Abstract
Three hundred and sixty woody litter samples naturally deposited in a stream and dammed section of the River Kali in southwest India were assessed for the diversity of lignicolous fungi (ascomycetes and anamorphic fungi) via damp chamber incubation and aquatic hyphomycetes (Ingoldian fungi) via bubble chamber incubation. Fifty-six fungal taxa were recorded with the highest of 47 taxa in the stream and 34 taxa in the dam. Twenty-five taxa were common to both sites. The core group (≥10%) comprised one ascomycete (Massarina australiensis), two anamorphic taxa (Acrogenospora sphaerocephala and Sporoschisma saccardoi) and 11 Ingoldian fungi (Anguillospora crassa, A. longissima, Clavariana aquatica, Cylindrocarpon sp., Flagellospora curvula, F. penicillioides, Lunulospora curvula, L. cymbiformis, Lunulospora sp., Triscelophorus acuminatus, T. monosporus). The species richness, average taxa per wood sample, richness of core group fungi and fungal diversity were higher in the Kaiga stream than in the Kadra dam. Species abundance curves showed higher frequency of occurrence of fungi in the stream than in the dam woody litter. The rarefaction indices of fungal species against number of wood samples revealed similar pattern of ascomycetes in both locations, while the richness of anamorphic and Ingoldian taxa was higher in the stream than in the dam. The anamorph/teleomorph ratio ranged between 2.4:1 (Kadra dam) and 3.3:1 (Kaiga stream), which is higher than the ratios reported from other geographical locations (0.7:1−2:1). This study reconfirmed that the method of incubation influences the assessment of fungal communities in woody litter and that Ingoldian fungi also constitute an important component of woody litter.
1. Introduction
Terrestrial woody litter (twigs, branches and trunks) deposited in lotic habitats increases the organic matter retention and provides suitable habitats for a wide range of organisms (e.g. fungi, insects and fish) (Triska and Cromack Citation1980; Parkyn et al. Citation2009). Such coarse and slow-degrading particulate organic matter constitutes the major nutrient source for different groups of aquatic fungi (e.g. ascomycetes, lignicolous hyphomycetes and Ingoldian hyphomycetes), which mediates in energy flow to higher trophic levels (Shearer et al. Citation2007; Gulis et al. 2008; Simonis et al. Citation2008). Studies on the diversity and distribution of fungi on woody litter was initiated and has been extensive in temperate regions (North America and Europe) (Willoughby and Archer Citation1973; Lamore and Goos Citation1978; Sanders and Anderson Citation1979; Shearer and von Bodman Citation1983; Révay and Gönczöl Citation1990; Shearer and Webster Citation1991; Shearer Citation1992 Citation1993; Hyde and Goh Citation1999; Kane et al. Citation2002; Raja et al. Citation2007, 2009; Gulis et al. 2008; Pearman et al. Citation2010). These studies have also been extended to tropical and subtropical regions (Australia, Brunei, China, India, Malaysia, Seychelles and Thailand) (Thomas et al. Citation1992; Hyde and Goh Citation1997, 1998a,b, 1999; Tsui et al. 2000; Ho et al. Citation2001; Sivichai et al. Citation2003; Fryar et al. Citation2004; Shearer et al. 2007; Ramesh and Vijaykumar Citation2006; Vijaykrishna et al. Citation2006; Jiang et al. Citation2008; Sridhar et al. Citation2010, 2011). Interestingly, Fryar et al. (Citation2004) evaluated fungal assemblage and diversity along a gradient of fresh, brackish and marine habitats of the River Tutong in Brunei. However, there have been a few reports of fungi occurring on woody litter in temperate and tropical lentic sources (Shearer and Crane Citation1986; Goh and Hyde Citation1999; Cai et al. Citation2002; Raja and Shearer Citation2007). Approximately 550 species of ascomycetes have been reported from freshwater habitats, and woody litter supported more species (60%) than herbaceous substrates (30%) and only a few species were common to both substrates (9−10%) (Raja et al. Citation2009). A total of 405, 320, 90 and 11 species of lignicolous ascomycetes/anamorphic taxa, Ingoldian fungi, aero-aquatic fungi and basidiomycetes, respectively, have been reported from freshwater habitats (Shearer et al. Citation2007; Marvanová 2010, pers. commun.).
Studies on freshwater fungi in India have mainly focused on the occurrence of Ingoldian fungi in water,
foam and leaf litter (Mer and Sati Citation1989; Sridhar et al. Citation1992; Chandrashekar et al. Citation1990; Raviraja et al. Citation1998, Rajashekhar and Kaveriappa Citation2003). However, a few studies are available on fungal diversity on naturally deposited and baited woody litter in the Western Ghats streams (Ramesh and Vijaykumar Citation2006; Sridhar et al. Citation2010, 2011). An inventory of aquatic bodies from virgin geographical locations is important in documenting fungal diversity and their exploitation. Similarly, fungal resources of human-influenced geographical locations also assumes importance in monitoring changes in patterns of fungal diversity. Usually, woody litter is assessed for colonization by lignicolous fungi (ascomycetes and anamorphic taxa) via damp incubation (Tsui et al. Citation2003). However, colonization of Ingoldian fungi on woody litter has also been demonstrated using bubble chamber incubation (Shearer and Webster Citation1991; Sridhar et al. Citation2010). Sridhar et al. (Citation2010) recommended that freshwater fungal assessment in woody litter requires both damp chamber and bubble chamber incubation techniques. Assessment of fungal diversity on naturally submerged woody litter in the River Kali at the foot-hill of the Western Ghats of India is the main objective of our study. To achieve this goal, we employed damp chamber and bubble chamber incubation techniques to stimulate growth and sporulation of the different groups of fungi.
2 Materials and methods
2.1 Study site
The Western Ghats mountain range is a hotspot of biodiversity stretching about 1600 km (140,000 km2) along the west coast of India. Several fast-flowing rivers originate from the Western Ghats and flow eastwards into the Bay of Bengal or westwards in to the Arabian Sea. The streams and rivers of the Western Ghats pass through a wide range of vegetation at different altitudes (grasslands, sholas, moist-dry deciduous forests, evergreen forests and scrub jungles). The River Kali originates in the Western Ghats (∼900 m asl) and flows westwards for 160 km and joins the Arabian Sea near Karwar City. The Kali River has two tributaries – Upper Kaneri and Tattihalla – and four dams are built across the river (Supa, Bommanahalli, Kodasalli and Kadra) for electricity generation.
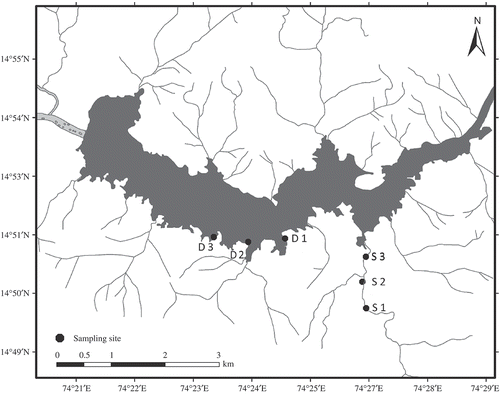
The sampling locations selected for study on the River Kali (Kaiga stream: S1, S2 and S3; Kadra dam, D1, D2 and D3) are situated adjacent to the village of Kaiga (∼35 km east of Karwar; ∼55−70 m asl; 14°50′−14°51′ N, 74°24′−74°27′ E) (). The Kaiga stream is a third-order stream with sandy loam and rocky bottom. The Kadra dam receives water from several streamlets passing through wetlands. The depth of sampling locations in the Kaiga stream during the study period ranged between 1.5 (S1) and 3.5 m (S3), while the depth of the dam sites ranged from 3.6 m (D3) to 13.8 m (D2). The sampling stream and dam locations were endowed with roots, wood and leaf detritus of forest tree species: Artocarpus heterophyllus, Ficus benghalensis, F. racemosa, Syzygium caryophyllatum, Terminalia arjuna, T. paniculata and Xylia xylocarpa.
2.2 Water analysis
Water samples were collected from the sampling sites during the post-monsoon season (November, 2008). Water temperature, pH, conductivity, dissolved oxygen and turbidity were assessed at the sampling sites (Water Analyzer 371, Systronics, Gujarat, India). Other parameters, such as COD, total alkalinity, total hardness, chloride, sulfate, nitrate, phosphate and silica, were assessed based on the methods outlined in APHA (Citation1995). Ca, Na and K of the water samples were estimated by flame-emission photometry (MK1/MK3; Systronics, India) (AOAC Citation1990). Other minerals, such as Fe, Mg, Cu, Pb, Zn, Ni and Cr, were detected using atomic absorption spectrophotometry (GBC 932 Plus) (AOAC, 1990).
2.3 Woody litter and fungal assessment
About 70−80 woody litter samples from each site were collected in clean polythene bags. They were rinsed in the laboratory to remove sediments and other debris. Within 24−36 h of sampling, a 15-cm length piece (∼1−1.5 cm diam.) was cut and 60 wood samples per sampling site were incubated individually on a wet sand bed in airtight polythene bags (23 ± 2°C). They were screened once a month for up to six months and lignicolous fungi (ascomycetes and anamorphic taxa) growing on the wood samples were identified using monographs and primary literature (Ellis 1970 Citation1976; Carmichael et al. Citation1980; Ellis and Ellis Citation1987; Cai et al. Citation2006; Bhat Citation2010). Wood bark pieces (if available) and cambium tissues (0.5 × 3 cm), selected for damp incubation, were separated, rinsed in water and four pieces per wood sample were incubated in 150 ml of sterile distilled water in 250-ml Erlenmeyer flasks. Water in the flasks was aerated though Pasteur pipettes by an aquarium pump for up to 48 h (23 ± 2 °C). Aerated water was filtered through a Millipore filter (5 μm) and stained with aniline blue in lactophenol (0.1%). Stained filters were excised into half to mount on a microscope slide with lactic acid, and the conidia of Ingoldian fungi were identified based on monographs and primary literature (Ingold Citation1975; Carmichael et al. 1980; Webster and Descals Citation1981; Nawawi Citation1985; Marvanová Citation1997; Santos-Flores and Betancourt-López Citation1997; Gulis et al. Citation2005).
2.4 Data analysis
The difference in water parameters of the Kaiga stream and Kadra dam were assessed based on Student's t-test (StatSoft, 2008).
The frequency of occurrence (%) and relative abundance (%) of each fungus on wood samples were determined as follows:
The Shannon's diversity (H′) (Magurran Citation1988) and Pielou's evenness (J′) (Pielou Citation1975) of ascomycetes, anamorphic taxa and Ingoldian fungi of wood samples were calculated as follows:
where, pi is the proportion of individuals that species i contributed to the total number of individuals.
where, H′max is the maximum value of diversity for the number of species present.
To compare the richness of fungi in wood samples based on number of isolations and number of wood samples assessed, the expected number of species was calculated by rarefaction indices (Ludwig and Reynolds Citation1988). The expected number of species, E (s), in a random sample of n isolations taken from a total population of N isolations was estimated as:
where, ni is the number of fungal isolations of the ith species.
3 Results
3.1 Water quality
Among the water parameters evaluated, minerals such as copper, lead, zinc, nickel and chromium were below detectable limits, while chloride, nitrate, sodium and magnesium did not differ significantly between the Kaiga stream and Kadra dam (). Water temperature, conductivity, turbidity, COD, total alkalinity, total hardness, sulfate, silica, calcium and potassium were significantly higher in dam than in stream water. The pH, dissolved oxygen, phosphate and iron were significantly higher in stream than in dam water.
Table 1. Physicochemical features of water (n = 9, mean, range in parenthesis)
3.2 Fungal taxa in damp chambers
A total of 16 ascomycetes were recovered via damp incubation of wood, with the highest number of species in Kaiga stream (11 species) (). Five species were common to both locations (Annulatascus velatispora, Eutypa flavovirens, Massarina australiensis, Massarina sp. and Savoryella lignicola). Six and five species were confined to stream and dam locations, respectively. Massarina australiensis was the only core group fungus dominant in both locations (15.8−24.6%). Eight taxa (Annulatascus velatispora, Aqualignicola hyalina, Eutypa flavovirens, Jahnula bipileata, Massarina sp., Phyllachora sylvatica, Savoryella lignicola and Torrentispora fibrosa) were frequent (5.2−8.8%) and the remaining seven taxa uncommon (3.5%). The average number of species per wood sample (0.71 versus 0.6) and Shannon diversity indices (3.459 versus 3.322) were higher in the stream than in the dam, while Pielou's evenness was higher in the dam than in the stream (0.935 versus 0.869) ().
Among the 25 anamorphic taxa recorded on damp incubation, species richness was higher in the stream than in the dam (21 versus 13 species) (). Nine species were common to both locations, while 12 and four species were found exclusively in wood samples from the stream and dam, respectively. Sporoschisma saccardoi (17.2−15.8%) was the most dominant core group fungus in both locations, followed by Acrogenospora sphaerocephala (12.1−12.3%). Nine taxa (Brachysporiella pulchra, Doratomyces microsporus, Drechslera sp., Helminthosporium velutinum, Phaeoisaria clematidis, Pleurothecium recurvatum, Sporidesmium rubi, Sporidesmium anglicum and Sporoschismopsis australiensis) were frequent (5.2−8.8%). The remaining 14 species were uncommon (<3.5%). The average number of species per wood sample (1 versus 0.82 species) and the Shannon diversity (4.392 versus 3.7) were higher in the stream than in the dam, while Pielou's evenness was higher in the dam than in the stream (0.935 versus 0.906) ().
Table 2. Frequency of occurrence (%) and relative abundance (%) (in parenthesis) of lignicolous and Ingoldian fungi in woody litter (fungi in each group for two locations arranged in descending order).
Table 3. Species richness, diversity and anamorph/teleomorph ratio in woody litter.
3.3 Fungal taxa recorded in bubble chambers
Fifteen Ingoldian taxa were recorded in bark and cambium of woody litter using bubble chamber incubation. All species were found in the stream, while only 11 species were found in the dam (). The number of core group taxa was higher in the stream than in the dam (11 versus 6 species). Overall, Lunulospora curvula was the most frequent core group fungus, followed by Flagellospora curvula, Anguillospora longissima, Triscelophorus acuminatus, Anguillospora crassa, Cylindrocarpon sp., Triscelophorus monosporus, Lunulospora sp., Flagellospora penicillioides, Clavariana aquatica and Lunulospora cymbiformis. The remaining four species were uncommon (<5.3%) (Campylospora chaetocladia, Flabellospora crassa, Nawawia filiformis and Triscelophorus konajensis). The average number of species per wood sample (3.1 versus 1.9 species), Shannon diversity (3.907 versus 3.459) and Pielou's evenness (0.876 versus 0.840) were higher in the stream than in the dam ().
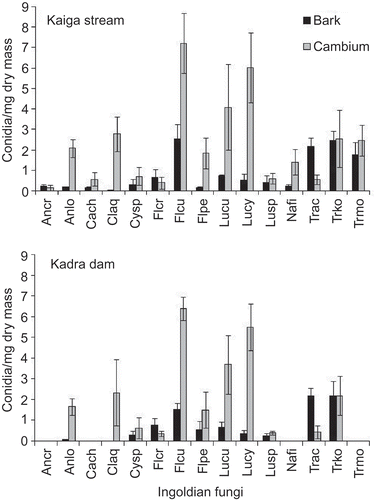
Bubble chamber incubation of cambium of woody litter yielded more conidia than bark (Kaiga stream, 33.2 versus 12.4 mg−1; Kadra dam, 25.1 versus 8.3 mg−1) (). Conidial output was slightly less in Kadra dam than Kaiga stream. Flagellospora curvula, Triscelophorus konajensis and T. acuminatus were the three top ranking species in bark, while Flagellospora curvula, Lunulospora cymbiformis, L. curvula and Clavariana aqauatica were the four top ranking species in cambium at both locations. Most of the species showed more conidial output from cambium than bark (except for Anguillospora crassa, Flabellospora crassa and Triscelophorus acuminatus).
Figure 2. Conidial output (conidia mg−1 dry mass ± SE) of Ingoldian fungi from bark and cambium of woody litter of Kaiga stream (bark n = 62; cambium n = 118) and Kadra dam (bark n = 29; cambium n = 151) using bubble chamber incubation (Ancr, Anguillospora crassa; Anlo, Anguillospora longissima; Cach, Campylospora chaetocladia; Claq, Clavariana aquatica; Cysp, Cylindrocarpon sp.; Flcr, Flabellospora crassa; Flcu, Flagellospora curvula; Flpe, Flagellospora penicillioides, Lucu, Lunulospora curvula; Lucy, Lunulospora cymbiformis; Lusp, Lunulospora sp., Nafi, Nawawia filiformis, Trac, Triscelophorus acuminatus; Trko, Triscelophorus konajensis; Trmo, Triscelophorus monosporus).
3.4 Overall occurrence of fungi
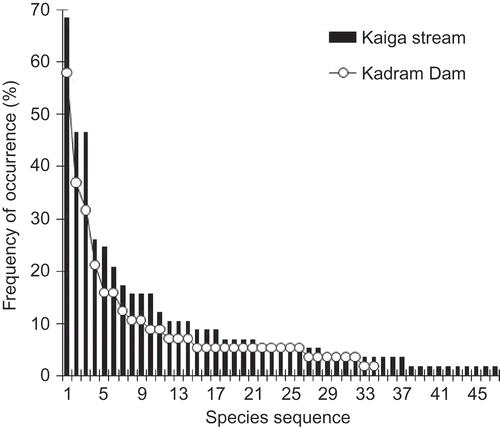
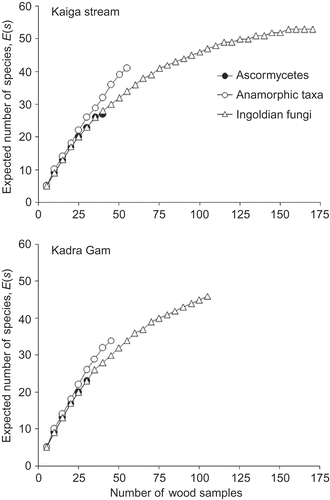
Overall, three groups of fungi were represented by 56 species from 360 wood samples, with a higher number of species from the stream than the dam (47 versus 34 species). A total of 25 species were common to both collecting sites (ascomycetes 5; anamorphic hyphomycetes 9; Ingoldian fungi 11) (). The anamorph to teleomorph ratio was also higher in the stream than the dam (3.27 versus 2.4) (). The species abundance curve shows a higher frequency of occurrence of fungi in the stream than in the dam (). A comparison of expected number of species of three groups of fungi of 30 random wood samples, i.e. rarefaction indices E(30) , showed that anamorphic hyphomycetes were higher in both locations than either ascomycetes or Ingoldian fungi (26 versus 23 species) (). The rarefaction indices also revealed higher species richness and an increasing trend in expected number of species of anamorphic and Ingoldian taxa in the stream than in dam wood samples.
4 Discussion
The present study reveals the importance of using more than one technique in a detailed evaluation of fungal diversity in freshwater woody litter. In addition to lignicolous fungi (ascomycetes and anamorphic hyphomycetes), Ingoldian fungi are also common in woody litter. It is assumed that a core group of fungi (≥10% occurrence) play an important role in wood degradation and energy transfer to higher trophic levels. However, whether all fungi found in wood reproduce under in situ conditions is unclear. There seems to be some stress in their habitat that induce sporulation (e.g. changes in water temperature, exposure of wood to air, grazing pressure by aquatic fauna and anoxic or extreme pH). For instance, Kaiga stream locations have fast flowing water, while the Kadra dam water shows less flow as the hydroelectric power plant works only during peak hours (5−6 h day−1). It is presumed that the fungi on woody litter in these locations might have adapted to such conditions (e.g. fluctuation in depth, oxygen saturation and exposure to air or other parameters indicated in ). The rarefaction indices of fungi revealed higher anamorphic and Ingoldian taxa in the Kaiga stream than in the dam, reflecting the conditions prevailing in these sampling sites.
4.1 Lignicolous taxa
Among the six lignicolous ascomycetes having worldwide distribution (Aniptodera chesapeakensis, A. lignatilis, Annulatascus velatispora, Natantispora retorquens, Massarina ingoldiana and Nais inornata) (Shearer Citation2001), Aniptodera chesapeakensis and Annulatascus velatispora were found in our study. Altogether, three core group lignicolous fungi were recorded in the present study, with a frequency of occurrence ranging between 12.1% (Acrogenospora sphaerocephala) and 24.6% (Massarina australiensis). Some core group fungi have been shown to be common on woody litter in other tropical and subtropical regions: Massarina australiensis (Hong Kong: Cai et al. Citation2006; India: Sridhar et al. Citation2010, Citation2011); Acrogenospora sphaerocephala (Australia: Hyde and Goh Citation1998a; Hong Kong and Thailand: Sivichai et al. Citation2000a; Tsui et al. Citation2000; Ho et al. Citation2002; Tsui and Hyde Citation2004; Philippines: Cai et al. Citation2003; Brunei: Fryar et al. Citation2004; India: Sridhar, Citation2011); Sporoschisma saccardoi (Australia: Hyde and Goh Citation1998a; Brunei, Hong Kong and Thailand: Tsui et al. Citation2000; Sivichai et al. Citation2000b; Ho et al. Citation2001, 2002; Fryar et al. Citation2004; India: Sridhar et al. Citation2010, Citation2011).
Some of the lignicolous ascomycetes of our study have also been reported from various tropical or subtropical regions: Aniptodera chesapeakensis (South Africa, Hyde et al. 1998a; Brunei, Hong Kong and Malaysia: Ho et al. Citation2001, 2002; Fryar et al. Citation2004; Hong Kong: Tsui et al. Citation2000; Tsui and Hyde Citation2004; India: Ramesh and Vijaykumar Citation2006; Sridhar et al. Citation2010); Annulatascus velatispora (South Africa, Hyde and Goh Citation1998b; Seychelles: Hyde and Goh Citation1998c; Hong Kong: Ho et al. Citation2002; Tsui and Hyde Citation2004; Philippines: Cai et al. Citation2003; Brunei: Fryar et al. Citation2004); Phyllochora sylvatica (India: Sridhar et al. Citation2010); Savoryella lignicola (Australia: Hyde and Goh Citation1997, 1998a; Seychelles: Hyde and Goh Citation1998c; Brunei, Hong Kong and Malaysia: Ho et al. Citation2001; Hong Kong: Tsui et al. Citation2000; Ho et al. Citation2002; Tsui and Hyde Citation2004; India: Udaiyan Citation1989; Udaiyan and Hosagoudar Citation1991; Ramesh and Vijaykumar Citation2006; Sridhar et al. Citation2010); Torentispora fibrosa (Hong Kong: Hyde et al. Citation2000; Tsui et al. Citation2000; Ho et al. Citation2001, 2002; Brunei: Fryar et al. Citation2004; India: Sridhar et al. Citation2010, 2011). Several ascomycetes found in our study are known from the above aquatic habitats (Cai et al. Citation2006).
Many of the lignicolous anamorphic taxa known in our study have also been reported from other tropical or subtropical locations: Dictyosporium digitatum (Hong Kong: Tsui et al. Citation2000; India: Sridhar et al. Citation2010, 2011); Sporoschimopsis australiensis (India: Sridhar et al. Citation2010); Pleurothecium recurvatum (Hong Kong: Tsui et al. Citation2000); Sporoschisma nigroseptatum (Thailand: Sivichai et al. Citation2000b); Sporoschisma uniseptatum (Seychelles: Hyde and Goh Citation1998c; Thailand: Sivichai et al. Citation2000b; Hong Kong: Ho et al. Citation2001; Tsui et al. Citation2000); Wiesneriomyces laurinus (Hong Kong: Ho et al. Citation2002). Lignicolous anamorphic taxa found in our study were also previously recorded in terrestrial habitats of the Western Ghats (Bhat Citation2010).
4.2 Ingoldian fungi
Altogether, 11 core group Ingoldian fungi were found on bark and cambium in our study, with a frequency of occurrence ranging between 10.3% (Clavariana aquatica, Lunulospora cymbiformis and Lunulospora sp.) and 68.4% (Lunulospora curvula). Shearer (Citation1992) provided a checklist of 46 Ingoldian fungi in woody litter mainly from the temperate locations. Among the 11 core group fungi in our study, Anguillospora crassa, A. longssima, Flagellospora curvula and Lunulospora curvula has also been shown to be widely distributed on woody litter in temperate regions (Willoughby and Archer Citation1973; Sanders and Anderson Citation1979; Révay and Gönczöl Citation1990; Shearer and Bartolata Citation1990; Shearer and Webster Citation1991; Shearer Citation1992) and the first two species were also common in woody litter of the Western Ghat streams (Sridhar et al. Citation2010) possessing exoenzymatic capabilities (Shearer Citation1992). Anguillospora longissima, Flagellospora curvula, Lunulospora curvula, L. cymbiformis, Triscelophorus acuminatus and T. konajensis were common on woody litter (Sridhar et al. Citation2010) and also leaf litter of the Western Ghats (Chandrashekar et al. Citation1990; Sridhar et al. Citation1992; Rajashekhar and Kaveriappa Citation2003).
In addition to the above Ingoldian fungi, 25 species (Alatospora acuminata, Anavirga dendromorpha, Anguillospora furtiva, Aticulospora tetracladia, Casaresia sphagnorum, Clavariopsis aquatica, Dendrospora erecta, Dimorphospora foliicola, Filosporella annelidica, Flabellospora acuminata, Heliscus lugdunensis, Lemonniera aquatica, L. terrestris, Mycocentrospora acerina, Tetrachaetum elegans, Tetracladium marchalianum, T. setigerum, Tricladium angulatum, T. chaetocladium, T. giganteum, T. gracile, T. splendens, Tumularia aquatica, Vargamyces aquaticus and Varicosporium elodeae) are also common on freshwater woody litter (see Shearer Citation1992). Surprisingly, many typical Ingoldian fungi have also recorded on damp chamber incubation. For instance, Anguillospora gigantea (Hong Kong: Tsui et al. Citation2000), Brachiosphaera tropicalis (Seychelles: Hyde and Goh Citation1998b; Hong Kong: Tsui et al. Citation2000) and Nawawia filiformis (Hong Kong, Thailand and Brunei: Sivichai et al. 2002a; Ho et al. Citation2001, 2002; Fryar et al. Citation2004) have been observed on woody litter following damp chamber incubation (see also Shearer Citation1992). Similarly, an aero-aquatic fungus (Helicomyces roseus) was common in bark and rare in cambium under bubble chamber incubation (Sridhar et al. Citation2010).
The conidial output from leaf litter of Kali River (Sridhar et al. Citation2011) was higher than from bark and cambium of woody litter in our study (leaf: 460−520 mg−1; bark: 8.3−12.4 mg−1; cambium: 25.1−33.2 mg−1). However, the conidial output from the woody litter in our study is higher than the woody litter reported from other Western Ghat streams (8.3−33.2 versus 0.05−1.14 mg−1) (Sridhar et al. Citation2010). Flagellospora curvula in bark, and Lunulospora cymbiformis, L. curvula and Flagellospora curvula observed from cambium tissues in this study were also the top ranked species on the woody litter of Western Ghat streams (Sridhar et al. Citation2010). Higher conidial output from the cambium than bark in our study corroborates an earlier study (Sridhar et al. Citation2010) and is possibly due to the inhibitory compounds produced in bark. Flabellospora curvula and Triscelophorus acuminatus, however, produced more conidia in bark than cambium in both sites, indicating a tolerance to the inhibitory compounds of bark.
Conclusions
The present study assessed different groups of fungi on submerged wood samples in a stream and dam using two traditional methods (damp chamber incubation and bubble chamber incubation). This study yielded more anamorphs (40 species) than teleomorphs (16 species) and the anamorph/teleomorph ratio ranged between 2.4:1 (Kadra dam) and 3.3:1 (Kaiga stream), which is higher than the ratios reported from other geographical locations (e.g. Malaysia, 0.7; Australia and South Africa, 1.1:1; Seychelles and UK, 1.8:1; China, 2:1) (Ho et al. Citation2001). The high anamorph/teleomorph ratio in our study may be due to employing two different methods of assessment of fungal diversity (Shearer et al. Citation2007). Besides lignicolous fungi, Ingoldian fungi also constitute an important component of woody litter. Assessment of lignicolous fungi by damp chamber incubation is a lengthy process, whereas the Ingoldian fungi in wood can be assessed within a few days using bubble chamber incubation. Woody litter may act as a refuge for Ingoldian fungi during scarcity of leaf litter and, thus, study of seasonal fluctuation of Ingoldian fungi in woody litter and leaf litter simultaneously may give more insight into this issue. As only bark and outer cambium were assessed for Ingoldian fungi in the current study, several questions arise: Are the Ingoldian fungi superficial colonizers of woody litter? Is their ability to penetrate wood dependent on hardness or softness of the wood? Do they compete with lignicolous fungi on wood for resource utilization? Besides damp and bubble chamber incubation, employing other methods of assessment (e.g. particle-plating method; Bills and Polishook Citation1994; whole DNA analysis; Bärlocher Citation2007) of freshly collected woody litter and damp incubated woody litter will improve our understanding of wood inhabiting fungi.
Acknowledgements
The authors are grateful to Mangalore University for permission to carry out this study at the Department of Biosciences and Nuclear Power Corporation of India Ltd. (NPCIL) Mumbai, for funding. NMS is indebted to the NPCIL for the award of a research fellowship. Authors are thankful to Drs K.S. Karamchand, Department of Biotechnology, St. Aloysius College, Mangalore; H.M. Somashekarappa, University Science Instrumentation Centre, Mangalore University; S.G. Ghadge, M. Kansal, P.M. Ravi, B.N. Dileep and S.K. Singh, NPCIL, Kaiga, Karnataka for support.
References
- AOAC . 1990 . Official Methods of Analysis , 15th , Washington, DC : Association of Official Analytical Chemists .
- APHA . 1995 . Standard Methods in Examination of Water and Waste Water , 19th , Washington, DC : American Public Health Association .
- Bärlocher , F. 2007 . Molecular approaches applied to aquatic hyphomycetes . Fung Biol Rev. , 21 : 19 – 24 .
- Bhat , DJ. 2010 . Fascinating Microfungi (Hyphomycetes) of Western Ghats , Goa, , India : Broadway Publishers .
- Bills , GF and Polishook , JD. 1994 . Abundance and diversity of microfungi in leaf litter of a lowland rainforest in Costa Rica . Mycologia , 86 : 187 – 198 .
- Cai , L , Tsui , CKM , Zhang , K and Hyde , KD. 2002 . Aquatic fungi from Lake Fuxian, Yunnan, China . Fung Divers. , 9 : 57 – 70 .
- Cai , L , Zhang , K , McKenzie , EHC and Hyde , KD. 2003 . Freshwater fungi from bamboo and wood submerged in the Liput River in the Philippines . Fung Divers. , 13 : 1 – 12 .
- Cai , L , Hyde , KD and Tsui , CKM. 2006 . Genera of freshwater fungi. Fungal Diversity Research Series # 18 , Hong Kong : Fungal Diversity Press .
- Carmichael , JW , Kendrick , WB , Conners , IL and Sigler , L. 1980 . Genera of Hyphomycetes , Edmonton, , Canada : The University of Alberta Press .
- Chandrashekar , KR , Sridhar , KR and Kaveriappa , KM. 1990 . Periodicity of water-borne hyphomycetes in two streams of Western Ghat forests (India) . Acta Hydrochim Hydrobiol. , 18 : 187 – 204 .
- Ellis , MB. 1971 . Dematiaceous Hyphomycetes , Kew : Commonwealth Mycological Institute .
- Ellis , MB. 1976 . More Dematiaceous Hyphomycetes , Kew : Commonwealth Mycological Institute .
- Ellis , MB and Ellis , JP. 1987 . Microfungi on Land Plants: An Identification Handbook , London : Croom Helm .
- Fryar , SC , Booth , W , Davies , J , Hodgkiss , IJ and Hyde , KD. 2004 . Distribution of fungi on wood in the Tutong River, Brunei . Fung Divers. , 17 : 17 – 38 .
- Goh , TK and Hyde , KD. 1999 . Fungi on submerged wood and bamboo in the Plover Cove Reservoir, Hong Kong . Fung Divers. , 3 : 57 – 85 .
- Gulis , V , Suberkropp , K and Rosemond , AD. 1998 . Comparison of fungal activities on wood and leaf litter in unaltered and nutrient-enriched headwater streams . Appl Environ Microbiol. , 74 : 1094 – 1101 .
- Gulis , V , Marvanová , L and Descals , E. 2005 . “ An illustrated key to the common temperate species of aquatic hyphomycetes ” . In Methods to Study Litter Decomposition: A Practical Guide , Edited by: Graça , M.A.S. , Baerlocher , F. and Gessner , M.O. 153 – 167 . Dordrecht : Kluwer Academic Publisher .
- Ho , WH , Hyde , KD , Hodgkiss , IJ and Yanna . 2001 . Fungal communities on submerged wood from streams in Brunei, Hong Kong and Malaysia . Mycol Res. , 105 : 1492 – 1501 .
- Ho , WH , Yanna , Hyde , KD and Hodgkiss , IJ. 2002 . Seasonality and sequential occurrence of fungi on wood submerged in Tai Po Kau Forest Stream, Hong Kong . Fung Divers. , 10 : 21 – 43 .
- Hyde , KD and Goh , TK. 1997 . Fungi on submerged wood in a small stream on Mt Lewis, North Queensland, Australia . Muelleria , 10 : 145 – 157 .
- Hyde , KD and Goh , TK . 1998a . Fungi on submerged wood in Lake Barrine, North Queensland, Australia . Mycol Res. , 102 : 739 – 749 .
- Hyde , KD and Goh , TK. 1998b . Fungi on submerged wood in the Palmiet River, Durban, South Africa . S Afr J Bot. , 64 : 151 – 162 .
- Hyde , KD and Goh , TK. 1998c . Fungi on submerged wood in the River St Marie-Louis, The Seychelles . S Afr J Bot. , 64 : 330 – 336 .
- Hyde , KD and Goh , TK. 1999 . Fungi on submerged wood from the River Coln, England . Mycol Res. , 103 : 1561 – 1574 .
- Hyde , KD , Ho , WH , Jones , EBG , Tsui , CKM and Wong , WSW. 2000 . Torrentispora fibrosa gen. sp. nov. (Annulatascaceae) from freshwater habitats . Mycol Res. , 104 : 1399 – 1403 .
- Ingold , CT. 1975 . An Illustrated Guide to Aquatic and Waterborne Hyphomycetes (Fungi Imperfecti).Scientific Publication # 30. , 1 – 96 . Ambleside-Cumbria, , UK : Freshwater Biological Association .
- Jiang , M , Wongsawas , M , Wang , HK , Lin , FC and Liang , YC. 2008 . Three new records of lignicolous freshwater hyphomycetes from Mainland, China . J Agric Technol. , 4 : 101 – 108 .
- Kane , DF , Tam , WY and Jones , EBG. 2002 . Fungi colonizing and sporulating on submerged wood in the River Severn, UK . Fung Divers. , 10 : 45 – 55 .
- Lamore , BJ and Goos , RD. 1978 . Wood-inhabiting fungi of a freshwater stream in Rhode Island . Mycologia , 70 : 1025 – 1034 .
- Ludwig , JA. and Reynolds , JF. 1988 . Statistical Ecology: A Primer on Methods and Computing , New York : Wiley .
- Magurran , A.E. 1988 . Ecological Diversity and its Measurement , Princeton, NJ : Princeton University Press .
- Marvanová , L. 1997 . “ Freshwater hyphomycetes: A survey with remarks on tropical taxa ” . In Tropical Mycology , Edited by: Janardhanan , KK , Rajendran , C , Natarajan , K and Hawksworth , DL . 169 – 226 . New York : Science Publishers .
- Mer , GS and Sati , SC. 1989 . Seasonal fluctuations in species composition of aquatic hyphomycetous flora in a temperate freshwater stream of Central Himalaya, India . Int Rev Gesamten Hydrobiol. , 74 : 433 – 437 .
- Nawawi , A. 1985 . Aquatic hyphomycetes and other water-borne fungi from Malaysia . Malaysian Nature J. , 39 : 75 – 134 .
- Parkyn , SM , Meleason , MA and Davies-Colley , RJ . 2009 . Wood enhances crayfish (Paranephrops planifrons) habitat in a forested stream . NZ J Mar Freshw Res. , 43 : 689 – 700 .
- Pearman , JK , Taylor , JE and Kinghorn , JR. 2010 . Fungi in aquatic habitats near St Andrews in Scotland . Mycosphere , 1 : 11 – 21 .
- Pielou , FD . 1975 . Ecological diversity , New York : Wiley InterScience .
- Raja , HA and Shearer , CA. 2007 . Freshwater ascomycetes: Aliquandostipite minuta (Jahnulales, Dothideomycetes), a new species from Florida . Mycoscience , 48 : 395 – 398 .
- Raja , HA and Shearer , CA. 2008 . Freshwater ascomycetes: new and noteworthy species from aquatic habitats in Florida . Mycologia , 100 : 467 – 489 .
- Raja , HA , Stchigel , AM , Miller , AN , Crane , JL and Shearer , CA. 2007 . Hyphomycetes from the Great Smoky Mountains National Park, including three new species . Fung Divers. , 26 : 271 – 286 .
- Raja , HA , Schmit , JP and Shearer , CA. 2009 . Latitudinal, habitat and substrate distribution patterns of freshwater ascomycetes in the Florida Peninsula . Biodivers Conserv. , 18 : 419 – 455 .
- Rajashekhar , M and Kaveriappa , KM. 2003 . Diversity of aquatic hyphomycetes in the aquatic ecosystems of the Western Ghats of India . Hydrobiologia , 501 : 167 – 177 .
- Ramesh , C and Vijaykumar , S. 2006 . “ Observation on water-borne fungi of Uttara Kannada region ” . In Recent Mycological Researches , Edited by: Sati , SC . 61 – 76 . New Delhi : IK International Publishing House Pvt Ltd .
- Raviraja , NS , Sridhar , KR and Bärlocher , F. 1998 . Fungal species richness in Western Ghat streams, (Southern India); is it related to pH, temperature or altitude? . Fung Divers. , 1 : 179 – 191 .
- Révay , A and Gönczöl , J. 1990 . Longitudinal distribution and colonization pattern of wood-inhabiting fungi in a mountain stream in Hungary . Nova Hedwigia , 51 : 505 – 520 .
- Santos-Flores , C and Betancourt-López , C. 1997 . Aquatic and Water-Borne Hyphomycetes (Deuteromycotina) in Streams of Puerto Rico , 1 – 116 . Puerto Rico : University of Puerto Rico. Caribbean J Sci Spec Publ. 2 .
- Sanders , PF and Anderson , JM. 1979 . Colonization of wood blocks by aquatic hyphomycetes . Trans Br Mycol Soc. , 73 : 103 – 107 .
- Shearer , CA. 1992 . “ Role of woody debris ” . In The Ecology of Aquatic Hyphomycetes , Edited by: Bärlocher , F . 77 – 98 . Berlin : Springer .
- Shearer , CA. 1993 . The freshwater ascomycetes . Nova Hedwigia , 56 : 1 – 33 .
- Shearer , CA. 2001 . “ The distribution of freshwater filamentous ascomycetes ” . In Trichomycetes and other Fungal Groups , Edited by: Misra , JK and Horn , BW . 225 – 292 . Enfield, , USA : Science Publishers .
- Shearer , CA and von Bodman , S. 1983 . Patterns of occurrence of ascomycetes associated with decomposing twigs in a Midwestern stream . Mycologia , 75 : 518 – 530 .
- Shearer , CA and Crane , JL. 1986 . Illinois fungi XII. Fungi and myxomycetes from wood and leaves submerged in Southern Illinois swamps . Mycotaxon , 25 : 527 – 538 .
- Shearer , CA and Bartolata , M. 1990 . “ Experimental determination of in situ competitive interactions among aquatic lignicolous fungi ” . In Abstracts of 4th International Mycological Congress , Edited by: Reisinger , A and Bresinsky , A . Regensburg (Germany), , Germany : University of Regensburg .
- Shearer , CA and Webster , J. 1991 . Aquatic hyphomycete communities in the River Teign. IV. Twig colonization . Mycol Res. , 95 : 413 – 420 .
- Shearer , CA , Descals , E , Kohlmeyer , B , Kohlmeyer , J , Marvanová , L , Padgett , D , Porter , D , Raja , HA , Schmit , JP , Thorton , HA and Voglymayr , H. 2007 . Fungal biodiversity in aquatic habitats . Biodivers Conserv. , 16 : 49 – 67 .
- Simonis , JL , Raja , HA and Shearer , CA. 2008 . Extracellular enzymes and soft rot decay: Are ascomycetes important degraders in fresh water? . Fung Divers. , 31 : 135 – 146 .
- Sivichai , S , Jones , EBG and Hywel-Jones , N. 2000a . Fungal colonization of wood in a freshwater stream Khoa Yai National Park, Thailand . Fung Divers. , 5 : 71 – 88 .
- Sivichai , S , Hywel-Jones , NL and Somrithipol , S. 2000b . Lignicolous freshwater Ascomycota from Thailand: Melanochaeta and Sporoschisma anamorphs . Mycol Res. , 104 : 478 – 485 .
- Sivichai , S , Jones , EBG and Hywel-Jones , NL. 2003 . Lignicolous freshwater Ascomycota from Thailand: Hymenoscyphus varicosporoides and its Tricladium anamorph . Mycologia , 95 : 340 – 346 .
- Sridhar , KR , Chandrashekar , KR and Kaveriappa , KM. 1992 . “ Research on the Indian Subcontinent ” . In The Ecology of Aquatic Hyphomycetes , Edited by: Bärlocher , F . 182 – 211 . Berlin : Springer .
- Sridhar , KR , Karamchand , KS and Hyde , KD. 2010 . Wood-inhabiting filamentous fungi in 12 high altitude streams of the Western Ghats by damp incubation and bubble chamber incubation . Mycoscience , 51 : 104 – 115 .
- Sridhar , KR , Arun , AB , Maria , GL and Madhyastha , MN. 2011 . Diversity of fungi on submerged leaf and woody litter in River Kali, Southwest India . Environ Res J. , 5 : 1 – 14 .
- Inc , StatSoft . 1995 . STATISTICA for Windows , Tulsa, OK : StatSoft Inc .
- Thomas , K , Chilvers , GA and Norris , RH. 1992 . Colonization and persistence of aquatic hyphomycetes on Eucalyptus viminalis bark and twig in an Australian stream . Nova Hedwigia , 55 : 407 – 417 .
- Triska , FJ and Cromack Jr , FJ . 1980 . “ The role of wood debris in forests and streams ” . In Forests: Fresh Perspectives from Ecosystem Analysis , Edited by: Waring , RH . 171 – 190 . Corvallis, OR : Oregon State University Press .
- Tsui , CKM and Hyde , KD. 2004 . Biodiversity of fungi on submerged wood in a stream and its estuary in the Tai Ho Bay, Hong Kong . Fung Divers. , 15 : 205 – 220 .
- Tsui , CKM , Hyde , KD and Hodgkiss , IJ. 2000 . Biodiversity of fungi on submerged wood in Hong Kong streams . Aquat Microb Ecol. , 21 : 289 – 298 .
- Tsui , CKM , Hyde , KD and Hodgkiss , IJ. 2003 . “ Methods for investigating the biodiversity and distribution of freshwater ascomycetes and anamorphic fungi on submerged wood ” . In Freshwater Mycology. Fungal Diversity Research Series # 10 , Edited by: Tsui , CKM and Hyde , KD . 195 – 209 . Hong Kong : Hong Kong University Press .
- Udaiyan , K. 1989 . Some interesting ascomycetes from water cooling towers . Kavaka , 17 : 11 – 16 .
- Udaiyan , K and Hosagoudar , VS. 1991 . Some interesting fungi from the industrial water cooling towers of Madras. II . J Econ Taxon Bot. , 15 : 649 – 665 .
- Vijaykrishna , D , Jeewon , R and Hyde , KD. 2006 . Molecular taxonomy, origins and evolution of freshwater ascomycetes . Fung Divers. , 23 : 351 – 390 .
- Webster , J and Descals , E. 1981 . “ Morphology, distribution and ecology of conidial fungi in freshwater habitats ” . In Biology of Conidial Fungi , Edited by: Cole , GT and Kendrick , B . Vol 1 , 295 – 355 . New York : Academic Press .
- Willoughby , LB and Archer , JF. 1973 . The fungal spora of a freshwater stream and its colonization pattern on wood . Freshw Biol. , 3 : 219 – 239 .