Abstract
Protein tyrosine phosphatase 1B (PTP1B) is an attractive therapeutic target for diabetes, playing a major role in negative regulation of the insulin signaling pathway. Bioassay-guided investigations of an MeOH extract of the Antarctic lichen, Lecidella carpathica, afforded three PTP1B inhibitory metabolites: hopane-6α,22-diol (1), brialmontin 1 (2), and atraric acid (3), along with two aromatic metabolites (4 and 5) previously isolated from a different Antarctic lichen species. Their structures were determined by analysis of NMR and MS data. Compounds 1–3 inhibited PTP1B activity in a dose-dependent manner with IC50 values of 3.7, 14.0 and 51.5 μM, respectively, and kinetic analyses of PTP1B inhibition by compounds 1 and 2 suggested that these compounds inhibit PTP1B activity in a competitive manner. In addition, 6,22-hopanediol (1) displayed some selectivity toward PTP1B over other protein tyrosine phosphatases, such as TCPTP (IC50 = 8.4 μM), SHP-2 (IC50 > 68 μM), LAR (IC50 > 68 μM), and CD45 (IC50 > 68 μM).
Introduction
Several protein tyrosine phosphatases (PTPs) play a critical role in the regulation of a variety of cellular processes, such as growth, proliferation and differentiation, metabolism, immune response, cell-cell adhesion, and cell-matrix contacts (Fischer et al. Citation1991; Hunt Citation1995). Among these, protein tyrosine phosphatase 1B (PTP1B) is a major nontransmembrane phosphotyrosine phosphatase in human tissues (Saltiel and Kahn Citation2001), and a number of genetic and biochemical studies have demonstrated that PTP1B is a major negative regulator of insulin receptor signaling (Elchebly et al. Citation1999; Klaman et al. Citation2000). In addition, recent studies have shown that the leptin signaling pathway can be attenuated by PTPs, and there is compelling evidence that PTP1B is also involved in this pathway (Klaman et al. Citation2000; Koren Citation2007). Therefore, PTP1B is now considered as an attractive target in efforts to develop new treatments for type 2 diabetes and related metabolic syndromes (Johnson et al. Citation2002; Dadke et al. Citation2003; Koren Citation2007; Lee and Wang Citation2007).
Lichens are symbiotic organisms composed of a fungus and its photosynthesizing partner that can be either an alga or a cyanobacterium (Huneck Citation1999). Most lichens are adapted to extreme ecological conditions and are known to respond very sensitively to changes in their microhabitat and to surrounding environment conditions, thereby leading to the production of antimicrobial and antiherbivore metabolites (Kumar and Műller Citation1999; Ingólfsdóttir Citation2002; Lohezic-Le Devehat et al. Citation2007). Regarding the utilization of lichen metabolites, several lichen extracts have been used as remedies in folk medicine, and a variety of biological activities of lichen metabolite, including antibiotic, antimycobacterial, antiviral, analgesic and antipyretic properties, have been reported. (Huneck Citation1999; Kumar and Műller Citation1999; Ernst-Russell et al. Citation2000). However, secondary metabolites from Antarctic lichens have rarely been the subject of chemical and / or pharmacological investigations.
In the course of our studies of secondary metabolites from Antarctic lichens and mosses (Seo et al. Citation2008a,b; Citation2009a,b), several metabolites have been identified as new naturally occurring PTP1B inhibitors. As a part of our on-going studies of Antarctic lichens, an MeOH extract of dried samples of Lecidella carpathica was selected for further bioassay-guided investigations based on the observation of significant PTP1B inhibitory (>90%) effect at a concentration of 30 μg / ml. This report describes the isolation, structure elucidation and biological activities of compounds 1–5 ().
Materials and methods
Experimental procedures
ESIMS data were obtained using an API-2000 ESI-MS instrument (Applied Bio-System, Foster City, CA, USA)at Pukyung National University, South Korea. NMR spectra (1D and 2D) were recorded in DMSO-d 6 and CDCl3 using a JEOL JNM ECP-400 spectrometer (400 MHz for 1H and 100 MHz for 13C), and chemical shifts were referenced relative to the respective residual solvents (δH / δC = 2.49 / 39.5 for DMSO-d 6; δH / δC = 7.26 / 77.0 for CDCl3). HMQC and HMBC experiments were optimized for 1 J CH = 140 Hz and n J CH = 8 Hz, respectively. Solvents for extractions and flash column chromatography were reagent grade and used without further purification. Solvents used for HPLC were analytical grade. Flash column chromatography was carried out using YMC octadecyl-functionalized silica gel (ODS-A, 75-μm particle size). HPLC separations were performed on a prep-C18 column (21.2 × 150 mm; 5 μm particle size) with a flow rate of 5 ml / min. Compounds were detected by UV absorption at 254 nm and / or ELSD (Evaporative Light Scattering Detector).
Specimen collection and identification
Lecidella carpathica was collected from Barton Peninsula around King Sejong Station (S 62°13′, W 58°47′) on King George Island, Antarctica, in January 2009. Voucher specimens (reference SL-8) have been deposited in the Korea Polar Research Institute.
Isolation and characterization of compounds 1–5
A dried sample of L. carpathica (4.6 g) was extracted with MeOH (1 l × 2) for 24 h. The resulting crude MeOH extract (634.6 mg) was subjected to C18-functionalized silica gel flash column chromatography (5 × 40 cm), eluting with a stepwise gradient of 20, 40, 60, 70, 80, 90 and 100 % (v / v) MeOH in H2O (500 ml each) followed by 50% MeOH in CH2Cl2 (500 ml).
The fraction eluted with 100% MeOH (97.8 mg) was then subjected to semi-preparative reversed-phase HPLC using a gradient from 80 to 100% MeOH in H2O (0.1% formic acid) over 35 min to yield 2 (3.9 mg; t R = 34.2 min), 3 (3.7 mg; t R = 11.7 min) and 5 (15.7 mg, t R = 11.7 min).
The fraction (186.2 mg) eluted with 50 % MeOH in CH2Cl2 was subjected to semi-preparative reversed-phase HPLC using a gradient from 80 to 100 % MeOH in H2O (0.1% formic acid) over 25 min to yield 1 (32.2 mg; t R = 43.2 min) and 4 (1.6 mg, t R = 28.3 min).
Hopane-6α,22-diol (1). White amorphous powder; ESIMS m / z 467 [M + Na] + ; 1H-NMR (400 MHz, DMSO-d 6) δ: 0.77 (1H, m, H-1), 1.56 (1H, m, H-1), 1.40 (1H, m, H-2), 1.60 (1H, m, H-2), 1.12 (1H, m, H-3), 1.24 (1H, m, H-3), 0.75 (1H, m, H-5), 3.74 (1H, m, H-6), 1.34 (1H, m, H-7), 1.18 (1H, m, H-9), 1.36 (1H, m, H-11), 1.60 (1H, m, H-11), 1.25 (1H, m, H-12), 1.48 (1H, m, H-12), 1.29 (1H, m, H-13), 1.06 (1H, m, H-15), 1.24 (1H, m, H-15), 1.53 (1H, m, H-16), 1.93 (1H, br d, J = 13.2 Hz, H-16), 1.32 (1H, m, H-17), 0.87 (1H, m, H-19), 1.43 (1H, m, H-19), 1.48 (1H, m, H-20), 1.64 (1H, m, H-20), 2.10 (1H, dd, J = 8.8 and 19.6 Hz, H-21), 1.11 (3H, s, H-23), 0.94 (3H, s, H-24), 0.81 (3H, s, H-25), 0.97 (3H, s, H-26), 0.91 (3H, s, H-27), 0.71 (3H, s, H-28), 1.03 (3H, s, H-29), 1.07 (3H, s, H-30), 3.92 (1H, d, J = 6.4 Hz, 6-OH), 3.85 (1H, s, 22-OH) ; 13C-NMR (100 MHz, DMSO-d 6) δ: 39.5 (C-1), 18.14 (C-2), 43.6 (C-3), 33.4 (C-4), 60.0 (C-5), 66.6 (C-6), 44.7 (C-7), 42.1 (C-8), 49.3 (C-9), 38.6 (C-10), 20.6 (C-11), 23.7 (C-12), 48.9 (C-13), 41.5 (C-14), 33.9 (C-15), 21.3 (C-16), 53.8 (c-17), 43.5 (C-18), 40.9 (C-19), 26.2 (C-20), 50.4 (C-21), 71.6 (C-22), 36.7 (C-23), 22.0 (C-24), 16.97 (C-25), 18.06 (C-26), 16.86 (C-27), 15.9 (C-28), 28.9 (C-29), 30.9 (C-30).
Brialmontin 1( 2 ). Brown amorphous powder; ESIMS m / z 395 [M + Na]+; 1H-NMR (400 MHz, CDCl3) δ: 2.18 (3H, s, H-8), 2.19 (3H, s, H-9), 2.31 (3H, s, H-10), 6.64 (1H, s, H-1′), 3.83 (3H, br s, 2-OCH3), 3.81 (3H, s, 4-OCH3), 3.71 (3H, s, 2′-OCH3), 2.21 (3H, s, H-7′), 2.27 (3H, s, H-8′), 2.35 (H-9′); 13C-NMR (100 MHz, CDCl3) δ: 120.9 (C-1), 158.8 (C-2), 116.7 (C-3), 154.6 (C-4), 124.7 (C-5), 135.1 (C-6), 167.0 (C-7), 17.2 (C-8), 12.6 (C-9), 9.6 (C-10), 61.9 (2-OCH3), 60.1 (4-OCH3), 110.2 (C-1′), 155.8 (C-2′), 122.2 (C-3′), 148.6 (C-4′), 126.4 (C-5′), 133.5 (C-6′), 20.5 (C-7′), 12.4 (C-8′), 9.6 (C-9′), 55.7 (2′-OCH3).
Atraric acid ( 3 ). Brown amorphous powder; ESIMS m / z 195 [M–H]-; 1H-NMR (CDCl3, 400 MHz) δ: 2.10 (3H, s, 3-CH3), 2.40 (3H, s, 6-CH3), 3.90 (3H, s, 7-OCH3), 5.24 (1H, s, 4-OH), 6.18 (1H, s, H-5), 12.02 (1H, s, 2-OH).
Atranorin ( 4 ). Brown amorphous powder; ESIMS m / z 373 [M–H]-; 1H-NMR (400 MHz, CDCl3) δ: 6.40 (1H, s, H-5), 10.36 (1H, s, H-8), 2.69 (3H, s, H-9), 6.52 (1H, s, H-6′), 2.09 (3H, s, H-7′), 2.55 (3H, s, H-9′), 3.99 (3H, s, 8′-OCH3), 12.51 (1H, s, 2-OH), 12.56 (1H, s, 4-OH), 11.96 (1H, s, 3′-OH,); 13C-NMR (100 MHz, CDCl3) δ: 102.8 (C-1), 169.1 (C-2), 108.5 (C-3), 167.5 (C-4), 112.8 (C-5), 152.4 (C-6), 169.7 (C-7), 193.8 (C-8), 25.6 (C-9), 151.9 (C-1′), 116.8 (C-2′), 162.9 (C-3′), 110.3 (C-4′), 139.9 (C-5′), 116.0 (C-6′), 9.4 (C-7′), 172.2 (C-8′), 24.0 (C-9′), 52.3 (8′-OCH3); HMBC data, H-5 → C-1, 3, 4, 9; H-8 → C-3, 4, 5; H-9 → C-1, 5, 6; H-6′ → C-1′, 2′, 4′, 9′; H-7′ → C-1′, 2′, 3′; H-9′ → C-4′, 5′, 6′; 2-OH → C-1, 2, 3; 4-OH → C-3, 4, 5; 3′-OH → C-2′, 3′, 4′; 8′-OCH3 → C-8′.
Methyl haematommate ( 5 ). Brown amorphous powder; ESIMS m / z 209 [M - H]-; 1H-NMR (400 MHz, CDCl3) δ: 6.29 (1H, s, H-5), 2.53 (3H, s, H-8), 3.96 (3H, s, 7-OCH3), 12.89 (1H, s, 2-OH), 12.41 (1H, s, 4-OH), 10.34 (3-CHO); 13C-NMR (100 MHz, CDCl3) δ: 103.9 (C-1), 168.3 (C-2), 108.4 (C-3), 166.6 (C-4), 112.1 (C-5), 152.3 (C-6), 172.0 (C-7), 25.2 (C-8), 194.0 (3-CHO), 52.3 (7-OCH3).
Assay procedures
Protein tyrosine phosphatase activity was measured using p-nitrophenyl phosphate (pNPP) as a substrate. Assays were performed in 100 μl volumes comprising 50 mM citrate (pH 6.0), 0.1 M NaCl, 1 mM EDTA and 1 mM dithiothreitol (DTT) (for PTP1B and TCPTP), 25 mM Tris–HCl pH 7.0, 5 mM DTT, 0.01% Brij-35 detergent solution (v / v), 0.1% BSA (w / v), 50 mM NaCl, 2 mM EDTA (for SHP-2), 25 mM Tris–HCl pH 7.0, 5 mM DTT, 0.01% Brij-35 detergent solution (v / v), 50 mM NaCl, 2 mM EDTA (for LAR), or 50 mM Hepes pH 7.2, 1 mM DTT, 0.05% NP-40 (v / v), 1 mM EDTA (for CD45). The substrate (pNPP) was used at the concentrations of 4 mM for PTP1B, 8 mM for TCPTP, 25 mM for SHP-2, 1 mM for LAR, or 5 mM for CD45. Assays were performed for different times (30–60 min at 37 oC) for different enzymes, ensuring linearity was maintained. The reaction was terminated by addition of 50 μl of 10 N NaOH, and the amount of produced p-nitrophenol was estimated by measuring the absorbance at 405 nm. The non-enzymatic hydrolysis of pNPP was corrected by measuring the absorbance at 405 nm in the absence of the enzymes.
Kinetic analysis
The reaction mixture consisted of different concentrations of pNPP as a PTP1B substrate in the absence or presence of compounds 1 and 2, and assayed as described above. Data were fitted by nonlinear regression analysis according to a Michaelis–Menten kinetic model (Graphpad Prism version 4.02).
Results and discussion
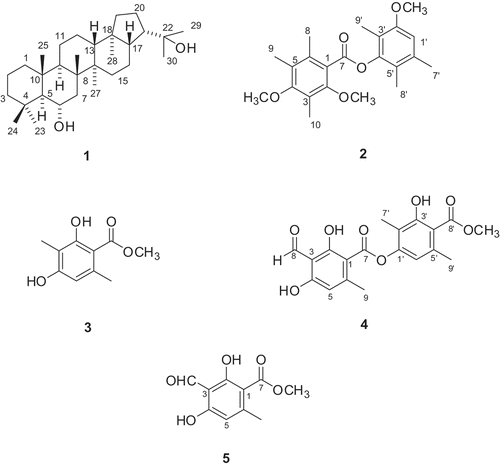
To isolate and identify the PTP1B inhibitory active principle(s) from the MeOH extract of the sample of L. carpathica, we performed bioassay and 1H-NMR guided fractionation and purification by using C18-functionalized silica gel column chromatography and HPLC, which led to the isolation of five lichen metabolites (compounds 1–5). The isolated compounds were identified as hopane-6α,22-diol (1) (König and Wright Citation1999), brialmontin 1 (2) (Vinet et al. Citation1990), atraric acid (3) (Ahad et al. Citation1991), atranorin (4) (König and Wright Citation1999; Athukoralage et al. Citation2001), and methyl haematommate (5) (Kouam et al. Citation2005) by analysis of NMR and MS data, along with a comparison of the literature.
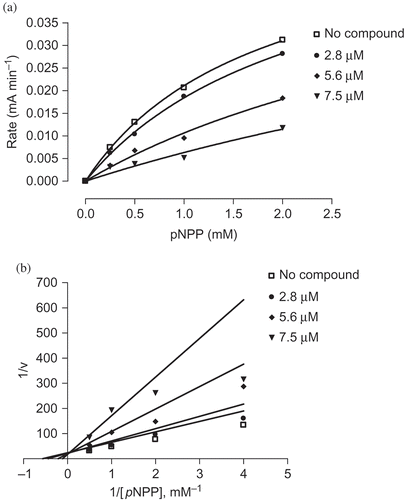
Among the isolated metabolites, compounds 1–3 were evaluated for their inhibitory effects against the activity of PTP1B in vitro. Compounds 4 and 5 had been encountered in our previous study of the Antarctic lichen, Stereocaulon alpinum (Seo et al. Citation2009b). In the enzyme inhibition assay, hopane-6α,22-diol (1) exhibited the most potent PTP1B inhibitory activity in a dose-dependent manner with an IC50 value of 3.7 μM, while brialmontin 1 (2) and atraric acid (3) displayed lower inhibitory effects, showing IC50 values of 14.0 and 51.5 μM, respectively. A known PTP1B inhibitor, ursolic acid (IC50 = 3.1 μM) was used as a positive control in the assay (Na et al. Citation2006; Zhang et al. Citation2006). The characteristics of the inhibition of PTP1B by compounds 1 and 2 were then analyzed. PTP1B was incubated with increasing concentrations of compounds 1 and 2 and full velocity curves were determined ( and ). Non-linear regression analysis showed that the data best fit a competitive model of inhibition, and re-plotting of the data as Lineweaver–Burk transformations confirmed this result, displaying the characteristic intersecting line pattern for competitive inhibition. Therefore, it was shown that both compounds 1 and 2 bind to the active site within PTP1B. In the development of PTP1B inhibitors from natural products or synthetic compounds, selectivity against other PTPs is one of the biggest issues (Bialy and Waldmnn Citation2005). Therefore, the specific inhibitory activities of compound 1 toward a small panel of PTPs including cytosolic PTPs such as T-cell protein tyrosine phosphatase (TCPTP) and src homology phosphatase-2 (SHP-2), as well as receptor-like PTPs such as leukocyte antigen-related phosphatase (LAR), and CD45 tyrosine phosphatase (CD45) were evaluated. As shown in , inhibition selectivity of compound 1 over TCPTP was not significant, but compound 1 appeared to possess no inhibitory effect against other PTPs such as SHP-2, LAR and CD45 up to the 78 μM level.
Table 1. Inhibitory activity of the 6,22-hopanediol against PTPs.
Figure 3. Substrate titration reveals that compound 2 is a classical competitive inhibitor that inhibits substrate binding (constant K m) but not substrate catalysis (V max). (a) Velocity curves performed with PIP1B in the presence of increasing concentrations of compound 2. (b) Lineweaver–Burk transformations of data from (a). Data are expressed as mean initial velocity for n = 3 replicates at each substrate concentration.
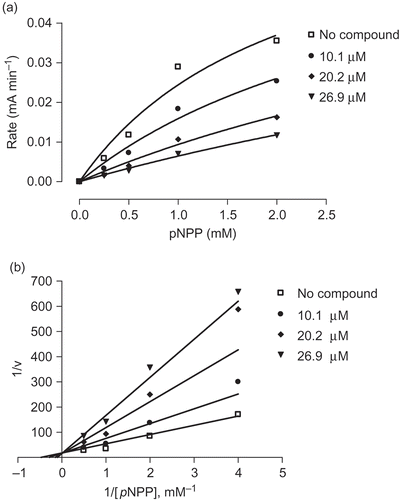
Figure 2. Substrate titration reveals that compound 1 is a classical competitive inhibitor that inhibits substrate binding (constant K m) but not substrate catalysis (V max). (a) Velocity curves performed with PIP1B in the presence of increasing concentrations of compound 1. (b) Lineweaver–Burk transformations of data from (a). Data are expressed as mean initial velocity for n = 3 replicates at each substrate concentration.
In summary, bioassay-guided fractionation of an MeOH extract of Antarctic lichen, L. carpathica, afforded two metabolites (1 and 2) as moderate to strong PTP1B inhibitory components, along with three additional lichen metabolites, atraric acid (3), atranorin (4) and methyl haematommate (5). Hopane-6α,22-diol (1) is a member of hopane-based triterpenes, which have taxonomic importance. Although its antimycobacterial activity has been reported (König et al. Citation2000), PTP1B inhibitory activity of this compound is now being reported for the first time. Several ursane- and oleanane-type triterpenoids from plant sources have been reported to have a similar magnitude of PTP1B inhibitory activity as that of compound 1 (Na et al. Citation2006; Zhang et al. Citation2006, Citation2008; Lin et al. Citation2008). In addition, these known plant metabolites are identified as competitive inhibitors of PTP1B, and most of these inhibitors commonly possess carboxylic acid functionality in the structures. Therefore, it is noteworthy that compound 1 represents an additional member of the PTP1B inhibitory tripenoids, yet with different carbon-skeleton and no carboxylic acid functionality. Brialmontin 1 (2) is a member of the lichen depsides originally isolated from L. brialmontii (Vinet et al. Citation1990), and no biological activity of this compound have been revealed. Therefore, compound 2 is an additional member of the phenolic PTP1B inhibitory lichen metabolites along with previously identified metabolites such as gyrophoric acid (Seo et al. Citation2008a) and lobaric acid (Seo et al. Citation2008b).
Acknowledgement
This work was supported by a grant from the KOPRI Project (PE11060 / utilization of novel metabolites from polar organisms).
References
- Ahad , AM , Goto , Y , Kiuchi , F , Tsuda , Y , Kondo , K and Sato , T . 1991 . Nematocidal principles in “Oakmoss Absolute” and nematocidal activity of 2,4-dihydroxybenzoates . Chem Pharm Bull. , 39 : 1043 – 1046 .
- Athukoralage , PS , Herath , HMTB , Deraniyagala , SA , Wijesundera , RLC and Weerasinghe , PA . 2001 . Antifungal constituent from Gordonia dassanayakei . Fitoterapia. , 72 : 565 – 567 .
- Bialy , L and Waldmann , H . 2005 . Inhibitors of protein tyrosine phosphatases: next-generation drugs? . Angew Chem Int Ed. , 44 : 3814 – 3839 .
- Dadke , S and Chernoff , J . 2003 . Protein-tyrosine phosphatase 1B as a potential drug target for obesity . Curr Drug Targets: Immune Endocr Metab Disord. , 3 : 299 – 304 .
- Elchebly , M , Payette , P , Michaliszyn , E , Cromlish , W , Collins , S , Loy , A L , Normandin , D , Cheng , A , Himms-Hagen , J , Chan , CC , Ramachandran , C , Gresser , MJ , Tremblay , ML and Kennedy , BP . 1999 . Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene . Science , 283 : 1544 – 1548 .
- Ernst-Russell , MA , Chai , CLL , Wardlaw , JH and Elix , JA . 2000 . Euplectin and coneuplectin, new naphthopyrones from the lichen Flavoparmelia euplecta . J Nat Prod. , 63 : 129 – 131 .
- Fischer , EH , Charbonneau , H and Tonks , NK . 1991 . Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes . Science , 253 : 401 – 406 .
- Hunter , T . 1995 . Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling . Cell , 80 : 225 – 236 .
- Huneck , S . 1999 . The significance of lichens and their metabolites . Naturwissenschaften , 86 : 559 – 570 .
- Ingólfsdóttir , K . 2002 . Usnic acid . Phytochemistry , 61 : 729 – 736 .
- Johnson , TO , Ermolieff , J and Jirousek , MR . 2002 . Protein tyrosine phosphatase 1B inhibitors for diabetes . Nat Rev Drug Discov. , 1 : 696 – 709 .
- Koren , S . 2007 . Inhibition of the protein tyrosine phosphatase PTP1B: potential therapy for obesity, insulin resistance and type-2 diabetes mellitus . Best Pract Res Clin Endocrinol Metab. , 21 : 621 – 640 .
- Klaman , LD , Boss , O , Peroni , OD , Kim , JK , Martino , JL , Zabolotny , JM , Moghal , N , Lubkin , M , Kim , YB , Sharpe , AH , Stricker-Krongrad , A , Shulman , GI , Neel , BG and Kahn , BB . 2000 . Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice . Mol Cell Biol. , 20 : 5479 – 5489 .
- Kumar , KCS and Műller , K . 1999 . Lichen metabolites. 1. Inhibitory action against leukotriene B4 biosynthesis by a non-redox mechanism . J Nat Prod. , 62 : 817 – 820 .
- Kouam , SF , Ngadjui , BT , Krohn , K , Wafo , P , Ajaz , A and Choudhary , MI . 2005 . Prenylated anthronoid antioxidants from the stem bark of Harungana madagascariensis . Phytochemistry , 66 : 1174 – 1179 .
- König , GM and Wright , AD . 1999 . 1H and 13C-NMR and biological investigations of four lichen-derived compounds . Phytochem Anal. , 10 : 279 – 284 .
- König , GM , Wright , AD and Franzblau , SG . 2000 . Assessment of antimycobacterial activity of series of mainly marine derived natural products . Planta Med. , 66 : 337 – 342 .
- Lee , S and Wang , Q . 2007 . Recent development of small molecular specific inhibitor of protein tyrosine phosphatase 1B . Med Res Rev. , 27 : 553 – 573 .
- Le , Lohezic- , Devehat , F , Tomasi , S , Elix , JA , Bernard , A , Rouaud , I , Uriac , P and Boustie , J . 2007 . Stictic acid derivatives from the lichen Usnea articulata and their antioxidant activities . J Nat Prod. , 70 : 1218 – 1220 .
- Lin , Z , Zhang , Y , Zhang , Y , Shen , H , Hu , L , Jiang , H and Shen , X . 2008 . Oleanolic acid derivative NPLC441 potently stimulates glucose transport in 3T3-L1 adipocytes via a multi-target mechanism . Biochem Pharmacol. , 76 : 1251 – 1262 .
- Na , M , Tang , S , He , L , Oh , H , Kim , BS , Oh , WK , Kim , BY and Ahn , JS . 2006 . Inhibition of protein tyrosine phosphatase 1B by ursane-type triterpenes isolated from Symplocos paniculata . Planta Med. , 72 : 261 – 263 .
- Saltiel , AR and Kahn , CR . 2001 . Insulin signalling and the regulation of glucose and lipid metabolism . Nature , 414 : 799 – 806 .
- Seo , C , Choi , YH , Sohn , JH , Ahn , JS , Yim , JH , Lee , HK and Oh , H . 2008a . Ohioensins F and G: protein tyrosine phosphatase 1B inhibitory benzonaphthoxanthenones from the Antarctic moss Polytrichastrum alpinum . Bioorg Med Chem Lett. , 18 : 772 – 775 .
- Seo , C , Sohn , JH , Park , SM , Yim , JH , Lee , HK and Oh , H . 2008b . Usimines A-C, bioactive usnic acid derivatives from the Antarctic lichen Stereocaulon alpinum . J Nat Prod. , 71 : 710 – 712 .
- Seo , C , Choi , YH , Ahn , JS , Yim , JH , Lee , HK and Oh , H . 2009a . PTP1B inhibitory effects of tridepside and related metabolites isolated from the Antarctic lichen Umbilicaria antarctica . J Enzyme Inhib Med Chem. , 24 : 1133 – 1137 .
- Seo , C , Sohn , JH , Ahn , JS , Yim , JH , Lee , HK and Oh , H . 2009b . Protein tyrosine phosphatase 1B inhibitory effects of depsidone and pseudodepsidone metabolites from the Antarctic lichen Stereocaulon alpinum . Bioorg Med Chem Lett. , 19 : 2801 – 2803 .
- Vinet , C , Quilhot , W , Gambaro , V and Garbarino , JA . 1990 . Studies on Chilean lichens, XIII. Polysubstituted depsides from Lecania brialmontii . J Nat Prod. , 53 : 500 – 502 .
- Zhang , W , Hong , D , Zhou , Y , Zhang , Y , Shen , Q , Li , J , Hu , L and Li , J . 2006 . Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake . Biochim Biophy Acta , 1760 : 1505 – 1512 .
- Zhang , YN , Zhang , W , Hong , D , Shi , L , Shen , Q , Li , JY , Li , J and Hu , LH . 2008 . Oleanolic acid and its derivatives: New inhibitor of protein tyrosine phosphatase 1B with cellular activities . Bioorg Med Chem. , 16 : 8697 – 705 .