Abstract
Arbuscular mycorrhizal (AM) fungus community composition and diversity among the forest, grassland, and cropland ecosystems in the Tibetan Plateau were carried out in this study. A total of 44 AM fungi belonging to six genera were isolated from 144 soil samples collected from forest, grassland, and cropland. Of these AM fungi, 40 taxa were found in forest, 19 in grassland, and 19 in cropland. Glomus was the dominant genus in the three ecosystems, as well as Acaulospora was dominant in forest and grassland. Acaulospora laevis and A. scrobiculata were the dominant species in forest. Acaulospora laevis and Glomus mosseae were dominant in grassland and cropland. The spore density, species richness, and Shannon diversity index of AM fungi from high to low were forest > grassland > cropland. The Sorenson's similarity coefficients of AM fungus community composition ranged from 0.58 to 0.63 between the forest, grassland, and cropland. The results revealed that there was high diversity of AM fungi and the AM fungal community composition varied among the three ecosystems.
Introduction
Arbuscular mycorrhizal (AM) fungi are widely distributed and form mycorrhizas with ca. 80% of vascular plants on earth (Smith and Read Citation1997). AM fungi can increase plant nutrient uptake, reduce pathogenic infection and enhance the resistance of host plants to abiotic stresses such as drought tolerance under certain conditions (Smith and Read Citation1997). AM fungi therefore play an important ecological role in potentialy influencing the plant diversity and species composition, soil aggregation, and carbon and nitrogen storage in terrestrial ecosystems (van der Heijden et al. Citation1998; Miller and Jastrow Citation2000). However, AM fungal communities have been shown to vary with plant community (Bever et al. Citation1996; Eom et al. Citation2000; Vandenkoornhuyse et al. Citation2002; Börstler et al. Citation2006; Aldrich-Wolfe Citation2007; Li et al. Citation2010), as well as abiotic factors (Jansa et al. Citation2002; Oehl et al. Citation2003, Citation2005; Hijri et al. Citation2006; Schalamuk et al. Citation2006; Su and Guo Citation2007). Therefore, understanding the differences in AM fungal communities in various habitats is key to understanding the ecology and function of fungus-plant associations in natural ecosystems.
The Tibetan Plateau (26°44'−36°32'N, 78°25'−99°06’E), as the highest plateau in the world, is known as the “roof of the world” or “the third pole”. Beginning about 70 million years ago, the Cenozoic collision between the Indian and Asian continents formed the Tibetan Plateau in southwestern China and it covers 120 km2 (Unsworth et al. Citation2005). The uplift of the Tibetan Plateau, with an average altitude of 4,500 m a.s.l., forms many high mountains including the Kunlun Mountains, the Kela Kunlun ranges, and the steep Hengduan ranges. Because of the complex geographic conditions, the weather differs sharply during day and night, and contrasting temperatures are found in the north and south of the Tibetan Plateau. The north has a continental climate, and the south is relatively warm and rainy. Simultaneously, the Tibetan Plateau also affects global climate (Raymo and Ruddiman Citation1992). Diverse ecosystems, e.g. forests, grasslands and croplands, form in the Tibetan Plateau.
Several surveys of AM fungi have been carried out in the high areas of altitude, e.g. in the Arctic (Bledsoe et al. Citation1990; Kohn and Stasovski Citation1990; Dalpé and Aiken Citation1998; Pietikäinen et al. Citation2007), the Antarctica (Christie and Nicholson Citation1983; DeMars and Boerner Citation1995), the Austrian Alps (Read and Haselwandter Citation1981), and the Colorado Front Range (Mullen and Schmidt Citation1993). However, as “the third pole”, little is known about the AM fungal diversity of forest, grassland, and cropland in the Tibetan Plateau, except for the AM status of some grassland plants (Gai et al. Citation2006a, Citationb; Citation2009). The lack of research into AM fungal diversity in the Tibetan Plateau has led to the idea that such symbiotic relationships are insignificant and rarely considered. Due to the diverse geographic environments and ecosystems, the Tibetan Plateau has high plant diversity in China.
In order to understand AM fungal diversity and community composition in different ecosystems, soil samples were collected from grasslands, croplands, and forests in the Tibetan Plateau. The basic aims of the present study were to understand 1) whether there is high diversity of AM fungi and 2) what difference of AM fungal community composition is between the forests, grasslands, and croplands in the Tibetan Plateau.
Methods
Study site and sampling procedure
The study was conducted in six sites, i.e. Bomi (29°51N, 95°46′), Gongbo gyamda (29°53′N, 93°14′E), Mainling (29°12′N, 94°12′E), Nyingchi (29°33′N, 94°31′E), Lhunze (28°24′N, 92°19′E), and Lhasa (29°39′N, 91°7′E), in the Tibetan Plateau, which included valley and alp. The altitude of the sampling sites is from 3000 to 4800 m a.s.l. The climate from north to south varies from a semiarid temperate to subtropical climate. The mean annual temperature is –1.2 to 8.5°C, and annual precipitation is about 300–900 mm. In natural forest the trees were dominant by species of Pinaceae, Cupressaceae, Fagaceae, Cuculidae, and Salicaceae. In grassland the plants were dominant by species of Gramineae, Cyperaceae, and Asteraceae. In cropland the main crops were Hordeum vulgare L. var. nudum Hook. f, Triticum aestivum L., Avena sativa L., Vicia faba L., Zea mays L., and Solanum tuberosum L.
A total of 144 soil samples (ca. 1 kg for each) were collected from forest, grassland, and cropland of each site in July 2004. In these soil samples, 48 were collected from forest, grassland and cropland, respectively. We collected soil samples from all forest, grassland and cropland ecosystems in the six sites, except for lack of soil samples from the forest in Lhasa. Soil samples were randomly collected in forest and grassland ecosystems, but soil samples from rhizosphere of crops were collected in cropland ecosystem. Soil samples were placed in sterilized cotton bags, labeled, and air-dried for one week. These soil samples were stored at 10°C for spore isolation.
Spore isolation and identification of AM fungi
Spores of AM fungi were isolated from 100 g air-dried soil for each soil sample using the wet-sieving and decanting method (Gerdemann and Nicolson Citation1963). AM fungi were identified following the current taxonomic criteria (Schenck and Pérez Citation1988), the information on the International Culture Collection of Arbuscular and Vesicular-Arbuscular Mycorrhizal Fungi (http://www.invam.caf.wdu.edu), and the original species descriptions with their emendations. At least 20 spores of each species were used for identification. Spores were mounted in water and morphological characteristics were measured. Melzer's reagent and cotton blue were used in species identification. Permanent slides were mounted in polyvinyl-lacto-glycerol, sealed with nail varnish, and stored in the Herbarium Mycologicum Academiae Sinicae (HMAS) in Beijing, China.
Data analysis
AM fungal composition in different ecosystems was evaluated based on isolation frequency, density, relative abundance, species richness, and importance value. Isolation frequency was calculated as the percentage of samples from which spores of a particular genus or species were isolated (Koske Citation1987). Spore density (spores per 100 g air-dried soil) was calculated from direct counts of spores. Relative abundance was calculated as the number of spores of a particular genus or species divided by the total number of spores. Species richness was defined as the number of AM fungal species per 100 g air-dried soil sample. Spore biovolume was calculated by multiplying the average spore density of a species by the average volume of an individual spore, and this value is expressed as volume of spores per 100 g air-dried soil (Dickman et al. Citation1984). The spore volume was calculated from the equation vol.. Relative spore biovolume was calculated as the biovolume of spores of a particular genus or species divided by the total biovolume of spores (Dickman et al. Citation1984). Important value was calculated as the sum of isolation frequency, relative abundance, and relative spore biovolume (Koske, Citation1987). The dominant genus and species were determined according to the important values.
The Shannon diversity index (H' ) was calculated according to the formula:
where k is the total species number of one site, and Pi is the relative abundance of AM fungus species of one site (Pielou Citation1975; Spellerberg and Fedor Citation2003). Sorenson's coefficient (CS ) was calculated according to the following formula: CS = 2j/(a + b), where j is the number of AM fungus species co-existing in two habitats, a is the total number of AM fungus species in one habitat, and b is the total number of AM fungus species in the other habitat. Data on AM fungal spore density and species richness were analyzed using one-way analysis of variance (ANOVA) to determine any significant difference (SPSS for windows, version 11.5, SPSS Inc, Chicago, USA). The statistically significant difference was determined at p < 0.05 level. A computer program Estimates version 8 was used to calculate species accumulation (rarefaction) curves.
Results
AM fungus composition
A total of 44 AM fungi belonging in six genera were obtained from 144 soil samples collected from forest, cropland, and grassland (). Of these AM fungi, 24 belonged to Glomus, 15 to Acaulospora, two to Pacispora, and one to each Archaeospora, Gigaspora, and Scutellospora, respectively.
Table 1. The importance value of AM fungi isolated from forest, grassland and cropland in the Tibetan Plateau
Acaulospora and Glomus were the dominant genera in forest and grassland, whilst Glomus was the only dominant genus in cropland (). Acaulospora laevis and Glomus mosseae were the overall dominant species in the Tibetan Plateau (, ). Acaulospora laevis and A. scrobiculata were the dominant species in forest (). Acaulospora laevis was dominant in grassland. Glomus mosseae was dominant in cropland.
Table 2. The importance value of AM fungal genera isolated from forest, grassland and cropland in the Tibetan Plateau
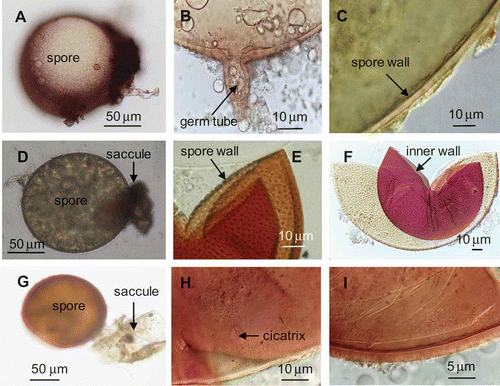
Figure 1. Morphological characteristics of the three dominant arbuscular mycorrhizal fungi. A–C, Glomus mosseae, D–F, Acaulospora scrobiculata, G–I, Acaulospora laevis.
Various numbers of AM fungi were recovered from the different ecosystems (). In the 44 AM fungi, 40 taxa were found in forest, 19 in grassland, and 19 in cropland. Of these AM fungi, 13 taxa were isolated from the three ecosystems, eight taxa from two ecosystems, and 23 taxa from one ecosystem. There were different Sorenson's similarity coefficients of AM fungus composition among the three ecosystems, i.e., between forest and grassland (0.59) > between grassland and cropland (0.55) > between forest and cropland (0.45).
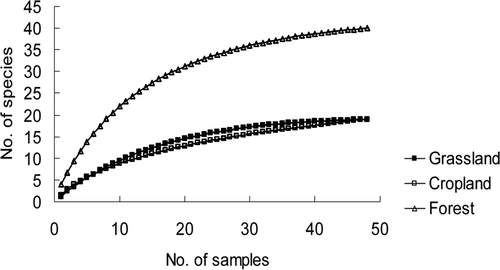
The species accumulation curves of AM fungi isolated from the grassland, cropland and forest nearly approached an asymptote, and the results indicated that most AM fungal species existed in the three ecosystems were isolated from the 48 soil samples ().
Spore density, species richness and diversity of AM fungi
The overall spore density of AM fungi was 73.9 ± 6.8 spores/100 g air-dried soil in the three ecosystems. The spore density of AM fungi from high to low was forest (108 ± 5.1 spores/100 g air-dried soil) > grassland (75.5 ± 8.9 spores/100 g air-dried soil) > cropland (52.5 ± 6.9 spores/100 g air-dried soil), and was significantly higher in forest than in grassland and cropland, but no significant difference between grassland and cropland (). The overall species richness of AM fungi was 2.4 ± 0.1 in the three ecosystems. Furthermore, the species richness of AM fungi from high to low was: forest (2.7 ± 0.2) > grassland (2.3 ± 0.4) > cropland (2.1 ± 0.2), but no significant difference among the forest, grassland, and cropland (). The overall Shannon diversity index of AM fungi was 3.18 and from high to low was: forest (2.78) > grassland (2.48) > cropland (2.2) ().
Table 3. The spore density, species richness and Shannon-Weiner diversity index (H') of AM fungi isolated from forest, grassland and cropland in the Tibetan Plateau
Discussion
The uplift of the Tibetan Plateau, with an average altitude of 4,500 m a.s.l., forms diverse geographic environments, climates, and ecosystems, it is therefore one of the regions with the highest plant diversity in China. Our results indicated that there was high AM fungal diversity (44 taxa) in the Tibetan Plateau compared to the Arctic (Bledsoe et al. Citation1990; Dalpé and Aiken, Citation1998; Pietikäinen et al. Citation2007) and Antarctic (Christie and Nicholson Citation1983; DeMars and Boerner Citation1995) regions. The possible reason is that there is higher plant diversity in the Tibetan Plateau than in the Arctic and Antarctic regions. Our data here may still represent an underestimation, as it is likely that not all AM fungi have sporulated at the sampling time (Bever et al. Citation1996). However, this observed unexpectedly high richness of AM fungi indicated the need to obtain comparable descriptive soil fungal community data from a more intensive sampling design in diverse ecosystems (Tchabi et al. Citation2008).
Our results indicated that the numbers of AM fungal taxa were higher in forest (40) than in grassland (19 taxa) and cropland (19 taxa). Similar results of different AM fungal species richness in various ecosystems have been reported in previous studies based on the analyses of spore morphology and DNA sequences amplified from root samples (Helgason et al. Citation1998; Daniell et al. Citation2001; Oehl et al. Citation2003, Citation2005; Öpik et al. Citation2003, Citation2006; Tchabi et al. Citation2008). For examples, high numbers of AM fungi (34 taxa) were found in subtropical forest of Sichuan province of China (Zhang et al. Citation2004) and in the roots of five host plant species in the Koeru boreal coniferous forest (Öpik et al. Citation2008). Thirty AM fungal species were isolated from Populus-Salix stands along the Verde River in America (Beauchamp et al. Citation2006), and more than 30 taxa were found in tropical forests in Hainan province of China (Shi et al. Citation2006), in Costa Rica (Aldrich-Wolfe Citation2007), and in sub-Saharan Savannas of west Africa (Tchabi et al. Citation2008).
Low numbers of AM fungi have been reported in grassland ecosystem in previous studies (Stutz et al. Citation2000; Öpik et al. Citation2006). For example, 16 AM fungal species were isolated from tallgrass prairie in north America (Eom et al. Citation2000), and 19 AM fungi were found in semi-arid temperate grassland of Inner Mongolia Plateau in China (Su and Guo Citation2007). Gai et al. (Citation2006a, Citationb; Citation2009) recovered 23, 25 and 26 taxa when they investigated the AM fungal diversity in the rhizosphere of dominant and common plants in three types of grassland, nine sedges and 22 plants in grassland ecosystem of Tibet. A similar range of AM fungal species numbers was reported in grassland ecosystem in Europe (Vandenkoornhuyse et al. Citation2002; Oehl et al. Citation2003, Citation2005; Öpik et al. Citation2003; Börstler et al. Citation2006), Africa (Stutz et al. Citation2000; Uhlmann et al. Citation2004), and Japan (Saito et al. Citation2004).
Low numbers of AM fungi were obtained in cropland (22 taxa, Gai et al. Citation2004) compared to wild plants in natural ecosystem (35 taxa, Gai et al. Citation2000) of north China. Tchabi et al. (Citation2008) recovered generally higher AM fungal species richness in natural forest than in crop field when they investigated AM fungi in different ecological zones in sub-Saharan Savannas of west Africa. The AM fungal community contained fewer species, e.g., 18 species in the lands with conventional agriculture and crop rotation and 8 to 13 species in the lands with monocroping in central European arable field (Oehl et al. Citation2003). A further study showed that there was a lower number of AM fungal species in maize field than in grassland (Oehl et al. Citation2005). Similar results of low numbers of AM fungi in cropland were reported in Europe (Daniell et al. Citation2001; Jansa et al. Citation2002; Hijri et al. Citation2006) and America (Franke-Snyder et al. Citation2001; Schalamuk et al. Citation2006; Alguacil et al. Citation2008).
Öpik et al. (Citation2006) summarized the number of AM fungal species per host plant species in different ecosystems detected using molecular techniques in previous studies and concluded that a significant higher richness was found in tropical forest, followed by grassland and arable fields. The difference in diversity of AM fungi observed in our study was assigned to a complex selective pressure such as ploughing, fertilization, and fungicide application in cropland (Jansa et al. Citation2002; Oehl et al. Citation2003; Schalamuk et al. Citation2006; Alguacil et al. Citation2008) and mowing, restoration, fertilization, and grazing management in grassland (Eom et al. Citation2000; Oehl et al. Citation2003; Börstler et al. Citation2006; Su and Guo Citation2007).
Glomus was the dominant genus in the three ecosystems in the present study. Glomus species, as a worldwide distribution fungi, have been commonly found in different ecosystems and geographical regions (Stutz et al. Citation2000; Öpik et al. Citation2006; Tchabi et al. Citation2008). Glomus mosseae was dominant in cropland in our study, and similar results of G. mosseae as the most common and typical AM fungus have been reported in arable fields (Helgason et al. Citation1998; Daniell et al. Citation2001; Oehl et al. Citation2003, Citation2005; Gai et al. Citation2004; Hijri et al. Citation2006; Öpik et al. Citation2006; Alguacil et al. Citation2008), as well as from tropical to temperate regions (Gai et al. Citation2000, Citation2006a, Citationb; Stutz et al. Citation2000; Zhao et al. Citation2001; Zhang et al. Citation2004; Beauchamp et al. Citation2006; Öpik et al. Citation2006; Su and Guo Citation2007) and in high arctic region (Dalpé and Aiken Citation1998).
Acaulospora was also the dominant genus in grassland and forest in the present study. This is in agreement with the observations in rhizosphere soils of some plants in grassland in the Tibetan Plateau (Gai et al. Citation2006a, Citationb; Citation2009), in a subtropical forest in Dujiangyan (Zhang et al. Citation2004), and in tropical rain forests of Xishuangbanna (Zhao et al. Citation2001) and Hainan (Shi et al. Citation2006) of southern China and Costa Rica (Lovelock et al. Citation2003). Acaulospora laevis was the dominant species in grassland and forest, as well as A. scrobiculata being dominant in forest in the present study. Similarly, some Acaulospora species (e.g. Acau 1 sequence type) was dominant in the woodland, but was rare in the arable field based on the analysis of DNA sequences amplified from plant roots (Helgason et al. Citation1999; Daniell et al. Citation2001). Lovelock et al. (Citation2003) showed that Acaulospora morrowiae Spain & N.C. Schenck, A. mellea and A. foveata Trappe & Janos were dominant in La Selva Reserve of tropical rain forest in Costa Rica. Furthermore, A. scrobiculata was dominant in grassland in the Tibetan Plateau (Gai et al. Citation2006a) and in natural forest in sub-Saharan savanna of west Africa (Tchabi et al. Citation2008). However, our results of the dominant species of AM fungi in grassland and forest were different from some previous studies (Oehl et al. Citation2003; Saito et al. Citation2004; Zhang et al. Citation2004; Gai et al. Citation2006b; Su and Guo Citation2007; Öpik et al. Citation2008). It is highly possible that the different habitat types and host species may select a suite of AM fungal species for colonization and sporulation (McGonigle and Fitter Citation1990; Bever et al. Citation1996; Helgason et al. Citation1998, Citation1999; Eom et al. Citation2000; Daniell et al. Citation2001; Vandenkoornhuyse et al. Citation2002; Öpik et al. Citation2003, Citation2006, Citation2008; Börstler et al. Citation2006).
There was different AM fungal community composition (Cs: 0.58-0.63) among the grassland, cropland, and forest, and most AM fungi (52.3% of the total taxa) occurred in one ecosystem and only one third taxa were found in the three ecosystems. The global survey of AM fungal taxa in different habitats was summarized by Öpik et al. (Citation2006), who concluded that AM fungi had a different distribution pattern, e.g., some taxa showed a globe range, others were limited to a few ecosystems only. Therefore, ecosystem type is an important determinant of AM fungal community composition (McGonigle and Fitter Citation1990; Öpik et al. Citation2003, Citation2006; Tchabi et al. Citation2008).
There were different spore densities of AM fungi among the three ecosystems (forest > grassland > cropland) in the Tibetan Plateau. Similarly, higher spore density of AM fungi in the natural forest than in cultivated cropland was found in sub-Saharan Savannas of west Africa (Tchabi et al. Citation2008), and higher spore density of AM fungi in grassland than in cropland was reported in central Europe by Oehl et al. (Citation2003, Citation2005). Öpik et al. (Citation2006) summarized the previous studies and concluded that the spore density of AM fungi per plant species had a similar trend in forest, grassland and cropland with our study. Spore production of AM fungi is known to vary greatly in different ecosystems, and is affected by many environmental and biological factors (Koske Citation1987; Oehl et al. Citation2003; Zhang et al. Citation2004; Öpik et al. Citation2006; Tchabi et al. Citation2008).
The persistence of AM fungi in the Tibetan Plateau depends on the survival of propagules, e.g., spores, soil mycelia, and colonized root systems. Fungal mycelia are known to survive and spread in the soil for several years (Dalpé and Aiken Citation1998). AM fungal spores are usually large, globose thick-walled structures filled with lipids for nutrient reserves (Sancholle and Dalpé Citation1993). They seem to be morphologically and physiologically well preserved in overwintering conditions and may profit from a dominancy period of 2–6 months at low temperate to improve their germination capability (Tommerup Citation1983; Addy et al. Citation1998; Dalpé and Aiken Citation1998; Klironomos et al. Citation2001). Therefore, extreme conditions in the Tibetan Plateau may not be deleterious for the long-term survival of spore populations and sporulating species of AM fungi.
In conclusion, there was high AM fungal diversity, and AM fungal community composition varied among the grassland, cropland, and forest. However, this is a primary study of AM fungal diversity and community composition in the three ecosystems in the Tibetan Plateau. Therefore, there is a need for further studies on the dynamics of AM fungal structure, colonization, and nutrient uptake in extreme natural environments to understand more information on the ecological significance in the Tibetan Plateau.
Acknowledgements
This work was supported by the National Natural Science Foundation of China Grants (Nos. 30499340 and 30870087) and Beijing Nova Program (2008A102).
References
- Addy , HD , Boswell , EP and Koide , RT. 1998 . Low temperature acclimation and freezing resistance of extraradical VA mycorrhizal hyphae . Mycol Res. , 102 : 582 – 586 .
- Aldrich-Wolfe , L. 2007 . Distinct mycorrhizal communities on new and established hosts in a transitional tropical plant community . Ecology , 88 : 559 – 566 .
- Alguacil , MM , Lumini , E , Roldan , A , Salinas-Garcia , JR , Bonfante , P and Bianciotto , V. 2008 . The impact of tillage practices on arbuscular mycorrhizal fungal diversity in subtropical crops . Ecol Appl. , 18 : 527 – 536 .
- Beauchamp , VB , Stromberg , JC and Stutz , JC. 2006 . Arbuscular mycorrhizal fungi associated with Populus-Salix stands in a semiarid riparian ecosystem . New Phytol. , 170 : 369 – 380 .
- Bever , JD , Morton , JB , Antonovics , J and Schultz , PA . 1996 . Host dependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland . J Ecol. , 84 : 71 – 82 .
- Bledsoe , C , Klein , P and Bliss , LC. 1990 . A survey of mycorrhizal plants on Truelove Lowland, Devon Island, N.W.T., Canada . Can J Bot. , 68 : 1848 – 1856 .
- Börstler , B , Renker , C , Kahmen , A and Buscot , F. 2006 . Species composition of arbuscular mycorrhizal fungi in two mountain meadows with differing management types and levels of plant biodiversity . Biol Fertil Soils , 42 : 286 – 298 .
- Christie , P and Nicholson , TH. 1983 . Are mycorrhizas absent from the Antarctic? . Trans Br Mycol Soc. , 80 : 557 – 560 .
- Dalpé , Y and Aiken , SG. 1998 . Arbuscular mycorrhizal fungi associated with Festuca species in the Canadian high Arctic . Can J Bot. , 76 : 1930 – 1938 .
- Daniell , TJ , Husband , R , Fitter , AH and Young , JPW. 2001 . Molecular diversity of arbuscular mycorrhizal fungi colonizing arable crops . FEMS Microbiol Ecol. , 36 : 203 – 209 .
- DeMars , BG and Boerner , RE. 1995 . Mycorrhizal status of Deschampsia Antarctica in the Palmer Station area, Antarctica . Mycologia , 87 : 451 – 453 .
- Dickman , LA , Liberta , AE and Anderson , RC. 1984 . Ecological interaction of little bluestem and vesicular-arbuscular mycorrhizal fungi . Can J Bot. , 62 : 2272 – 2277 .
- Eom , AH , Hartnett , DC and Wilson , GWT. 2000 . Host plant species effects on arbuscular mycorrhizal fungal communities in tallgrass prairie . Oecologia , 122 : 435 – 444 .
- Franke-Snyder , M , Douds , DD , Galvez , L , Phillips , JG , Wagoner , P , Drinkwater , L and Morton , JB. 2001 . Diversity of communities of arbuscular mycorrhizal (AM) fungi present in conventional versus low-input agricultural sites in eastern Pennsylvania, USA . Appl Soil Ecol. , 16 : 35 – 48 .
- Gai , JP , Cai , XB , Feng , G , Christie , P and Li , XL. 2006a . Arbuscular mycorrhizal fungi associated with sedges on the Tibetan plateau . Mycorrhiza , 16 : 151 – 157 .
- Gai , JP , Christie , P , Cai , XB , Fan , JQ , Zhang , JL , Feng , G and Li , XL. 2009 . Occurrence and distribution of arbuscular mycorrhizal fungal species in three types of grassland community of the Tibetan Plateau . Ecol Res. , 24 : 1345 – 1350 .
- Gai , JP , Feng , G , Cai , XB , Christie , P and Li , XL. 2006b . A preliminary survey of the arbuscular mycorrhizal status of grassland plants in southern Tibet . Mycorrhiza , 16 : 191 – 196 .
- Gai , JP , Liu , RJ and Li , XL. 2000 . Ecological distribution of arbuscular mycorrhizal fungi on wild plants in different vegetation regions of Shandong . Chin J Ecol. , 19 : 18 – 22 . (Chinese)
- Gai , JP , Feng , G and Li , XL. 2004 . Diversity of arbuscular mycorrhizal fungi in field soils from North China . Biodiversi Sci. , 12 : 435 – 440 .
- Gerdemann , JW and Nicolson , TJ. 1963 . Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting . Trans Br Mycol Soc. , 46 : 235 – 244 .
- Helgason , T , Daniell , TJ , Husband , R , Fitter , AH and Young , JPW. 1998 . Ploughing up the wood-wide web? . Nature , 394 : 431
- Helgason , T , Fitter , AH and Young , JPW. 1999 . Molecular diversity of arbuscular mycorrhizal fungi colonizing Hyacinthoides non-scripta (bluebell) in a seminatural woodland . Mol Ecol. , 8 : 659 – 666 .
- Hijri , I , Sykorovazu , Z , Oehl , F , Ineichen , K , Mader , P , Wiemken , A and Redecker , D. 2006 . Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity . Mol Ecol. , 15 : 2277 – 2289 .
- Jansa , J , Mozafar , A , Anken , T , Ruh , R , Sanders , IR and Frossard , E. 2002 . Diversity and structure of AMF communities as affected by tillage in a temperate soil . Mycorrhiza , 12 : 225 – 234 .
- Klironomos , JN , Hart , MM , Gurney , JE and Moutoglis , P. 2001 . Interspecific differences in the tolerance of arbuscular mycorrhizal fungi to freezing and drying . Can J Bot. , 79 : 1161 – 1166 .
- Kohn , LM and Stasovski , E. 1990 . The mycorrhizal status of plants at Alexandre Flord Ellesmere Island, Canada, a high arctic site . Mycologia , 82 : 23 – 35 .
- Koske , RE. 1987 . Distribution of VA mycorrhizal fungi along a latitudinal temperature gradient . Mycologia , 79 : 55 – 68 .
- Li , LF , Li , T and Zhang , Y. 2010 . Molecular diversity of arbuscular mycorrhizal fungi and their distribution patterns related to host-plants and habitats in a hot and arid ecosystem, southwest China . FEMS Microbiol Ecol. , 71 : 418 – 427 .
- Lovelock , CE , Andersen , K and Morton , JB. 2003 . Arbuscular mycorrhizal communities in tropical forests are affected by host tree species and environment . Oecologia , 135 : 268 – 279 .
- McGonigle , TP and Fitter , AH. 1990 . Ecological specificity of vesicular-arbuscular mycorrhizal associations . Mycol Res. , 94 : 120 – 122 .
- Miller , RM and Jastrow , JD. 2000 . “ Mycorrhizal fungi influence soil structure ” . In Arbuscular mycorrhizas: physiology and function , Edited by: Kapulnik , Y Jr and Doudls , DD . 3 – 18 . Dordrecht : Kluwer. p .
- Mullen , RB and Schmidt , SK. 1993 . Mycorrhizal infection, phosphorus uptake and phenology in Ranunculus adoneus: implications for the functioning of mycorrhizas in alpine systems . Oecologia , 94 : 229 – 234 .
- Oehl , F , Sieverding , E , Ineichen , K , Mäder , P , Boller , T and Wiemken , A. 2003 . Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of central Europe . Appl Environ Microbiol. , 69 : 2816 – 2824 .
- Oehl , F , Sieverding , E , Ineichen , K , Ris , EA , Boller , T and Wiemken , A. 2005 . Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems . New Phytol. , 165 : 273 – 283 .
- Öpik , M , Moora , M , Liira , J , Kõljalg , U , Zobel , M and Sen , R. 2003 . Divergent arbuscular mycorrhizal fungal communities colonize roots of Pulsatilla spp. in boreal Scots pine forest and grassland soils . New Phytol. , 160 : 581 – 593 .
- Öpik , M , Moora , M , Liira , J and Zobel , M. 2006 . Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe . J Ecol. , 94 : 778 – 790 .
- Öpik , M , Moora , M , Zobel , M , Saks , Ü , Wheatley , R , Wright , F and Daniell , T. 2008 . High diversity of arbuscular mycorrhizal fungi in a boreal herb-rich coniferous forest . New Phytol. , 179 : 867 – 876 .
- Pielou , EC. 1975 . Ecological Diversity , N.Y. : John Wiley and Sons Inc .
- Pietikäinen , A , Kytöviita , MM , Husband , R and Young , JPW. 2007 . Diversity and persistence of arbuscular mycorrhizas in a low-Arctic meadow habitat . New Phytol. , 176 : 691 – 698 .
- Raymo , ME and Ruddiman , WF. 1992 . Tectonic forcing of late Cenozoic climate change . Nature , 359 : 117 – 122 .
- Read , DJ and Haselwandter , K. 1981 . Observation on the mycorrhizal status of some alpine plant communities . New Phytol. , 88 : 341 – 353 .
- Saito , K , Suyama , Y , Sato , S and Sugawara , K. 2004 . Defoliation effects on the community structure of arbuscular mycorrhizal fungi based on 18S rDNA sequences . Mycorrhiza , 14 : 363 – 373 .
- Sancholle , M and Dalpé , Y. 1993 . Taxonomic relevance of fatty acids of vesicular-arbuscular mycorrhizal fungi and related species . Mycotaxon , 49 : 187 – 193 .
- Schalamuk , S , Velazquez , S , Chidichimo , H and Cabello , M. 2006 . Fungal spore diversity of arbuscular mycorrhizal fungi associated with spring wheat: effects of tillage . Mycologia , 98 : 16 – 22 .
- Schenck , NC and Pérez , Y. 1988 . Manual for identification of vesicular-arbuscular mycorrhizal fungi, INVAM , Gainesville, Fla, , USA : University of Florida .
- Shi , ZY , Chen , YL , Feng , G , Liu , RJ , Christie , P and Li , XL. 2006 . Arbuscular mycorrhizal fungi associated with the Meliaceae on Hainan island, China . Mycorrhiza , 16 : 81 – 87 .
- Smith , SE and Read , DJ. 1997 . Mycorrhizal symbiosis , 2nd , London : Academic Press .
- Spellerberg , IF and Fedor , PJ. 2003 . A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index . Global Ecol Biogeogr , 12 : 177 – 179 .
- Stutz , JC , Copeman , R , Martin , CA and Morton , JB. 2000 . Patterns of species composition and distribution of arbuscular mycorrhizal fungi in arid regions of southwestern North America and Namibia, Africa . Can J Bot. , 78 : 237 – 245 .
- Su , YY and Guo , LD. 2007 . Arbuscular mycorrhizal fungi in non-grazed, restored and over-grazed grassland in the Inner Mongolia steppe . Mycorrhiza , 17 : 689 – 693 .
- Tchabi , A , Coyne , D , Hountondji , F , Lawouin , L , Wiemken , A and Oehl , F. 2008 . Arbuscular mycorrhizal fungal communities in sub-Saharan Savannas of Benin, West Africa, as affected by agricultural land use intensity and ecological zone . Mycorrhiza , 18 : 181 – 195 .
- Tommerup , I. 1983 . Spore dominancy in vesicular-arbuscular mycorrhizal fungi . Trans Br Mycol Soci. , 81 : 37 – 45 .
- Uhlmann , E , Gorke , C , Petersen , A and Oberwinkler , F. 2004 . Arbuscular mycorrhizae from semiarid regions of Namibia . Can J Bot. , 82 : 645 – 653 .
- Unsworth , MJ , Jones , AG , Wei , W , Marquis , G , Gokarn , SG , Spratt , JE and the INDEPTH-MT team . 2005 . Crustal rheology of the Himalaya and southern Tibet inferred from magnetotelluric data . Nature , 438 : 78 – 81 .
- van der Heijden , MGA , Klironomos , JN , Ursic , M , Moutoglis , P , Streitwolf-Engel , R , Boller , T , Wiemken , A and Sanders , IR. 1998 . Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity . Nature , 396 : 69 – 72 .
- Vandenkoornhuyse , P , Husband , R , Daniell , TJ , Watson , IJ , Duck , JM , Fitter , AH and Young , JPW. 2002 . Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem . Mol Ecol. , 11 : 1555 – 1564 .
- Zhang , Y , Guo , LD and Liu , RJ. 2004 . Survey of arbuscular mycorrhizal fungi in deforested and natural forest land in the subtropical region of Dujiangyan, southwest China . Plant Soil , 261 : 257 – 263 .
- Zhao , ZW , Xia , YM , Qin , XZ , Li , XW , Cheng , LZ , Sha , T and Wang , GH. 2001 . Arbuscular mycorrhizal status of plants and the spore density of arbuscular mycorrhizal fungi in the tropical rain forest of Xishuangbanna, southwest China . Mycorrhiza , 11 : 159 – 162 .