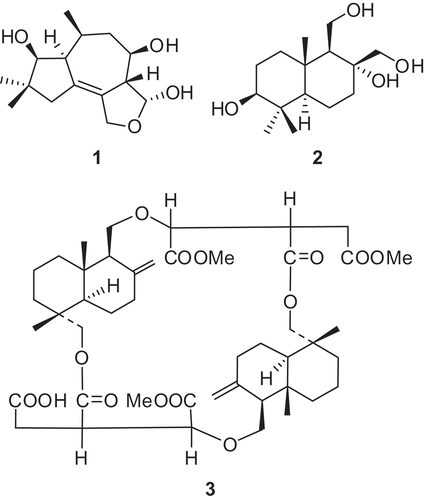
Abstract
Graphical abstract
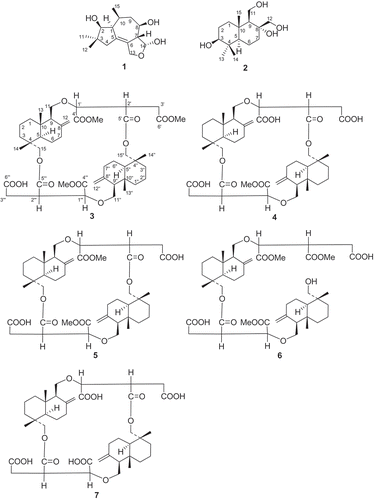
Four new sesquiterpenes, including a tremulane sesquiterpene (1), a drimane sesquiterpene (2), and two dimeric drimane sesquiterpenes (3 and 4), plus previously known dimeric drimane sesquiterpenes, cryptoporic acid D (5), cryptoporic acid E (6) and dimeric 15-hydroxyl cryptoporic acid H (7), were isolated from a fermentation extract of Marasmius cladophyllus F070624009. This strain was isolated from a spore suspension of a Phylloporus sp. collected from the Wuyi Mountain Conservation Area in Fujian Province, China. The chemical structures of 1–7 were elucidated by spectroscopic analyses, including 1D- and 2D-NMR, and on the basis of HR Q-TOF MS data.
1. Introduction
Mushrooms are known to be a rich source of natural products (Bose Citation1953; Monro Citation2003; Li and Oberlies Citation2005; Zaidman et al. Citation2005; Zheng and Shen Citation2009). Marasmius cladophyllus belongs to the family Tricholontataceae, and closely related genera have long been known to produce novel and active secondary metabolites (Kavanagh et al. Citation1949; Dugan et al. Citation1966; Hoefle et al. Citation1976; King et al. Citation1977). Previously, two sesquiterpenes, namely alliacolide (King et al. Citation1977) and marasmic acid (Dugan et al. Citation1966), have been isolated from the genus Marasmius. Due to limitations in obtaining wild mushrooms, which usually replicate in small quantity and have a very short season annually, we employed the sustainable mycelia fermentation approach to mine for macrofungal products, especially sesquiterpenes, from the genus Marasmius. In this paper, we report the isolation and structural elucidation of a new tremulane sesquiterpene (1) and six drimane sesquiterpenes (2–7), including three new ones (2–4), from Marasmius cladophyllus F070624009.
2 Methods
2.1 General
Column chromatography (CC): silica gel (SiO2, 200–300 and 80–100 mesh; Qingdao Marine Chemical Factory, Qingdao, China), SiO2 GF254 (Merck, Darmstadt, Germany), RP-18 (Merck), and Sephadex LH-20 (Amersham Biosciences, Little Chalfont, UK) were used. TLC: precoated SiO2 GF254 plates (0.20–0.25 mm; Qingdao Marine Chemical Factory). Optical rotations: Perkin-Elmer 341 ploarimeter with MeOH as solvent. IR spectra: Nicolet FT-IR 360 in KBr. HR-Q-TOF-MS: Bruker Daltonios BioTOF-Q mass spectrometers; in m/z (rel. %). 1H- and 13C-NMR spectra: Bruker DRX-600 spectrometer, at 600 (1H), and 150 (13C) MHz; in MeOD; δ in ppm relative to Me4Si, J in Hz.
2.2 Isolation and fermentation of the fungal strain
The fungal strain F070624009 was isolated from a spore suspension of a Phylloporus sp., collected from the Wuyi Mountain Conservation Area in Fujian Province, China. Both traditional morphological assessment and internal transcribed spaces (ITS) sequence analysis were performed to characterize it as Marasmius cladophyllus and named as F070624009. A nucleotide-to-nucleotide BLAST query of the NCBI database yielded Marasmius cladophyllus AY216475 as the closest match to the ITS rDNA of F070624009 (99%). The strain F070624009 was cultivated on potato dextrose agar (PDA) plates for 14 days at 25°C in darkness. Every plate contained 20 ml PDA media and, in total, 4 l were cultivated under the above fermentation conditions.
2.3 Extraction and Isolation
The cultured agar was chopped, diced and extracted with 4 l EtOAc/MeOH/AcOH (80:15:5, v/v) at room temperature overnight. The organic solution was collected through filtration and the remaining agar residue was extracted successively with the same solvent until the filtrate was colorless. The combined filtrate, upon evaporation, yielded a crude yellow syrup extract (1.98 g). The extract was subjected to MPLC [(RP-18 (80 g); gradient aq. (MeOH 0%, 30%, 50%, 70% and 100% resp., 1 l each)] to obtain 10 fractions, i.e. Fr.1–Fr.10.
Fr.1 (210.9 mg) was separated by CC (Sephadex LH-20 (80 g); MeOH) to obtain four fractions Fr.1.1–Fr.1.4. Fr.1.3 (56.7 mg) was further purified by MPLC [(RP-18 (30 g); gradient aq. MeOH (15%, 20%, 25% and 100% resp.,100 ml each)] to obtain Fr.1.3.1–Fr1.3.2. Fr1.3.2 (8.4 mg) was further purified by CC (SiO2; petroleum ether (PE)/ethyl acetate (EA), 5:1, 3:1 and 1:1, v/v) to obtain 1 (1.2 mg) and 2 (1.2 mg).
Fr.7 (970 mg) was subjected to MPLC (RP-18 (80 g); gradient aq. MeOH (80%, 85%, 90% and 100% resp., 1 l each) to obtain six fractions. Fr.7.5 (31.4 mg) was further purified by CC (SiO2; PE/EA, 10:1, 5:1 and 3:1, v/v) to obtain 6 (8.6 mg); Fr.7.6 (23.2 mg) was purified by CC (SiO2; PE/EA, 10:1, 3:1 and 2:1, v/v) to obtain 3 (22 mg). Fr.7.2 (72.1 mg) was subjected to MPLC (PR-18, 30 g; 82% MeOH) to obtain three fractions Fr.7.2.1–Fr.7.2.3. Fr.7.2.2 (40 mg) was further purified by CC (SiO2; PE/EA, 5:1, 3:1, 2:1 and 1:1, v/v) to obtain 7 (3 mg) and 4 (10.1 mg). Fr.7.3 (506.2 mg) was subjected to MPLC (PR-18 (80 g); 82% MeOH) to obtain five fractions Fr.7.3.1–Fr.7.3.5. Fr.7.3.1 (142.4 mg) was purified by CC (Sephadex LH-20 (80 g); MeOH) to obtain 5 (3.7 mg).
Cladophyllol
(=(3R*,3aR*,4R*,6S*,6aS*,7S*)-6,8,8-trimethyl-1,3,3a,4,5,6,6a,7,8,9-decahydroazuleno[4,5-c]furan-3,4,7-triol; 1). White powder; UV (MeOH): vmax (lgϵ): 224 nm (2.17); IR (KBr) λmax: 3285, 1650 cm−1; HR Q-TOF-MS m/z: 291.1575 ([M + Na]+, C15H24O4; calcd. 291.1567); 1H- and 13C- NMR: see .
Table 1. 1H- and 13C-NMR data (600 and 150 MHz, resp.; in CD3OD) of 1 (δ in ppm, J in Hz).
Drimane-3,8,11,12-tetraol
( = (2S*,4aS*,5S*,6R*,8aR*)-5,6-bis(hydroxymethyl)-1,1,4a-trimethyldecahydronaphthalene-2,6-diol; 2). White powder. UV (MeOH): vmax (lgϵ): 216 nm (1.42); [α]20 D = +15 (c = 0.05, MeOH); IR (KBr) λmax: 3285 cm−1; HR Q-TOF-MS m/z: 295.1881 ([M + Na]+, C15H28O4; calcd. 295.1880); 1H- and 13C-NMR: see .
Table 2. 1H- and 13C-NMR data (600 and 150 MHz, resp.; in CD3OD) of 2 (δ in ppm, J in Hz).
Cryptoporic acid J
( = (2R,3S)-2-(((1S*,4aR*,5R*,8aS*)-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)met hoxy)-3-(methoxycarbonyl)pentanedioic acid; 3). White powder, [α]20 D = −6.6 (c = 0.033, CHCl3); UV (MeOH): vmax (lgϵ): 207 nm (1.57); IR (KBr) λmax: 3415 cm−1; HR Q-TOF-MS m/z: 848.4812 ([M + NH4]+, C45H66O14; calcd. 848.4791); 1H- and 13C-NMR: see .
Table 3. 1H- and 13C-NMR data (600 and 150 MHz, resp.; in CD3OD) of 3 and 4 (δ in ppm, J in Hz).
3 Results and discussion
3.1 Structural elucidation
The morphological properties of isolate F070624009 were examined after incubation for 14 days at 25°C on PDA plates. This organism was identified to be Marasmius cladophyllus according to the ITS rDNA sequence (ITS1-5.8S-ITS2). The fermentation culture was extracted with EtOAc/MeOH/AcOH (80:15:5, v/v). The extract was separated by repeated column chromatography (RP-18, Sephadex LH-20 and SiO2) to afford compounds 1–7 ().
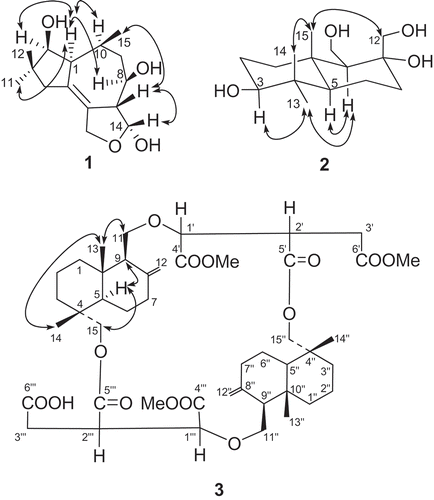
Compound 1 was isolated as a white powder. The molecular formula of 1 was determined to be C15H24O4 according to the HR Q-TOF-MS and NMR data. The IR absorptions at 3285, 1650 and 1300 cm − 1 indicated the presence of OH, C = C and ether groups, respectively. Inspection of the 1H- and 13C-NMR spectra () revealed the presence of three methyl groups (two singlets at δ 1.05 and 0.96, one doublet at δ 1.06), three methylenes, six methines and three quaternary carbons, including one oxymethylene and three oxymethine groups that were attributed to four oxygen substitutions in 1. The HMBC correlations from the protons of the two methyl groups at δ 1.05 (s, H-C(11)) and 0.96 (s, H-C(12)) to corresponding carbons determined one 5-carbon moiety (including C(2), C(3), C(4), C(11) and C(12)) (). This 5-carbon moiety was composed of two methyls, one methylenes and one oxymethine, indicating the presence of gem-dimethyl groups. The 1H-1H COSY correlations determined the structure of one more 8-carbon moiety including C(2), C(1), C(10), C(15), C(9), C(8), C(7) and C(14) (). The connection between C(1) and C(5) was assigned based on the HMBC correlations between H-C(4) and C(1) and C(5), and between H-C(10) and C(5), and H-C(2) and C(5). The connection between C(6) and C(7) was assigned according to the 1H-13C long-range correlations between H-C(14) to C(6), C(7) and C(13). The four oxygen substitutions was assigned at C(2), C(8), C(14) and C(13) based on their downshift of chemical shifts. The presence of three free hydroxyl groups was determined by the ESI–MS m/z 417.2 [M + Na]+ of the fully acetylated 1, which further supported the elucidation of an ether bond between C(13) and C(14). Based on the above, a tetrahydrofuran, cycloheptane and cyclopentane ring system was assigned to 1, which represents a tremulane sesquiterpene (Ayer and Cruz Citation1993; Wu et al. Citation2010). The relative configurations of 1 were determined by analysis of the ROESY spectrum. The presence of NOE correlations between H-C(1) and H-C(2), and H-C(1) and H-C(8), H-C(1) and H-C(10), and H-C(1) and H3-C(11) indicated the α-orientation of these protons and Me(11) (). Further, the NOESY cross-peaks between H-C(7) and H-C(14), and H-C(7) and Me(15) established the β-orientation of H-C(7), H-C(14) and Me(15) (). Thus, the structure of 1 was established to be (3R*,3aR*,4R*,6S*,6aS*,7S*)-6,8,8-trimethyl-1,3,3a,4,5,6,6a,7,8,9-decahydroazuleno[4,5-c]furan-3,4,7-triol, named as cladophyllol.
Figure 1. Structures of compounds 1, 2, and 3, the selected HMBC correlations (H → C) and 1H,1-COSY (bold line).
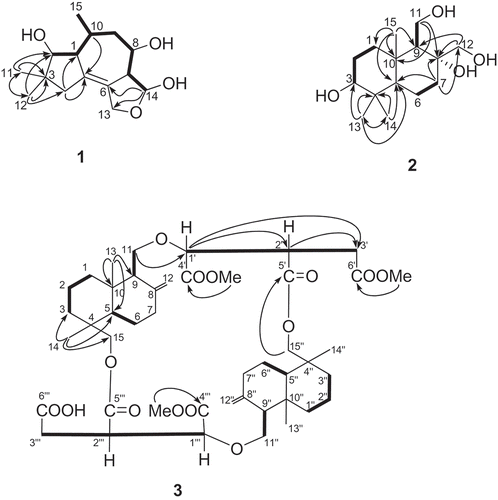
Compound 2 was obtained as a white powder. The molecular formula of 2 was determined to be C15H28O4 according to the HR Q-TOF-MS and NMR data. [α]20 D = + 15 (c = 0.05, MeOH). The IR absorption at 3285 cm − 1 indicated the presence of hydroxyl groups. The 13C-NMR and DEPT spectra of 2 () revealed the presence of 15 resonance signals attributed to 3 Me, 6 CH2 (two oxygenated), three CH (one oxygenated) and three quaternary carbons (one oxygenated), indicating a two-ring sesquiterpene backbone. The HMBC correlations from the protons of the three methyl groups at δ 1.00 (s, Me(13)), 0.80 (s, Me(14)) and 0.90 (s, Me(15)) to corresponding carbons determined a 9-carbon moiety (including C(3), C(4), C(5), Me(13) and Me(14), and C(1), C(10), C(9), C(15) and C(5)) (), which determined a six-membered ring together with the 1H-1H COSY correlations between H-C(1)/H-C(2)/H-C(3). The 1H-1H COSY correlations (H-C(6)/H-C(7), H-C(9)/H-C(11)), and the HMBC correlations from H-C(7), H-C(11) and H-C(12) to corresponding carbons determined the substructure of another six-membered ring (). The NOEs between H-C(3) and Me(13), H-C(9) and Me(13), H-C(5) and H-C(9) indicated the α-orientation of these protones and Me(13) (). Furthermore, the NOESY cross peaks between H-C(12) and Me(15), and Me(14) and Me(15) indicated the β-orientation of C(12), Me(14) and Me(15) (). Therefore, a drimane-type backbone was assigned to 2 (Cortèet al. Citation1998), and the relative configuration of 2 was established. Thus, the structure of 2 was established to be (2S*,4aS*,5S*,6R*,8aR*)-5,6-bis(hydroxymethyl)-1,1,4a-trimethyldecahydronaphthalene-2,6-diol, named as drimane-3,8,11,12- tetraol.
Compound 3 was obtained as a white powder. The molecular formula of 3 was determined to be C45H66O14 according to the HR Q-TOF-MS and NMR data (). [α]20 D = −6.6 (c = 0.033, CHCl3). The IR absorptions at 3450 and 1740, and 1725 cm−1 were in accordance with the presence of carboxylic and ester groups, respectively. The 13C-NMR and DEPT spectra of 3 revealed the presence of 45 resonance signals attributed to seven methyl, eighteen methylene, eight methine and twelve quaternary carbons including six carboxylic carbon atoms. The 1H-NMR data of 3 showed the presence of four tertiary methyls and three methoxyl groups. The 1H- and 13C-NMR spectra of 3 resembled with those of cryptoporic acid D (5), except for the prescence of a methoxyl group at δC 51.1 (OMe) and δH 3.68 (OMe), and the upfield shift of one of the carbonyl groups (Asakawa et al. Citation1992). The upfield shifted carbonyl was determined to be C (6’) by comparing with the13 C-NMR spectrum of cryptoporic acid D, determining the position of the extra methoxyl group at C(6’), which was further supported by the HMBC correlation from δH 3.68 (OMe) to C(6’) (). The NOEs between H-C(11) and H-C(13), and H-C(13) and H-C(14), indicated the β-orientation of those protones (). Furthermore, the NOESY cross peaks between H-C(5) and H-C(9), H-C(5) and H-C(15) indicated the α-orientation of the protons (). Therefore, the chemical structure of 3 was established and named as cryptoporic acid J.
The molecular formula of compound 4 was deduced as C43H62O14 according to NMR data and comparison with 3. The structure of 4 was elucidated on the basis of 1D- and 2D-NMR, and by comparing with those of cryptoporic acid D (5). The NMR spectra of 4 () were similar to those of 5. The only difference was the methoxyl group at C(4’) in 5 was replaced by a hydroxyl group in 4. Therefore, the structure of 4 was elucidated as cryptoporic acid K.
3.2 Biological study
The antibacterial activities of compounds 1–3 were tested against bacteria Escherichia coli, Bacillus subtilis and Mycobacterial smegmatis via the filter paper disc diffusion method. Each compound was performed at a concentration of 1.0 mg/ml with the loading volume 30 μl. Compound 3 had an inhibitory zone ca. 8 mm against Bacillus subtilis, while other compounds had no effects on the growth of tested bacteria at 30 μg/disc.
4 Conclusions
In this paper, we report the isolation and structure elucidation of a new tremulane sesquiterpene (1) and six drimane sesquiterpenes (2–7). Cladophyllol (1) was elucidated as a new tremulane-type sesquiterpenoid. The tremulanes, a class of unusual sesquiterpenes, first isolated in 1993 from the aspen tree rotting fungus Phellinus tremulae, were isolated recently from cultures of the basidiomycete Conocybe siliginea and cultures of Phellinusig niarius (Ayer and Cruz Citation1993; Zhou et al. 2008; Wu et al. Citation2010). Tremulane sesquiterpenoids have been assessed for their significant vascular-relaxing activities against PE-induced vasoconstriction (Wu et al. Citation2010). Compounds 2–7 were elucidated as drimane-type sesquiterpenoids. Cryptoporic acid J (3) and cryptoporic acid K (4) are new dimmers of drimane-type sesquiterpenes, belonging to the subfamily of cryptoporic acids (CAs) as a type of unusual esters of citric/isocitric acid. Our work was the first isolation of CAs from the cultures of higher fungi, rather than from fruiting bodies. CAs showed inhibitory effect on the release of superoxide anion radicals and displayed diverse bioactivities, including anticarcinogenic and the prevention human diseases caused by ischemia and inflammation (Asakawa et al. Citation1992). Thus, the biological activities of novel compounds 1–4 need to be further explored.
Acknowledgments
This work was financially supported by the Independent Innovation Foundation of Shandong University to C.-H. Lu (No. 2010TB016) and the 973 program (2010CB833802).
References
- Asakawa , Y , Hashimoto , T , Mizuno , Y , Tori , M and Fukazawa , Y . 1992 . Cryptoporic acids A-G, drimane-type sesquiterpenoid ethers of isocitric acid from the fungus . Cryptoporus volvatus. Phytochemistry , 31 : 579 – 592 .
- Ayer , WA and Cruz , ER . 1993 . The tremulanes, a new group of sesquiterpenes from the aspen rotting fungus . Phellinus tremulae. J Org Chem. , 58 : 7529 – 7534 .
- Bose , SR . 1953 . Antibiotics from higher fungi . Arch Mikrobiol. , 18 : 349 – 355 .
- Cortés , M , Moreno , L and López , J . 1998 . Partial Synthesis of (−)-11,12-Dinordriman-8-one and the (−)-Enantiomer of Polywood . J Chem Res. , S : 36 – 37 .
- Dugan , JJ , de Mayo , P , Nisbet , M , Robinson , JR and Anchel , M . 1966 . Terpenoids. XIV. The constitution and biogenesis of marasmic acid . J Am Chem Soc. , 88 : 2838 – 2844 .
- Hoefle , G , Gmelin , R and Luxa , HH . N'Galamulume-Treves M, Hatanaka SI. 1976. Structure of lentinoic acid: 2-(-glutamylamino)-4,6,8,10,10-pentaoxo-4,6,8,10-tetrathiaundecanoic acid . Tetrahedron Lett. , 17 3129 – 3132 .
- Kavanagh , F , Hervey , A and Robbins , WJ . 1949 . Antibiotic Substances from Basidiomycetes: IV . Marasmius Conigenus. Proc Natl Acad Sci USA , 35 : 343 – 349 .
- King TJ Farrell IW Halsall TG Thaller V 1977 Alliacolide, a new bicyclic sesquiterpene epoxy-lactone with a novel carbon skeleton from cultures of the fungus Marasmius alliaceus (Jacques ex Fr.)Fr: X-ray structure. J Chem Soc Chem Commun. 727 728
- Li , C and Oberlies , NH . 2005 . The most widely recognized mushroom . chemistry of the genus Amanita. Life Sci. , 78 : 532 – 538 .
- Monro , JA . 2003 . Treatment of cancer with mushroom products . Arch Environ Health , 58 : 533 – 537 .
- Wu , X , Lin , S , Zhu , C , Yue , Z , Yu , Y , Zhao , F , Liu , B , Dai , J and Shi , J . 2010 . Homo- and heptanor-sterols and tremulane sesquiterpenes from cultures of . Phellinus igniarius. J Nat Prod. , 73 : 1294 – 1300 .
- Zaidman , BZ , Yassin , M , Mahajna , J and Wasser , SP . 2005 . Medicinal mushroom modulators of molecular targets as cancer therapeutics . Appl Microbiol Biotechnol. , 67 : 453 – 468 .
- Zheng , Y and Shen , Y . 2009 . Clavicorolides A and B, Sesquiterpenoids from the Fermentation Products of Edible Fungus Clavicorona pyxidata . Org Lett. , 11 : 109 – 112 .