 : 61 – 77 .
- Rodríguez , O and Guzmán-Dávalos , L. 2001 . Clave dicotômica de las espécies del gênero Pluteus Fr. (Pluteaceae) conocidas de la región de Nueva Galicia y algunas áreas aledañas, Mexico . Acta Bot Mex , 57 : 23 – 36 .
- Rodríguez , O , Guzmán-Dávalos , L and Horak , E. 2008 . Pluteus neotropicalis (Pluteaceae, Agaricales), a new species from tropical-subtropical Mexico . Mycotaxon , 103 : 273 – 278 .
- Saccardo , PA and Trotter , A. 1912 . Sylloge Fungorum XXI , Padua : Edwards .
- Singer , R. 1953 . Type Studies on Basidiomycetes VI . Lilloa , 26 : 57 – 159 .
- Singer , R. 1956 . Contributions towards a monograph of the genus Pluteus . Trans Br Mycol Soc , 39 : 145 – 232 .
- Singer , R. 1958 . Monographs of South American Basidiomycetes, especially those of the east slope of the Andes and Brazil. 1. The genus Pluteus in South America . Lloydia , 21 : 195 – 299 .
- Singer , R. 1961 . Monographs of South American Basidiomycetes, especially those of the East Slope of the Andes and Brazil. 4. Inocybe in the Amazone region, whit a Supplement to part 1 (Pluteus in South America) . Sydowia , 15 : 112 – 132 .
- Singer R. 1969. Mycoflora Australis. Beih Nova Hedwigia 29:1–405.
- Singer , R. 1973 . Diagnoses Fungorum Novorum Agaricalium III . Beih Sydowia , 7 : 1 – 106 .
- Singer R. 1984. Adaptation of higher fungi to várzea conditions. Amazoniana 8:311–319.
- Singer , R. 1986 . The Agaricales in Modern Taxonomy Koenigstein, Koeltz Scientific Books
- Singer , R. 1989 . New taxa and new combinations of Agaricales (Diagnoses Fungorum Novorum Agaricalium IV) . Fieldiana Bot , 21 : 1 – 133 .
- Singer , R and Digilio , APL. 1951 . Pródomo de la flora Agaricina Argentina . Lilloa , 25 : 5 – 461 .
- Singh , SK , Yadav , MC , Upadhyay , RC , Kamal , S , Rai , RD and Tewari , RP. 2003 . Molecular characterization of specialty mushroom germplasm of the National Mushroom Repository . Mushroom Res , 12 : 67 – 78 .
- Smith , AH and Stuntz , DE. 1958 . Studies on the genus Pluteus I. Redescriptions of American species based on a study of type specimens . Lloydia , 21 : 115 – 136 .
- Spegazzini , C. 1925 . Observaciones y adiciones a la micología Argentina . Bol Acad Nac Cienc Córdoba , 28 : 266 – 406 .
- Stijve T. 1995. Worldwide occurence of psychoactive mushrooms – an update. Czech Mycol 48:11–19.
- Stijve , T and Meijer AAR de . 1993 . Macromycetes from the state of Paraná, Brazil, 4. The psychoactive species . Arq Biol Tecnol , 36 : 313 – 329 .
- Swofford , D. 2003 . PAUP* Phylogenetic analysis using parsimony (* and other methods), version 4 0b10 , Sunderland, MA : Sinauer Associates .
- Thompson , JD , Higgins , DG and Gibson , TJ. 1994 . Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice . Nucleic Acids Res , 22 : 4673 – 80 .
- Vargas , O , Guzmán-Dávalos , L and Vazquez , LS. 1993 . Observations on some little known macrofungi from Jalisco (Mexico) . Mycotaxon , 49 : 437 – 447 .
- Vellinga , EC and Schreurs , J. 1985 . Notulae ad floram agaricinam neerlandicam – VIII Pluteus Fr. in West-Europe . Persoonia , 12 : 337 – 373 .
- Vilgalys , R and Hester , M. 1990 . Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species . J Bacteriol , 172 : 4238 – 4246 .
- Wartchow , F , Cortez , VG and Coelho , G. 2004 . Pluteus thomsonii (Pluteaceae): A northern agaric found in South America . Mycotaxon , 89 : 349 – 353 .
- Wartchow , F , Cortez , VG and Coelho , G. 2006 . New records of Pluteus (Pluteaceae, Agaricales) from Brazil . Mycotaxon , 96 : 241 – 252 .
- White , TJ , Bruns , T , Lee , S and Taylor , JW. 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR Protocols: A Guide to Methods and Applications , Edited by: Innis , MA , Gelfand , DH , Sninsky , JJ and White , TJ . 315 – 322 . New York : Academic Press . In:editorsp
- Zolan , ME and Pukkila , PJ. 1986 . Inheritance of DNA methylation in Coprinus cinereus . Mol Cell Biol , 6 : 195 – 200 .